Abstract
Distant metastases (MET) are for most solid cancers decisive life-threatening events. Data about MET-free survival and survival after MET show a strong dependency on the kind of cancer and the prognostic features. Nonetheless, within biological subgroups, the MET process is very homogenous. Therefore, the growth rate can be estimated from initiation of MET to MET diagnosis and to time of death. Based on the known volume doubling time of breast cancer, the time of the first possible dissemination can also be estimated. Important consequences of these MET-initiation estimates are the hypotheses that almost all MET are initiated before removal of the primary tumor and that MET do not metastasize in a clinically relevant magnitude. Although breast cancer data were primarily used to form these hypotheses, the discussed MET process can be generalized to all solid cancers. The impact of these hypotheses on diagnostic, curative and palliative treatment, aftercare, and especially on clinical research would be important.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Distant metastasization (MET) is for most solid tumors the decisive, life-threatening event. The multistage MET process assumes increasing complexity. Tumor cells (TC) must escape from the primary tumor (PT), must invade and survive in the circulation, arrive in a distant organ, survive in the new environment, and must persistently grow [1, 2]. In this review, it is our goal to describe the distant MET process, from the time of initiation of MET, the growth rate up to MET diagnosis, and the sequence of MET locations. Patient survival after MET will be presented from the Munich Cancer Registry [3], especially for breast cancer (BC). Approximately 33,000 BC patients were registered since 1988 from a current recruitment area of 4.5 Mio inhabitants [4]. The availability of death certificates and of progressive events allows a detailed description of the course of disease. BC is most suitable for a quantitative description because the tumor diameter is available as a continuous variable in millimeters, which can be correlated with the growth rate of the PT and with regional and distant progressions. Additionally, BC does not grow through different morphological structures in comparison, for example, to colorectal cancer. The focus on BC simplifies this review and the interpretation of the data. Nonetheless, comparable data are available for all frequent tumors because the pathogenetic process seems to be identical for all cancers [5, 6]. Despite the increasing complexity of MET and the resulting individualization of treatment, we present an uncomplicated MET model with two very simple hypotheses (Fig. 1 H1): First, all MET are initiated before the removal of the PT and second, as a corollary, MET do not metastasize [7, 8]
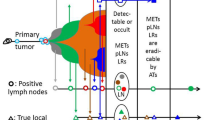
The hypotheses of parallel and metachronous (H1) versus cascade-like (H2) tumor cell dissemination and initiation of secondary tumor foci. Arrows indicate the successfully disseminated tumor cells (only a few from millions) from a source to an initiated focus (circle). Each arrow has a different point in time (t i) whereby the difference between the extremes can span many years. In Fig. 1.H1, further initiations of tumor foci can be prevented with the removal of the primary tumor
2 Survival
The frequency of MET and, therefore, survival depend upon prognostic factors like pT according to the TNM classification [9] and the number of positive lymph nodes (pLN), the most important classical factor (Fig. 2) [10]. Regarding tumor size, Fig. 2a suggests that a PT gains increasing neoplastic potency with each TC doubling. Figure 2b shows the dependency of survival on the number of pLN and is illustrative of more of a stable process. In reality, the percentage of a positive LN status (pN+) and the 10-year tumor-related mortality (the complement of the relative survival as an estimation for the disease-specific survival [11]) are both linear and dependent on the tumor thickness with a similar slope (mortality in% = −2.44 + 1.21 × d, pN + in% = 11.9 + 1.22 × d (d, diameter in millimeter)). With every millimeter increase of the BC diameter, the mortality increases about 1.2%. This is a suitable approximation for the interval between 7.5 and 47.5 mm, with 5-mm incremental steps [12]. The dependency beyond this interval is of course S-shaped. Quite a different approach is based on a Poisson process, with millions of disseminated tumor cells of the PT and only few initiated foci, which achieves comparable result in this reduced diameter interval of 7.5–47.5 mm [13]. Is there evidence for such a deterministic model?
