Abstract
The fatality of cancer is mainly bestowed to the property of otherwise benign tumor cells to become malignant and invade surrounding tissues by circumventing normal tissue barriers through a process called metastasis. S100A4 which is a member of the S100 family of calcium-binding proteins has been shown to be able to activate and integrate pathways both intracellular and extracellular to generate a phenotypic response characteristic of cancer metastasis. A large number of studies have shown an increased expression level of S100A4 in various types of cancers. However, its implications in cancer metastasis in terms of whether an increased expression of S100A4 is a causal factor for metastasis or just another after effect of several other physiological and molecular changes in the body resulting from metastasis are not clear. Here we describe the emerging preclinical and clinical evidences implicating S100A4 protein, in both its forms (intracellular and extracellular) in the process of tumorigenesis and metastasis in humans. Based on studies utilizing S100A4 as a metastasis biomarker and molecular target for therapies such as gene therapy, we suggest that S100A4 has emerged as a promising molecule to be tested for anticancer drugs. This review provides an insight in the (1) molecular mechanisms through which S100A4 drives the tumorigenesis and metastasis and (2) developments made in the direction of evaluating S100A4 as a cancer biomarker and drug target.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 S100 protein family
S100 proteins, named based on the observation that they are soluble in 100% saturated ammonium sulfate, are low molecular weight (10–12 kDa) acidic proteins that normally exist as symmetric homodimers stabilized by noncovalent interactions [1]. There are about 25 members in this family with each member having its unique features with the general property of calcium (Ca) binding. S100 proteins which mostly exist as monomers are composed of two EF-hand calcium-binding domains (helix–loop–helix motif) [2]. The N-terminal EF-hand (also known as the S100 hand, pseudo EF-hand, or half EF-hand) encompasses 14 amino acids and coordinates calcium weakly via backbone carbonyl oxygen atoms, while the C-terminal EF-hand (also known as the canonical EF-hand) is composed of 12 amino acid residues and coordinates calcium with a higher affinity (Kd = 2.6 μM) via side chain carboxylate oxygens [3]. For most S100 proteins, those are capable of binding calcium, the apo state is known as the inactive, closed conformation, and the calcium-bound state is known as the active open conformation. Upon calcium binding, a large conformational change results in the exposure of a hydrophobic binding pocket in each monomer, which is capable of binding to intracellular or extracellular proteins [3–5].This calcium-dependent conformational change is necessary for S100 proteins to interact with their protein targets and generates biological effects [6].
2 Nomenclature, genomic location, structural organization, and transcriptional regulation of S100A4
S100A4 is known by several aliases such as metastatin (Mts1), fibroblast-specific protein (FSP1), p9Ka, 18A2, pEL98, 42A, CAPL, and calvasculin ([6] and references therein). S100A4 is a polypeptide of 101 amino acids with a molecular mass of ∼11.5 kd [7].The human S100A4 gene is located at position 1q21 on chromosome 1 whereas it is placed at 3f3 and 2q34 position in mouse and rat chromosome, respectively [6]. The human S100A4 gene contains four exons two of which are non-coding at 5′ UTR position and may have a role in transcript regulation [6]. In addition, two splice variants have also been reported [7]. A study has shown that S100A4 promoter contains an ErbB2 response element [8]. Transcription of S100A4 gene is reported to be controlled by both positive and negative regulatory elements located within the first intron, which bind several transcription factors including I-κB [9, 10]. Recent studies have shown that enhancer and silencer elements of S100A4 gene may be strongly affected by the methylation, and this status of gene might have an association with its role in human cancer [11–13].
3 S100A4: protein structure
S100A4 consists of an X-type four-helical bundle (Fig. 1a). Helices I and IV of each subunit are responsible for the formation of the dimer interface of S100A4 protein [14]. The amino- and the carboxy-terminal calcium-binding loops of S100A4 comprise 14 and 12 amino acid residues, respectively. The amino-terminal EF-hand is built by the helix I, loop I (N-term calcium-binding loop) and helix II, while the carboxy-terminal EF-hand is formed by helix III, loop III (C-term calcium-binding loop) and helix IV(Fig. 1a). The two EF-hands are connected by a loop II termed the hinge region. Helix IV is followed by the carboxy-terminal tail (Fig. 1b) [14]. Dukhanina et al. demonstrated the presence of two types of hydrophobic regions in S100A4 protein [15]. One of the hydrophobic regions is reported to be accessible to probe in the absence of calcium while the other required calcium for its interaction with its target protein. This study suggested that S100A4 protein is capable of both calcium-dependent and calcium-independent interactions with the target molecule [15].
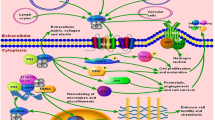
a Structural domains of S100A4 protein presented in the linear mode; I, II, III, and IV represent structural domains as explained in the text. b The ribbon diagram represents the molecular structure of S100A4 molecular protein. The figure is a slightly modified version of the original that was adapted from the web site of the protein data bank which is a public domain (http://www.pdb.org/pdb/results/results.do?qrid=95038868&tabtoshow=Current). c The role of S100A4 in the pathogenesis of several human diseases. S100A4 is associated with several diseases owing to its involvement in fibrotic, inflammatory, tissue-remodeling, and mesenchymal transition pathway (see text for details). d Established and potential molecular targets of S100A4 protein. S100A4 is described to mediate its metastatic action via interaction with several intracellular and extracellular effector molecules (see text for details)
4 Cell- and tissue-specific expression of S100A4 protein
S100A4 protein expression has been shown in human monocytes, macrophages, and polymorphonuclear granulocytes [16]. Boni R. has reported faint expression levels of S100A4 in keratinocytes, melanocytes, Langerhans’ cells, and sweat glands [17]. S100A4 protein was shown to be present at detectable limits in (a) a subset of cells of the normal ovary and prostate and (b) tissues such as spleen, thymus, bone marrow, T lymphocytes, neutrophils, and macrophages [18–20]. However, detectable levels of S100A4 protein are very low in the normal tissue of the pancreas, colon, thyroid, lungs, and kidneys [18]. This might be possible due to the low detection limits of assays used to detect this protein. Studies conducted in rats show that S100A4 protein is expressed in absorptive and keratinized epithelia, in the parietal cells of the stomach, and in a subset of cells of the immune system, bone marrow, spleen, and lymph nodes [21]. S100A4 is reported to be highly expressed in embryonic macrophages and differentiating mesenchymal tissues during mouse development [22].