Breast cancer: relative survival in dependence of a pT category (n = 33,475) and b number of positive lymph nodes (n = 31,316). The relative survival is the quotient of observed survival in each cohort and the survival of an identical group in the normal population and estimates the tumor-related survival
3 Survival after MET
Clinical data support this deterministic model. Patient survival after metastasization is depicted in Fig. 3. Following MET, the survival curves, regardless of the prognostic factor, show little variability in comparison to Fig. 2. The survival time is largely independent of the PT prognostic factors, such as tumor diameter (Fig. 3a). Even the MET-free interval, which is repeatedly interpreted as a measure of growth rate, has little influence on survival time (Fig. 3b). The remaining variability is in part dependent on clinicopathological features (Fig. 3c) [14, 15]. The dependency of survival on pT and the number of pLN (Fig. 2) are convincing. This dependency after MET nearly disappears (Fig. 3a–c). In the subgroup with negative receptor status, not even an influence of the most important prognostic factor (pLN) for BC is observable (Fig. 3d). Therefore, the disseminated TC, the seed which causes MET, is stable for small-sized BC with a 10-year survival >90%, and for large BC, less than 30%. This can already be concluded from the linear dependency of survival on PT diameter. Nevertheless, the seed stability is noteworthy because growing PTs show dramatic changes over time [16]. Perhaps, the stem cell concept explains this contradiction [17, 18]. In summary, an important fact is that the growth rate of MET after detection is quite homogeneous, i.e., it varies maximally by twofold between biological subgroups and in no case by five- or tenfold.
Breast cancer: survival after the first metastasization in dependence on a the pT category (n = 5,382), b the length of the MET-free interval (the middle curve is M1, n=8,801), c the disjoint subgroups receptor-negative, receptor-positive and grade 1-2, receptorpositive and grade 3, and Her2new positive (n = 4,772), and d the number of positive lymph nodes within the subgroup receptor-negative tumors (n = 979). The variability observed between a and b is due, in part, to the distribution of the four biologically distinct subgroups
4 Timeline of metastasization
A MET which begins with a single TC or a cluster of TCs can be detected at a size of 5 mm or about 1.5 x 108 TCs, and can achieve some 1012 TCs in the remaining survival time of about 2 years. This is outlined in Fig. 4. About 3/4 of MET growth, considered as volume doublings (VD), occurs before MET detection, and 1/4 afterwards. It is plausible that tumor growth up to MET has the same marginal variability as afterwards. However, this assumption does not imply that volume doubling time (VDT) is constant after the first MET cell doubling, as shown in Fig. 4. The development of MET must follow a Gompertzian growth with a potent exponential regression as known from the VD of liver or lung MET foci with different sizes [19, 20]. The growth of MET is also dependent on cancer type and the involved distant organ. A discontinuous growth e.g. with a balanced phase of division and apoptosis is possible but without influence on the widely homogeneous variability of the growth rate up to the detection of MET. The next important question is the temporal relation between initiation and detection of MET and the diagnosis of PT.
The distant metastasization (MET) process from initiation to the diagnosis of MET and until the time of death constitutes the time scale on which the diagnosis of a primary tumor can be positioned (1–5). In addition, the number of TC of the MET and the corresponding volume doublings are stated. The primary tumor (PT) process outlines the PT growth for the case of a primary M1 with an early MET initiation (at approx. 1 x 10^6 TC) years before the detection of PT
5 Time to metastasization
It may be misleading to characterize MET based on the time differential between pT and MET diagnosis. The time of PT diagnosis depends partly on methods of early detection and therefore, slides along the timeline for courses of disease with MET. This is illustrated in Fig. 4 with the well-known fact that a PT can indirectly appear only when the initiated MET occurs as cancer of unknown primary (Fig. 4 (1)), as primary M1 (2), or with primary M0 and tumor size-dependent intervals up to MET (3). Figure 4 (4–5) illustrates also the question of whether a MET can be initiated shortly before the PT diagnosis (4.1), during PT treatment (4), or even after the removal of the PT (5). The additional two time scales of the MET, based on either the number of VD or the TC, beg the question about the growth rate of PT and MET if both are simultaneously detected with comparable sizes. The PT process depicted at the bottom of Fig. 4 illustrates an individual course of disease. The dotted arrow left of circle 2 points to the first TC division, the tiny circle marks a very early MET initiation, perhaps at a PT size of 106 TC. After years, a concurrent detection of PT and its MET may occur. It is consequential that a PT cannot originate simultaneously or after initiation of its MET and therefore, the VD of MET must be shorter than that of PT. What is needed first are data for the synchronization of the two timescales and growth rates for PT and MET.