5 Role of S100A4 in human diseases
As summarized in Fig. 1c, S100A4 is reported to be involved in the pathogenicity of several diseases. The role of S100A4 in the pathogenesis of diseases is comprehensively discussed in recently published articles [23, 24]. S100A4 overexpression has been reported to be positively correlated with rheumatoid arthritis, kidney fibrosis, liver fibrosis, peritoneal fibrosis, corneal dystrophy, neural diseases, and cardiac and lung disease [23, 24]. In this review, we will present the accumulated evidences correlating the role of S100A4 in tumor progression and metastasis.
6 S100A4 in tumor progression and metastasis: clinical evidences
S100A4 is a highly expressed transcript in growth-stimulated cultured cells [25] and metastatic tumor cell lines [26]. S100A4 is highly expressed in cells representing morphogenic transition from an epithelial to mesenchymal phenotype [27]. S100A4 expression levels are reported to be upregulated during oncogenic transformation [28, 29]. S100A4 protein level has been shown to be elevated in human cancer patients. Further, testing of biopsy samples and tumor specimens of human cancer patients has shown that malignant tissues express greater S100A4 protein level than adjacent normal tissues [29]. These clinical data are summarized in Table 1.
A recent study by Wang et al. conducted in gastric cancer patients showed that the expression of S100A4 in regions of lymph node and peritoneal metastasis is significantly higher than in tumor tissues [30].This study shows that the expression levels of S100A4 messenger RNA (mRNA) and protein differed significantly among gastric tumor tissue, matched normal gastric mucosa, lymph node, and peritoneal metastases among gastric cancer patients [30]. Yonemura et al. demonstrated that 55% of gastric cancer patients exhibit elevated S100A4 levels which were found to be positively associated with high incidence of metastasis. This study also showed that patients with low expression of S100A4 have lower number of metastatic lesions [31]. Studies conducted in pancreatic cancer patients show that 51–93% patients exhibit elevated S100A4 levels [32, 33]. In a study conducted in patients suffering from pancreatic ductal adenocarcinoma disease, 57 patients out of 61 exhibited elevated S100A4 expression levels [32]. Similarly, in another study of primary pancreatic cancer, tumor specimens of 56 (out of 72) tested positive for S100A4 expression [33].
S100A4 protein levels have been shown to be increased in colorectal cancer (CCR) in humans [34]. About 57% of total CCR patients have been found to test positive for S100A4 expression [34]. Kim et al. reported that 82% of CCR patients exhibit increased S100A4 mRNA expression levels [35]. This study suggested that upregulation of S100A4 is significantly related to invasion, nodal status, and distal metastasis among CCR patients [35]. Another study conducted in 195 CCR patients showed that ∼62% of tumor specimens tested positive for S100A4 protein [36]. This study also showed that S100A4 expression is more prevalent in patients with CCR of invasive phenotype [36].
S100A4 is reported to be overexpressed in advanced stage of thyroid carcinoma and associated with the poor prognosis in human patients [37, 38]. Thyroid carcinoma patients (62–86%) have shown positive correlation of S100A4 expression and advancement of disease [37, 38]. Moriyama et al. investigated the status of S100A4 in the pathogenesis of oral squamous cell carcinoma (SCC) [39]. This study involved the evaluation of clinical specimen for 41 SCC patients [39]. S100A4 expression was found to be increased in 27% of cases. This study also established a significant positive correlation between the increased S100A4 expression, the rate of invasion (p < 0.0001), and lymph node metastasis (p value, <0.01) in SCC patients [39]. Chen et al. reported that an upregulated S100A4 expression in non-small cell lung cancer (NSCLC) is positively related to tumor size, lymphatic metastasis, and TNM stage, suggesting an important role of this protein in development and metastasis of NSCLC [40]. Studies conducted by Qi Rx et al. [41] and Kimura et al.[42] in 250 lung cancer patients showed a positive correlation between S100A4 expression and metastasis in 60% of lung cancer patients [41, 42].
Recent studies have shown that S100A4 protein levels are elevated in prostate cancer patients [13, 43]. Gupta et al. have shown that S100A4 protein levels increase with the progressive stages of prostate cancer in humans [43]. S100A4 levels were reported to be high in prostate cancer patients with higher Gleason score [43]. S100A4 expression was detected in both epithelial and stromal cells in prostatic tissue [43]. In contrast, Rehman et al. showed that S100A4 is predominantly detected in stromal cells of prostate cancer patients [13]. However, the detection of S100A4 in prostate epithelial cells is debatable. Studies from our lab show that moderate S100A4 levels are present in DU-145 and PC-3 prostate cancer cell lines; however, LNCAP cells test negative for S100A4 expression (unpublished data, Saleem’s Lab). Recently, Bandiera et al. reported an overexpression of S100A4 protein in the peritumoral infiltrate of primary renal cell carcinoma in human patients [44]. The association of S100A4 expression and breast cancer progression is also reported in several studies [45–47]. These studies showed a positive correlation between S100A4 level and metastasis in 45–65% of breast cancer patients [45–47].
7 S100A4 in animal models of tumor and metastasis
Although the clinical correlation between S100A4 expression and tumor metastasis is more conclusive, some of the early indications of their plausible association came from some excellent studies conducted in experimental animal models. Transgenic mice which overexpress S100A4 in the mammary epithelium are phenotypically indistinguishable from wild-type mice and exhibit increasing metastasis [48]. MMTV-neu and GRS/A animals are characterized by a high incidence of mammary tumors that rarely metastasize; however, overexpression of S100A4 in the mammary epithelium of these animals has been shown to cause more invasive primary tumors which metastasize to the lungs [48, 49]. The association between S100A4 and metastasis is further supported by knockdown experiments where inhibition of S100A4 (by antisense or anti-ribozyme) suppressed the metastatic potential of tumor cells in animal models of lung carcinoma and osteosarcoma [50, 51]. We previously showed that expression of S100A4 at mRNA and protein levels is increased with the progression of age and prostate cancer growth in TRAMP mice, an autochthonous mouse model of human prostate cancer [52]. Studies conducted in our laboratory showed that heterozygous deletion in S100A4 gene causes a decrease in tumor development and metastatic progression in TRAMP mice (unpublished data, Saleem Lab). Taken together, these observations establish a positive association of S100A4 with the phenomenon of metastasis as well as development of tumor.
8 Association of S100A4 with survival of human cancer patients
Some of the earliest examples of S100A4 association with survival rate in cancer patients were reported in breast cancer patients [53, 54]. Rudland et al. [53] reported a significant difference in the 19-year survival rate for patients with invasive but operable stage I and stage II breast cancer depending on the S100A4 status of their tumors. The median survival of the S100A4-negative group was 228 months compared to 47 months for the S100A4-positive group [53]. In another study, Lee et al. [54] reported S100A4 expression as an indicator of poor prognosis in T1-N0M0 breast cancer. This study showed that S100A4 expression could be an early indicator of metastatic progression in breast cancer patients [54]. Ikenaga et al. [33] have recently recorded low survival rate in pancreatic ductal carcinoma patients with high S100A4 expression levels in comparison to those with low expression of S100A4 (p value = 0.023). The median survival time of the patients with high and low S100A4 expressions was reported to be 12 and 23 months, respectively [33]. Another study reported that S100A4 expression along with lymph node and distant metastases, tumor node metastasis (TNM) stage, acts as an independent prognostic factor in gastric cancer patients [31]. In this study, a 5-year survival rate was reported in gastric cancer patients, and high S100A4 levels were significantly correlated with low incidence of survival (p value, <0.05). The role of S100A4 in the outcome of therapy and survival of cancer patients is further strengthened by a clinical study conducted in humans suffering from renal cancer [44]. In this study, a survival rate of 5 years was reported in renal carcinoma patients, and the survival rate was found to have positive correlation with S100A4 levels [44].
9 S100A4: molecular mechanism
Studies to determine the mechanistic basis for S100A4 function have shown a potential role for S100A4 in cell motility, invasion, and apoptosis [29, 55]. S100A4 protein has been reported to interact with multiple molecular targets (Fig. 1d) which are known to play a role in various metabolic and regeneration pathways in cells. S100A4 has no known enzymatic activity; however, its interactions with other proteins, both intracellularly and extracellularly, have been shown to be functionally critical [56]. Several proteins have been identified as target for S100A4 protein. These includes liprin β1 [57], methionine aminopeptidase [58], and the p53 tumor suppressor protein [59]. S100A4 is also known to interact with proteins involved in cytoskeletal rearrangement and cell motility such as F-actin, tropomyosin, and the heavy chain of nonmuscle myosin II [55, 60, 61]. The interaction of S100A4 with most of the target proteins is found to be calcium dependent [55, 57–61]. However, several of the other molecular targets of S100A4 are known to follow an alternate path for their activities and in a calcium-independent manner, thus facilitating multiple ways of regulating the process of metastasis. Here, we discuss major binding partners of S100A4 which are known to play a key role in tumor progression and metastasis.
9.1 Binding with nonmuscle myosin-IIA protein
S100A4 is reported to bind nonmuscle myosin-IIA, inhibit the assembly of myosin-IIA monomers into filaments, and promote the disassembly of pre-existing myosin-IIA filaments [62].The S100A4 binding site on nonmuscle myosin-IIA has been shown to map between 1909 and 1924 in the C-terminal end of the myosin-IIA heavy chain [63, 64]. Although the S100A4 binding site overlaps a PKC phosphorylation site at Ser-1917 on myosin-IIA, the binding process is shown to be not affected by PKC phosphorylation [64]. However, phosphorylation on the CK2 site at Ser-1944, which is located downstream of the S100A4 binding site in the tail piece of myosin-IIA, is reported to inhibit its capacity to bind with S100A4 [65]. These findings demonstrate that heavy chain phosphorylation at the CK2 site of myosin-IIA provides (in addition to calcium binding) another regulatory mechanism for the S100A4-myosin-IIA interaction [65]. Li ZH demonstrated that S100A4 modulates cellular motility by affecting cell polarization during chemotaxis [62].S100A4-overexpressing cells have been shown to display side protrusions and extensive forward protrusions during chemotaxis as compared with cells that do not express S100A4 [62].
9.2 Interaction with p53
The tumor suppressor protein p53 has been identified as a target for the S100A4 protein [59]. S100A4 is reported to bind to the extreme end of the C-terminal regulatory domain of p53 in vitro and inhibit phosphorylation of the C-terminal peptide of p53 [59]. Activation of S100A4 in cell lines expressing wild-type p53 has been shown to modulate transcription of p53 target genes including p21/WAF, Bax, thrombospondin-1, and Mdm-2[6]. S100A4 through interaction with p53 is reported to induce apoptotic cell death in tumor cells harboring wt p53, thus contributing to selection of cells with mutated TP53 [59]. In contrast, a recent report by Berge et al. suggested that experimental up- or downregulation of S100A4 exerted no effect on p53 protein expression and did not influence the stabilization of p53 [66].
9.3 Interaction with cytoskeletal proteins
The perturbation in normal cell motility and cytoskeletal arrangement is an important event in the development of metastasis. There are reports which suggest that S100A4 interacts with cytoskeletal elements such as actin, tubulin, and nonmuscle tropomyosin thus establishing a direct role of S100A4 in regulating cell motility and cytoskeletal rearrangement [67–69]. Although the interaction of S100A4 and cytoskeletal proteins is well established, the calcium dependency of this interaction is strongly debated. While some studies [67–69] support these interactions to be calcium dependent (due to the calcium-dependent nature of S100A4), other reports contradict this notion and show calcium-independent interaction of S100A4 with cytoskeletal protein [70, 71]. More importantly, it has been documented that S100A4 has the potential to interact with other members of the S100 family [56]. S100A4 has been shown to form heterodimers with S100A1 [70]. Studies have shown that the interaction between S100A4 and S100A1 proteins reduces motility and metastatic ability of S100A4 transformed cells [71]. However, the mechanism by which interaction of these two S100 proteins reduces metastatic potential is not yet clear.