6 Lead time effects
Figure 4 (2–5) describes, along with the sizes of PT, the knowledge that a primary MET is normally more frequent in large BCs, while long MET-free survival times can be observed in small BC. Because of increasingly early detection, PT can be detected closer towards MET initiation. This produces a lead time effect and is illustrated in Fig. 5 with the cumulative distribution of time to MET. The time is stratified according to pT category. The step at time zero shows the percentage of primary M1 findings within each subgroup of MET patients. The dotted line suggests the distribution of delayed versus advanced detection of primary M1 findings. Particularly, the pT4 subgroup demonstrates, for one, that more than 50% are M1 findings, and secondly, that the fraction of delayed detection, or rather a long asymptomatic growth of MET, seems to prevail.
Cumulative distribution of the MET-free survival time of all MET patients: the step at zero shows the percentage of primary M1 from all known metastasized patients, up to 15 years, within each primary tumor (pT) category. The dotted line combines the lead time effect and delay for the MET detection. Numbers 1–3 assign cases with very early, median, and just before the PT diagnosis initiated MET with the assumption of a widely homogeneous MET growth, respectively
We can combine the stratified data from Fig. 5 with the concept from Fig. 4, showing that MET growth is comparable for pT1 and pT4 tumors, to create the schematic in Fig. 6. The course of diseases for pT1, pT2, and pT4 is synchronized onto an identical MET growth timeline with the sequential occurrences of MET initiation, diagnosis of PT, diagnosis of MET, and death. This illustration demonstrates that, with the delayed detection of a PT, continuously more MET are initiated and are simultaneously diagnosed with the PT. For a pT2 PT, we observe about 4.2% primary M1, approximately 45.7% MET in 15 years, and a median time to MET for primary M0 cases of 2.9 years. If the PT is detected as pT1, the corresponding figures are 1.4% for M1, 20.5%, and 3.8 years. Reservations about Fig. 6 are reasonable if the decreasing MET-free intervals suggest the idea of tumor aggressiveness, growing MET potential of a PT, or increasing cancer-like characteristics, instead of a homogeneous early acquisition of MET tendency.
Characteristics of the breast cancer distant metastasization (MET) process according to pT category: The identical time scale represents the homogeneous MET process with varying detection of PT. According to MCR data for pT1, the 15-year tumor-specific mortality is 20.6% and 1.5% are already primary M1 findings due to the early detection the MET-free survival time, which is 3.8. The two percentages (25.2%/27.9%) between pT1 and pT2 and pT3 describe the increase of MET and therefore, the additional MET initiation during the growth of PT from pT1 to pT3–4. A synchronization on the diagnosis of PT reflects the known view: tiny PT, low MET and long MET-free survival and large PT, frequent MET and a short MET-free survival
7 MET on timeline of PT
Figure 6 depicts an important corollary of the lead time effect. Cancer-related death adds up to 20.5% for pT1 and to 45.7% for pT2. From this, it follows that the difference, 25.2% of MET, will be initiated during the growth from pT1 to pT2. The linear relationship between PT size and MET and the known VDT for BC enable a more precise calculation of MET initiation. The VDT has been shown to have a mean of 157 days for women aged 50–70 [21, 22]. From this, it follows that a PT with a diameter of 30 mm has doubled the volume in 157 days and reached a diameter of about 38 mm. Therefore, after some 20 days, a diameter of 31 mm has been reached, and if therapy is delayed by 20 days, new MET will have been initiated in 1.2% of the patients [23]. In the case of a 10-mm PT, an additional 1.2% of newly initiated MET would occur after a 2-month delay in treatment. In principle, every day, a growing PT can initiate its first MET up to the very day of its removal.
The consideration of the time scale for the PT process improves our understanding of the time of initiation and the duration of MET. In about 1% of BC patients with 5-mm tumors, there are primary M1 findings (2 of Fig. 4). Such early MET must be initiated years before the detection of the BC. Assuming minimal variation in the MET growth, this unknown duration of MET growth time in M1 findings must be comparable with the MET-free survival time in patients where initiation of MET occurs just before PT removal (4.1 of Fig. 4 ). This poses the question for an estimation of the growth time of MET.