9.4 Extracellular S100A4: role in degradation of extracellular matrix and metastasis
S100A4 has been shown to exhibit its prometastatic role via affecting angiogenesis, cytoskeletal integrity, matrix metalloproteinases (MMPs), tumor-related transcription factors, and stromal factors [72, 73]. Angiogenesis is a crucial step for cancer progression, because it supplies the proliferating tumor cells with necessary nutrients and oxygen and at the same time provides an escape route for invading tumor cells [74]. MMPs promote metastasis both by degradation of extracellular matrix (ECM) and by liberating factors that promote and maintain the angiogenic character. Studies from our laboratory and others have shown that metastatic function of S100A4 is associated with its ability to upregulate the expression of several MMPs [72, 75]. We previously reported that S100A4 gene suppression significantly decreased the expression of MMP-9 and that overexpression of the S100A4 gene significantly increased MMP-9 expression [72]. We have also shown that suppression of the S100A4 gene caused a decrease in the proteolytic activity of MMP-9 protein, whereas overexpression of S100A4 caused a reverse effect [72]. Furthermore, a recent study has indicated tissue inhibitor metalloproteinase (TIMP) expression in the cells to be driven by the expression of MMP, and overall, the balance between their activities determines their net proteolytic activity [74]. We observed that S100A4 gene suppression decreased, and its overexpression increased, the expression of TIMP-1 in prostate cancer cells [72]. These observations lead us to conclude that S100A4 gene influences the ratio of MMP-9 to TIMP-1, leading to ECM degradation [72].
There is accumulating evidence that S100 proteins have extracellular functions; however, their mechanism of secretion remains unknown. S100A4 does not have any of the known signal sequences to target it for secretion through a classic vesicle transport pathway. Instead, it is hypothesized that S100A4 is released from the cell through an atypical pathway [56]. S100A4 is reported to be present in the stromal region of the human prostatic tissue [43]. We observed that S100A4 is a secretary protein present in extracellular secretion of prostatic cell lines (unpublished data, Saleem Lab).
Although extracellular S100A4 is increasingly recognized as a key player in metastasis, the question is still not very clear about which cell types are key producers of extracellular S100A4 and the exact nature of signal transduction events during the process of release of S100A4. It is also quite likely that S100A4 promotes metastatic progression by influencing both stromal and tumor cells, and the mere presence of S100A4 in tumor microenvironment may be a prerequisite factor instead of its specific release from any particular cell type [56]. It has been suggested that the receptor for advanced glycation endproducts (RAGE) may act as a putative receptor for several S100 proteins [76, 77] including S100A4. RAGE has been described to play an important role in the pathogenesis of many diseases such as diabetes, rheumatoid arthritis, alzheimer’s, neuronal degeneration, and also in cancer. Upregulation of RAGE is shown to be associated with many of these disease conditions. Soluble peptide of RAGE hence may block intracellular translocation of S100A4, S100A12, S100A13, and S100B in endothelial cells. Logsdon et al. [78] suggested that RAGE ligands were secreted by cancer cells into their microenvironment, and these in turn might act in an autocrine fashion to induce cell proliferation, invasion, and subsequently metastasis. Further, they also suggested that RAGE ligands could also influence other cell types such as fibroblast, leukocytes, and endothelial cells in the tumor microenvironment to promote angiogenesis by enhancing the production of matrix-degrading enzymes by tumors and/or endothelial cells.
Conclusively, extracellular S100A4 targets tumor or stromal cells by binding to receptors and triggering a signal transduction cascade and/or perhaps by internalization followed by interaction with the intracellular target proteins. As a result, tumor cells acquire a more metastatic phenotype with altered cell adhesion, motility, high proteolytic activity, and increased survival potential.
10 S100A4: a potential target for cancer therapy
Efforts utilizing S100A4 as a target for anticancer therapy mainly focused on inhibiting/suppressing S100A4 expression. The methods tested include siRNA-mediated gene silencing [79], ribozyme-driven S100A4 downregulation[50], exposing tumor cells to hyperthermic condition [80], and epigenetic silencing using altered methylation of S100A4 [81]. Maelendesmo et al. were successful in suppressing S100A4 protein level by specifically directing hammerhead ribozymes against S100A4 mRNA [50]. When injected into mice, the S100A4 ribozyme reduces metastasis significantly [50]. A study by Basile et al. showed that S100A4 expression level was reduced by hyperthermia [80]. A recent study shows that methyl transferase inhibitors (5′-aza-2′- deoxycytidine) do alter S100A4 expression in leukemia cells by altering methylation status of S100A4, thus decreasing metastatic potency of the cells [81].
We recently showed that targeted inhibition of S100A4 by adopting gene therapy approach resulted in the reduction in the invasive potential and tumorigenic potential of prostate cancer cells [72]. Our institute has embarked upon a broad program aimed to evaluate the potential and usefulness of S100A4 as a molecular target for human diseases. We developed specific S100A4 small molecule inhibitors and tested these under in vitro and in vivo conditions (unpublished data, Saleem Lab). Small molecule inhibitors of S100A4 were observed to inhibit the tumorigenic and metastatic potential of prostate, pancreatic, and skin cancer cells in vitro and in relevant animal models (unpublished studies, Saleem Lab). Taken together, these studies show that S100A4 has the potential of developing as an anticancer drug target, and molecules efficiently targeting its expression may act as future anticancer drugs.
11 Future perspective
The substantial number of experiments carried in the last two decades has established S100A4 to be a key player in cancer metastasis. The evidences have been generated which indicate the important role of S100A4 in the crucial steps of the metastatic cascade including migration, invasiveness, and angiogenesis. Although several targets of S100A4 are described both intracellularly as well as extracellularly, it remains to be determined how these interactions affect the function of the binding partners and whether these interactions are indeed relevant to the metastatic phenotype. A more thorough identification and characterization of the molecular mechanisms by which S100A4 regulates its effectors will provide the biochemical foundation for understanding the contribution of S100A4 to normal and metastatic processes and will provide important new insights into the molecular basis for the invasive behavior of tumor cells. We firmly believe this greater understanding will ideally lead to the use of S100A4 as not only a diagnostic and prognostic marker but also as a target for therapeutic design.
References
Moore, B. W. (1965). A soluble protein characteristic of the nervous system. Biochemical and Biophysical Research Communications, 19(6), 739–744.
Heizmann, C. W., & Cox, J. A. (1998). New perspectives on S100 proteins: a multifunctional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. BioMetals, 11(4), 383–397.
Otterbein, L. R., Kordowska, J., Witte-Hoffmann, C., Wang, C. L., & Dominguez, R. (2002). Crystal structures of S100A6 in the Ca(2+)-free and Ca(2+)-bound states: the calcium sensor mechanism of S100 proteins revealed at atomic resolution. Structure, 10(4), 557–567.
Drohat, A. C., Baldisseri, D. M., Rustandi, R. R., & Weber, D. J. (1998). Solution structure of calcium-bound rat S100B(betabeta) as determined by nuclear magnetic resonance spectroscopy. Biochemistry, 37(9), 2729–2740.
Wright, N. T., Varney, K. M., Ellis, K. C., Markowitz, J., Gitti, R. K., Zimmer, D. B., & Weber, D. J. (2005). The three-dimensional solution structure of Ca(2+)-bound S100A1 as determined by NMR spectroscopy. Journal of Molecular Biology, 353(2), 410–426.
Garrett, S. C., Varney, K. M., Weber, D. J., & Bresnick, A. R. (2006). S100A4, a mediator of metastasis. Journal of Biological Chemistry, 281(2), 677–680.
Mazzucchelli, L. (2002). Protein S100A4: too long overlooked by pathologists? American Journal of Pathology, 160(1), 7–13.
Hernan, R., Fasheh, R., Calabrese, C., Frank, A. J., Maclean, K. H., Allard, D., Barraclough, R., & Gilbertson, R. J. (2003). ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Research, 63(1), 140–148.
Tulchinsky, E. M., Georgiev, G. P., & Lukanidin, E. M. (1996). Novel AP-1 binding site created by DNA-methylation. Oncogene, 12(8), 1737–1745.
Cohn, M. A., Hjelmso, I., Wu, L. C., Guldberg, P., Lukanidin, E. M., & Tulchinsky, E. M. (2001). Characterization of Sp1, AP-1, CBF and KRC binding sites and minisatellite DNA as functional elements of the metastasis-associated mts1/S100A4 gene intronic enhancer. Nucleic Acids Research, 29(16), 3335–3346.
Li, Y., Liu, Z. L., Zhang, K. L., Chen, X. Y., Kong, Q. Y., Wu, M. L., Sun, Y., Liu, J., & Li, H. (2009). Methylation-associated silencing of S100A4 expression in human epidermal cancers. Experimental Dermatology, 18(10), 842–848.
Xie, R., Loose, D. S., Shipley, G. L., Xie, S., Bassett, R. L., Jr., & Broaddus, R. R. (2007). Hypomethylation-induced expression of S100A4 in endometrial carcinoma. Modern Pathology, 20(10), 1045–1054.
Rehman, I., Goodarzi, A., Cross, S. S., Leiblich, A., Catto, J. W., Phillips, J. T., & Hamdy, F. C. (2007). DNA methylation and immunohistochemical analysis of the S100A4 calcium binding protein in human prostate cancer. The Prostate, 67(4), 341–347.
Tarabykina, S., Griffiths, T. R., Tulchinsky, E., Mellon, J. K., Bronstein, I. B., & Kriajevska, M. (2007). Metastasis-associated protein S100A4: spotlight on its role in cell migration. Current Cancer Drug Targets, 7(3), 217–228.
Dukhanina, E. A., Dukhanin, A. S., Lomonosov, M. Y., Lukanidin, E. M., & Georgiev, G. P. (1997). Spectral studies on the calcium-binding properties of Mts1 protein and its interaction with target protein. FEBS Letters, 410(2–3), 403–406.
Takenaga, K., Nakanishi, H., Wada, K., Suzuki, M., Matsuzaki, O., Matsuura, A., & Endo, H. (1997). Increased expression of S100A4, a metastasis-associated gene, in human colorectal adenocarcinomas. Clinical Cancer Research, 3(12 Pt 1), 2309–2316.
Boni, R., Burg, G., Doguoglu, A., Ilg, E. C., Schafer, B. W., Muller, B., & Heizmann, C. W. (1997). Immunohistochemical localization of the Ca2+ binding S100 proteins in normal human skin and melanocytic lesions. British Journal of Dermatology, 137(1), 39–43.
Ilg, E. C., Schafer, B. W., & Heizmann, C. W. (1996). Expression pattern of S100 calcium-binding proteins in human tumors. International Journal of Cancer, 68(3), 325–332.
Duarte, W. R., Iimura, T., Takenaga, K., Ohya, K., Ishikawa, I., & Kasugai, S. (1999). Extracellular role of S100A4 calcium-binding protein in the periodontal ligament. Biochemical and Biophysical Research Communications, 255(2), 416–420.
Ambartsumian, N., Klingelhofer, J., Grigorian, M., Karlstrom, O., Sidenius, N., Georgiev, G., & Lukanidin, E. (1998). Tissue-specific posttranscriptional downregulation of expression of the S100A4(mts1) gene in transgenic animals. Invasion & Metastasis, 18(2), 96–104.