8 Growth of distant metastases
An estimation of MET growth time is possible if we know the time of MET initiation and of MET detection. Clinical data provide this information. In addition, we need information about the variation of growth time that is dependent on biological characteristics, e.g., for receptor negative or positive BC (Fig. 3c). According to what is known, no evidence exists for a distribution with a tenfold variation or more between minimal and maximal growth rates. Considering this, Fig. 5 allows an additional interpretation. The time distributions are almost identically shaped which is more easily seen without the primary M1 cases. The translation between the curves describes, as previously mentioned, the magnitude of the lead time effect. Again, the conformity of the distributions demonstrates the homogeneity of the MET process. Due to the small variability of MET growth, the growth time can be directly read from Fig. 5. The MET initiation around label 1 has occurred very early in the history of the PT. Therefore, most of them appear as primary M1 cases. However, the cases around label 3 must be initiated just before the removal of the PT, and therefore, demonstrates the time required for MET growth [24].
The median MET-free time (label 2) is not influenced by outliers. The patients with a pT1 PT have half of the MET growth time after and half before the detection of the PT, each about 3.5 years. This is a total of 7.0 years and together with a survival time of 2.4 years after MET for pT1 (Fig. 3a). This results in approximately 9.4 years from MET initiation to death. If we consider the prolonged tumor growth before and after MET in Fig. 3b, also late events after ten and more years can be explained. For receptor-negative BC with a twofold VDT, 5 years could be assumed (Fig. 3d). With this reasoning, we can explain more than 90–95% of the courses of disease in BC without auxiliary and complex hypotheses such as dormancy with a temporary growth stop or even with recirculation [25]. Comparable interpretations for colorectal, gastric, or lung cancers can also explain nearly 100% of the cases with MET. An important conclusion results for the VDT of the MET. If we assume a MET foci of 16 mm with about 4 × 109 TC, or 32 VD, then we get a VDT of about 80 days for MET or twice the growth rate of a PT in 157 days.
9 MET initiation relative to PT
These MET growth rate estimations now provides the necessary information to connect the timescales of PT and MET. If we assume a 7.0-year growth time from MET initiation up to MET detection, then it follows that the MET of a pT1 tumor with primary M1 was initiated 7.0 years before the detection of the PT. A 16-mm PT has about 4 × 109 TC and needed about 32 VD, and as previously stated, one VD requires about 157 days [21, 22]. Therefore, the PT has accomplished 16.3 VD in 7.0 years. This synchronization of the two time scales results in a first initiation of MET by the PT after 16 VD with about 216 or about 66,000 TC.
These are estimations based on clinical data which largely apply to MET because we know the time of MET initiation (label 3 of Fig. 5). However, for PT, we do not know the VDT of a micro-sized PT, especially before the angiogenic switch [26]. Perhaps, PT and MET share a common early developmental process. This consideration implies that the first MET can be initiated very early up until the R0 resection, most likely when a PT has between 105 and 106 TCs or a diameter of 200–1,000 μm. Therefore, MET is a very early event in cancer progression [27]. This very early initiation of MET poses the obvious question of whether very late MET initiations are also possible, for example, during or even after the removal of the PT (4–5 of Fig. 4).
10 No MET during or after removal of the primary tumor
It is risky to hypothesize that MET initiation cannot occur after R0 resection. This implies that the migration time of TCs to regional or distant organs is very short and may be measured in hours rather than in days. At first glance, this is a contradiction to the detection of TC in blood and bone marrow years after the removal of the PT [28–30]. However, a delayed activation of TCs out of such niches with subsequent MET initiation has not been observed, only hypothesized [31, 32]. The initiation of MET through TCs transferred with an organ transplant is a special case and apparently not clinically relevant [33]. Moreover, delayed activation is not plausible because it has to be assumed that millions of TCs are disseminated by the PT that remain viable and yet few of these cause MET. It is nearly impossible that these disseminated TCs migrate, during a great space of time, with information about the duration of a clinically relevant dormancy phase which is dependent on the time of removal. Equally improbable is a highly sophisticated communication between millions of TCs disseminated over the years that remain viable in blood, bone marrow, and distant organs [30, 34, 35], and their orchestration up to or by the removal of a pT1c/pT2 PT with about 30%/50% MET within 18 years (Fig. 2a). In addition, no excessive risk of MET has been observed due to the surgical removal of the PT [36]. Therefore, even the dormancy hypothesis that TC can recirculate from an interim storage after a long delay [37] can be questioned in solid tumors. Clearly, R0 resection stops further MET initiation. However, in those patients whose tumors will metastasize during the course of the disease, the initiation of MET has already occurred before diagnosis and removal of PT [38, 39].