Gibbs, F. E., Barraclough, R., Platt-Higgins, A., Rudland, P. S., Wilkinson, M. C., & Parry, E. W. (1995). Immunocytochemical distribution of the calcium-binding protein p9Ka in normal rat tissues: variation in the cellular location in different tissues. Journal of Histochemistry and Cytochemistry, 43(2), 169–180.
Klingelhofer, J., Ambartsumian, N. S., & Lukanidin, E. M. (1997). Expression of the metastasis-associated mts1 gene during mouse development. Developmental Dynamics, 210(2), 87–95.
Schneider, M., Hansen, J. L., & Sheikh, S. P. (2008). S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases? Journal of Molecular Medicine, 86(5), 507–522.
Grigorian, M., Ambartsumian, N., & Lukanidin, E. (2008). Metastasis-inducing S100A4 protein: implication in non-malignant human pathologies. Current Molecular Medicine, 8(6), 492–496.
Goto, K., Endo, H., & Fujiyoshi, T. (1988). Cloning of the sequences expressed abundantly in established cell lines: identification of a cDNA clone highly homologous to S-100, a calcium binding protein. Journal of Biochemistry, 103(1), 48–53.
Ebralidze, A., Tulchinsky, E., Grigorian, M., Afanasyeva, A., Senin, V., Revazova, E., & Lukanidin, E. (1989). Isolation and characterization of a gene specifically expressed in different metastatic cells and whose deduced gene product has a high degree of homology to a Ca2+-binding protein family. Genes & Development, 3(7), 1086–1093.
Strutz, F., Okada, H., Lo, C. W., Danoff, T., Carone, R. L., Tomaszewski, J. E., & Neilson, E. G. (1995). Identification and characterization of a fibroblast marker: FSP1. The Journal of Cell Biology, 130(2), 393–405.
De Vouge, M. W., & Mukherjee, B. B. (1992). Transformation of normal rat kidney cells by v-K-ras enhances expression of transin 2 and an S-100-related calcium-binding protein. Oncogene, 7(1), 109–119.
Takenaga, K., Nakamura, Y., Endo, H., & Sakiyama, S. (1994). Involvement of S100-related calcium-binding protein pEL98 (or mts1) in cell motility and tumor cell invasion. Japanese Journal of Cancer Research, 85(8), 831–839.
Wang, Y. Y., Ye, Z. Y., Zhao, Z. S., Tao, H. Q., & Chu, Y. Q. (2010). High-level expression of S100A4 correlates with lymph node metastasis and poor prognosis in patients with gastric cancer. Annals of Surgical Oncology, 17(1), 89–97.
Yonemura, Y., Endou, Y., Kimura, K., Fushida, S., Bandou, E., Taniguchi, K., Kinoshita, K., Ninomiya, I., Sugiyama, K., Heizmann, C. W., Schafer, B. W., & Sasaki, T. (2000). Inverse expression of S100A4 and E-cadherin is associated with metastatic potential in gastric cancer. Clinical Cancer Research, 6(11), 4234–4242.
Rosty, C., Ueki, T., Argani, P., Jansen, M., Yeo, C. J., Cameron, J. L., Hruban, R. H., & Goggins, M. (2002). Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. American Journal of Pathology, 160(1), 45–50.
Ikenaga, N., Ohuchida, K., Mizumoto, K., Yu, J., Fujita, H., Nakata, K., Ueda, J., Sato, N., Nagai, E., & Tanaka, M. (2009). S100A4 mRNA is a diagnostic and prognostic marker in pancreatic carcinoma. Journal of Gastrointestinal Surgery, 13(10), 1852–1858.
Wang, H. Y., Zhang, J. Y., Cui, J. T., Tan, X. H., Li, W. M., Gu, J., & Lu, Y. Y. (2010). Expression status of S100A14 and S100A4 correlates with metastatic potential and clinical outcome in colorectal cancer after surgery. Oncology Reports, 23(1), 45–52.
Kim, J. H., Kim, C. N., Kim, S. Y., Lee, J. S., Cho, D., Kim, J. W., & Yoon, S. Y. (2009). Enhanced S100A4 protein expression is clinicopathologically significant to metastatic potential and p53 dysfunction in colorectal cancer. Oncology Reports, 22(1), 41–47.
Cho, Y. G., Kim, C. J., Nam, S. W., Yoon, S. H., Lee, S. H., Yoo, N. J., Lee, J. Y., & Park, W. S. (2005). Overexpression of S100A4 is closely associated with progression of colorectal cancer. World Journal of Gastroenterology, 11(31), 4852–4856.
Zou, M., Famulski, K. S., Parhar, R. S., Baitei, E., Al-Mohanna, F. A., Farid, N. R., & Shi, Y. (2004). Microarray analysis of metastasis-associated gene expression profiling in a murine model of thyroid carcinoma pulmonary metastasis: identification of S100A4 (Mts1) gene overexpression as a poor prognostic marker for thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism, 89(12), 6146–6154.
Zou, M., Al-Baradie, R. S., Al-Hindi, H., Farid, N. R., & Shi, Y. (2005). S100A4 (Mts1) gene overexpression is associated with invasion and metastasis of papillary thyroid carcinoma. British Journal of Cancer, 93(11), 1277–1284.
Moriyama-Kita, M., Endo, Y., Yonemura, Y., Heizmann, C. W., Miyamori, H., Sato, H., Yamamoto, E., & Sasaki, T. (2005). S100A4 regulates E-cadherin expression in oral squamous cell carcinoma. Cancer Letters, 230(2), 211–218.
Chen, X. L., Zhang, W. G., Chen, X. Y., Sun, Z. M., & Liu, S. H. (2006). Correlations of S100A4 protein expression to invasion and metastasis of non-small cell lung cancer. Ai Zheng, 25(9), 1134–1137.
Qi, R. X., & Xu, X. Y. (2007). Inverse correlation of S100A4 and E-cad protein expression and their clinical significance in non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi, 29(9), 681–684.
Kimura, K., Endo, Y., Yonemura, Y., Heizmann, C. W., Schafer, B. W., Watanabe, Y., & Sasaki, T. (2000). Clinical significance of S100A4 and E-cadherin-related adhesion molecules in non-small cell lung cancer. International Journal of Oncology, 16(6), 1125–1131.
Gupta, S., Hussain, T., MacLennan, G. T., Fu, P., Patel, J., & Mukhtar, H. (2003). Differential expression of S100A2 and S100A4 during progression of human prostate adenocarcinoma. Journal of Clinical Oncology, 21(1), 106–112.
Bandiera, A., Melloni, G., Freschi, M., Giovanardi, M., Carretta, A., Borri, A., Ciriaco, P., & Zannini, P. (2009). Prognostic factors and analysis of S100a4 protein in resected pulmonary metastases from renal cell carcinoma. World Journal of Surgery, 33(7), 1414–1420.
Ismail, N. I., Kaur, G., Hashim, H., & Hassan, M. S. (2008). S100A4 overexpression proves to be independent marker for breast cancer progression. Cancer Cell International, 8, 12.
Pedersen, K. B., Nesland, J. M., Fodstad, O., & Maelandsmo, G. M. (2002). Expression of S100A4, E-cadherin, alpha- and beta-catenin in breast cancer biopsies. British Journal of Cancer, 87(11), 1281–1286.
Platt-Higgins, A. M., Renshaw, C. A., West, C. R., Winstanley, J. H., de Silva, R. S., Barraclough, R., & Rudland, P. S. (2000). Comparison of the metastasis-inducing protein S100A4 (p9ka) with other prognostic markers in human breast cancer. International Journal of Cancer, 89(2), 198–208.
Ambartsumian, N. S., Grigorian, M. S., Larsen, I. F., Karlstrom, O., Sidenius, N., Rygaard, J., Georgiev, G., & Lukanidin, E. (1996). Metastasis of mammary carcinomas in GRS/A hybrid mice transgenic for the mts1 gene. Oncogene, 13(8), 1621–1630.
Davies, M. P., Rudland, P. S., Robertson, L., Parry, E. W., Jolicoeur, P., & Barraclough, R. (1996). Expression of the calcium-binding protein S100A4 (p9Ka) in MMTV-neu transgenic mice induces metastasis of mammary tumours. Oncogene, 13(8), 1631–1637.
Maelandsmo, G. M., Hovig, E., Skrede, M., Engebraaten, O., Florenes, V. A., Myklebost, O., Grigorian, M., Lukanidin, E., Scanlon, K. J., & Fodstad, O. (1996). Reversal of the in vivo metastatic phenotype of human tumor cells by an anti-CAPL (mts1) ribozyme. Cancer Research, 56(23), 5490–5498.
Takenaga, K., Nakamura, Y., & Sakiyama, S. (1997). Expression of antisense RNA to S100A4 gene encoding an S100-related calcium-binding protein suppresses metastatic potential of high-metastatic Lewis lung carcinoma cells. Oncogene, 14(3), 331–337.
Saleem, M., Adhami, V. M., Ahmad, N., Gupta, S., & Mukhtar, H. (2005). Prognostic significance of metastasis-associated protein S100A4 (Mts1) in prostate cancer progression and chemoprevention regimens in an autochthonous mouse model. Clinical Cancer Research, 11(1), 147–153.
Rudland, P. S., Platt-Higgins, A., Renshaw, C., West, C. R., Winstanley, J. H., Robertson, L., & Barraclough, R. (2000). Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Research, 60(6), 1595–1603.
Lee, W. Y., Su, W. C., Lin, P. W., Guo, H. R., Chang, T. W., & Chen, H. H. (2004). Expression of S100A4 and met: potential predictors for metastasis and survival in early-stage breast cancer. Oncology, 66(6), 429–438.
Kriajevska, M. V., Cardenas, M. N., Grigorian, M. S., Ambartsumian, N. S., Georgiev, G. P., & Lukanidin, E. M. (1994). Non-muscle myosin heavy chain as a possible target for protein encoded by metastasis-related mts-1 gene. Journal of Biological Chemistry, 269(31), 19679–19682.
Helfman, D. M., Kim, E. J., Lukanidin, E., & Grigorian, M. (2005). The metastasis associated protein S100A4: role in tumour progression and metastasis. British Journal of Cancer, 92(11), 1955–1958.
Kriajevska, M., Fischer-Larsen, M., Moertz, E., Vorm, O., Tulchinsky, E., Grigorian, M., Ambartsumian, N., & Lukanidin, E. (2002). Liprin beta 1, a member of the family of LAR transmembrane tyrosine phosphatase-interacting proteins, is a new target for the metastasis-associated protein S100A4 (Mts1). Journal of Biological Chemistry, 277(7), 5229–5235.
Endo, H., Takenaga, K., Kanno, T., Satoh, H., & Mori, S. (2002). Methionine aminopeptidase 2 is a new target for the metastasis-associated protein, S100A4. Journal of Biological Chemistry, 277(29), 26396–26402.
Grigorian, M., Andresen, S., Tulchinsky, E., Kriajevska, M., Carlberg, C., Kruse, C., Cohn, M., Ambartsumian, N., Christensen, A., Selivanova, G., & Lukanidin, E. (2001). Tumor suppressor p53 protein is a new target for the metastasis-associated Mts1/S100A4 protein: functional consequences of their interaction. Journal of Biological Chemistry, 276(25), 22699–22708.
Takenaga, K., Nakamura, Y., Sakiyama, S., Hasegawa, Y., Sato, K., & Endo, H. (1994). Binding of pEL98 protein, an S100-related calcium-binding protein, to nonmuscle tropomyosin. The Journal of Cell Biology, 124(5), 757–768.
Watanabe, Y., Usada, N., Minami, H., Morita, T., Tsugane, S., Ishikawa, R., Kohama, K., Tomida, Y., & Hidaka, H. (1993). Calvasculin, as a factor affecting the microfilament assemblies in rat fibroblasts transfected by src gene. FEBS Letters, 324(1), 51–55.
Li, Z. H., & Bresnick, A. R. (2006). The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Research, 66(10), 5173–5180.