11 Efficient migration process
This last conclusion leads to an astonishing consequence. The MET process is inefficient [40] if we consider that only few initiated MET foci result from millions of disseminated TCs [41]. This is compatible with the short lifetime of TCs in the blood [28]. With this understanding, more than 90% of MET that occur up to 10 years after diagnosis of BC can be explained without interim storages in blood, bone marrow, or distant organs, and nearly 100% for other frequent tumors. Therefore, a very efficient migration process to a distant organ, survival in the new environment, and perhaps a delayed growth of disseminated TCs have to be assumed for posteriori successfully established MET. There are two alternative explanations for this gigantic difference between numbers of initiated foci and disseminated TC. Either TCs are very heterogeneous, and, to be a successful seed, a TC must be comparable to a decathlon champion [2] or, more probably, TCs are “stable seeds” only as a consequence of the successful interaction between seed and the distant organ microenvironment, a factor which is the Achilles’ heel of the insufficient MET process. Cell division and apoptosis are decentralized affairs. However, the fact that gene expression analyses of the PT can predict MET, independent of the LN status, provides evidence that a PT must be genetically predisposed for a successful MET process [42–45].
12 MET progression pattern
Up to now, we have considered MET-free survival time independent of the organ involved. Further aspects of the MET process can be gleaned from the MET progression pattern. Table 1 shows the MET distribution for primary M1 versus M0 findings; unilocular and multilocular MET are shown for frequently occurring MET. Cancer registries receive these data if they are integrated within the health care network, and MET-related symptoms are treated. About 42% of the M1 findings are detected in at least two organs and 28% after M0. This supports the previously mentioned advances in MET detection through staging diagnostics and the improved prognosis of primary M1 tumors (Fig. 3b). Most often, the disease course begins with bone MET, alone or in any combination with other locations [46]. MET in other organs appears thereafter, normally within short time intervals, too short for a cascade-like initiation with the afore-estimated VD for MET (Fig. 1 H2). The pattern of MET progression also depends on the characteristics of the seed. Table 1 combines very different MET patterns for estrogen-receptor positive and negative BC, which mostly differ in bone and CNS MET progression patterns. Such a dependency on the seed is well known if we consider for example, adenocarcinomas of the breast, colon, kidney, or lung and their very different growth times, or the affinity of small cell lung cancer with CNS and adrenal gland MET. Nonetheless, the multistage MET process is not influenced by the velocity of PT and MET growth.
13 Clonal origin of metastases
It has long been known that MET foci are initiated from different TCs [47, 48]. The MET progression pattern considered together with a VDT of about 80 days provides with delivers the same message. Furthermore, the spatial distribution of the foci e.g. in different segments of liver or lung, or the synchronous detection of MET in different organs supports the initiation of MET by different TCs. Also, the time differences between metachronous MET are too short in comparison with the estimated VDT of MET for a sequential dissemination. The size of different foci and their sequential appearance in one organ is the reflection of the clonal and sequential origin of each MET foci. In addition, the observed difference in responses of multiple foci to systemic treatment supports the idea of seed heterogeneity. This influence may not be relevant in the complex MET process until anchoring in a distant organ occurs. Parallel MET initiations are implicit to this proposal [49], while a cascade-like MET initiation is excluded [50].
14 The special case: CNS metastases
The uniqueness of CNS MET has to be mentioned. Survival after CNS MET is known to be very short. About 50% of patients with CNS MET die within 6 months after MET detection. At first CNS MET, with such a short survival expectancy, contradicts our reasoning and the hypothesis of MET initiation before R0 resection. The conflict arises due to the assumption of a continuous development of MET, with a quarter of the growth occurring after, and ¾ before the detection of a MET. Additionally, the appearance of CNS MET generally occurs after three or more years of follow-up. The existence of vital yet non-dividing TC could provide an answer which bridges longer time intervals without tumor growth [51]. This hypothesis is plausible. An adjuvant prophylactic CNS irradiation for small cell lung cancer provides better local control of CNS MET. Also, a whole-brain irradiation after radiosurgical removal of smaller CNS foci is advantageous [52, 53]. This better CNS control can be explained by the existence of radiosensitive isolated TC or clusters and by a very asymmetric CNS MET growth.