Li, Z. H., Spektor, A., Varlamova, O., & Bresnick, A. R. (2003). Mts1 regulates the assembly of nonmuscle myosin-IIA. Biochemistry, 42(48), 14258–14266.
Kriajevska, M., Tarabykina, S., Bronstein, I., Maitland, N., Lomonosov, M., Hansen, K., Georgiev, G., & Lukanidin, E. (1998). Metastasis-associated Mts1 (S100A4) protein modulates protein kinase C phosphorylation of the heavy chain of nonmuscle myosin. Journal of Biological Chemistry, 273(16), 9852–9856.
Dulyaninova, N. G., Malashkevich, V. N., Almo, S. C., & Bresnick, A. R. (2005). Regulation of myosin-IIA assembly and Mts1 binding by heavy chain phosphorylation. Biochemistry, 44(18), 6867–6876.
Berge, G., Costea, D. E., Berg, M., Rasmussen, H., Grotterod, I., Lothe, R. A., et al. (2010). Coexpression and nuclear colocalization of metastasis-promoting protein S100A4 and p53 without mutual regulation in colorectal carcinoma. Amino Acids, 42(4), 875–884.
Chen, H., Fernig, D. G., Rudland, P. S., Sparks, A., Wilkinson, M. C., & Barraclough, R. (2001). Binding to intracellular targets of the metastasis-inducing protein, S100A4 (p9Ka). Biochemical and Biophysical Research Communications, 286(5), 1212–1217.
Kim, E. J., & Helfman, D. M. (2003). Characterization of the metastasis-associated protein, S100A4. Roles of calcium binding and dimerization in cellular localization and interaction with myosin. Journal of Biological Chemistry, 278(32), 30063–30073.
Tarabykina, S., Kriajevska, M., Scott, D. J., Hill, T. J., Lafitte, D., Derrick, P. J., Dodson, G. G., Lukanidin, E., & Bronstein, I. (2000). Heterocomplex formation between metastasis-related protein S100A4 (Mts1) and S100A1 as revealed by the yeast two-hybrid system. FEBS Letters, 475(3), 187–191.
Wang, G., Rudland, P. S., White, M. R., & Barraclough, R. (2000). Interaction in vivo and in vitro of the metastasis-inducing S100 protein, S100A4 (p9Ka) with S100A1. Journal of Biological Chemistry, 275(15), 11141–11146.
Wang, G., Zhang, S., Fernig, D. G., Martin-Fernandez, M., Rudland, P. S., & Barraclough, R. (2005). Mutually antagonistic actions of S100A4 and S100A1 on normal and metastatic phenotypes. Oncogene, 24(8), 1445–1454.
Saleem, M., Kweon, M. H., Johnson, J. J., Adhami, V. M., Elcheva, I., Khan, N., Bin, H. B., Bhat, K. M., Sarfaraz, S., Reagan-Shaw, S., Spiegelman, V. S., Setaluri, V., & Mukhtar, H. (2006). S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proceedings of the National Academy of Sciences of the United States of America, 103(40), 14825–14830.
Bjornland, K., Winberg, J. O., Odegaard, O. T., Hovig, E., Loennechen, T., Aasen, A. O., Fodstad, O., & Maelandsmo, G. M. (1999). S100A4 involvement in metastasis: deregulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in osteosarcoma cells transfected with an anti-S100A4 ribozyme. Cancer Research, 59(18), 4702–4708.
Adhami, V. M., Siddiqui, I. A., Ahmad, N., Gupta, S., & Mukhtar, H. (2004). Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Research, 64(23), 8715–8722.
Schmidt-Hansen, B., Ornas, D., Grigorian, M., Klingelhofer, J., Tulchinsky, E., Lukanidin, E., & Ambartsumian, N. (2004). Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene, 23(32), 5487–5495.
Hofmann, M. A., Drury, S., Fu, C., Qu, W., Taguchi, A., Lu, Y., Avila, C., Kambham, N., Bierhaus, A., Nawroth, P., Neurath, M. F., Slattery, T., Beach, D., McClary, J., Nagashima, M., Morser, J., Stern, D., & Schmidt, A. M. (1999). RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell, 97(7), 889–901.
Huttunen, H. J., Kuja-Panula, J., Sorci, G., Agneletti, A. L., Donato, R., & Rauvala, H. (2000). Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. Journal of Biological Chemistry, 275(51), 40096–40105.
Logsdon, C. D., Fuentes, M. K., Huang, E. H., & Arumugam, T. (2007). RAGE and RAGE ligands in cancer. Current Molecular Medicine, 7(8), 777–789.
Sauter, W., Rosenberger, A., Beckmann, L., Kropp, S., Mittelstrass, K., Timofeeva, M., Wolke, G., Steinwachs, A., Scheiner, D., Meese, E., Sybrecht, G., Kronenberg, F., Dienemann, H., Chang-Claude, J., Illig, T., Wichmann, H. E., Bickeboller, H., & Risch, A. (2008). Matrix metalloproteinase 1 (MMP1) is associated with early-onset lung cancer. Cancer Epidemiology, Biomarkers & Prevention, 17(5), 1127–1135.
Basile, A., Biziato, D., Sherbet, G. V., Comi, P., & Cajone, F. (2008). Hyperthermia inhibits cell proliferation and induces apoptosis: relative signaling status of P53, S100A4, and Notch in heat sensitive and resistant cell lines. Journal of Cellular Biochemistry, 103(1), 212–220.
Lindsey, J. C., Lusher, M. E., Anderton, J. A., Gilbertson, R. J., Ellison, D. W., & Clifford, S. C. (2007). Epigenetic deregulation of multiple S100 gene family members by differential hypomethylation and hypermethylation events in medulloblastoma. British Journal of Cancer, 97(2), 267–274.
Acknowledgments
The unpublished studies cited in the manuscript from the corresponding author’s laboratory are supported by The Hormel Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, S.K., Siddique, H.R. & Saleem, M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer Metastasis Rev 31, 163–172 (2012). https://doi.org/10.1007/s10555-011-9338-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9338-4