Actually, the CNS MET process and response to treatment supports the hypothesized early initiation of MET along with the assumption that MET will most likely be stopped in the distant organ shortly after TCD, rather than as TC in isolated niches which are believed to be less accessible to adjuvant treatment [5, 54, 55]. This would also be supported by survival curves which would have to open like scissors after 7 years if adjuvant treatment were able to control ITC in niches or blood, yet such results do not exist. We observe the opening to occur after 2–3 years which supports the elimination of micro MET by adjuvant therapy [56, 57]. It is possible that an angiogenic switch provides the limit, up to which curative interventions are possible [26]. Our estimated VDT goes with agrees these observations.
15 Metastases do not metastasize
Many arguments are available to support the hypothesis that an established MET cannot further disseminate. This applies to all distant foci as well as to pLN. A MET initiation by remaining pLN after R0 removal of PT can be excluded based solely on the nearly linear increase of survival with the number of pLN (Fig. 2b). Additionally, a multiplicity of randomized trials has supported this hypothesis [58–61].
The observed spatial and temporal distribution of distant MET already excludes the possibility of a cascade-like dispersion. Liver MET can have a volume more than five hundredfold larger than the PT. However, this tumor mass, founded by a single TC that was a successful seed in the liver, is unable to generate satellite foci in the surrounding tissue (Fig. 1 H2). These are the foci distant from the removed ones, or in other organs which limit the benefit of MET surgery. Because MET do not metastasize, MET surgery offers a chance for selected patients [62]. The generalization for all solid tumors and for all foci, true local recurrences, pLN, and distant organs has great relevance for this hypothesis (Fig. 1 H1). Even pLNs which are regional, for example in lung MET, are not in conflict with this hypothesis. It has been known for a long time that migrating TCs can traverse the interstitial space, extravascularly, invade the lymphatics, and follow the same course as TC which were initially disseminated via the lymphatics [63]. Therefore, clinical data do not provide any indication that a cascade-like initiation could be a relevant clinical problem, even if, of course, a reactivation of TC motility cannot be excluded if pluripotent stem cells already can be generated from fibroblasts [64]. With this reservation, the ultimate abstraction follows: true local, regional and distant MET do not metastasize (Fig. 1 H1).
16 MET model
Figure 7 presents a synopsis of the discussed MET process. First, the clinical view shows the development of a PT with a growth of up to about 1 cm, or 109 TC. At this size, the PT will be detected and removed. The risk of MET increases up to the R0 resection and is depicted by the horizontal arrows, 25.2% MET risk for PT at stage pT1 to pT2 versus 27.9% for >pT2. The dotted horizontal lines indicate the parallel development of MET with the early initiation, the growth, the time of detection of possible MET, and the cause of death. For BC with 109 TCs, the probability of MET is about 10% (Fig. 2a (pT1b)). Six alternative MET events are outlined in Fig. 7.
Clinical view on metastasization during the course of disease: possible distant metastasization (MET) initiation, growth time, and detection are depicted. The first MET is hypothesized to be initiated by tumor cell dissemination, when the PT attains a diameter of about 1 mm or 106 cells (1). A fast growing MET can pass the PT and appear as CUP (2). Late MET initiation results in long survival (3). MET in different organs can appear at different times during the course of disease in dependence of initiation and growth (5). Multifocal MET with different sizes of foci reflect different initiation times (6)
If a successful TCD occurs very early, the initiated MET may grow autonomously and in parallel to the PT. Therefore, the first variant in the course of disease is the diagnosis of primary M1 (1 of Fig. 7). Because PT and MET are perhaps largely autonomous processes and MET has a twofold VDT, it can get ahead of the PT and become a carcinoma of unknown primary (CUP; 1 of Fig. 7). The PT can, if sufficient survival time remains, be discovered later. This is considerable even with regressing or temporarily stationary PT [65]. The increased incidence caused by screening or hormone substitution in BC is perhaps only explainable with this explanation of CUP [66].
Primary M1 is the earliest possible MET initiation in about 1.5% of the pT1 BC subgroup. The third extreme variant is a MET initiation just before the removal of the PT with subsequently a very long MET-free survival (3 of Fig. 7). The normal course of MET disease is a primary M0 finding, with about 20% MET initiations occurring over the whole time interval up to the removal of the PT (4 of Fig. 7). Number 5 of Fig. 7 represents sequentially initiated MET foci in multiple organs. A concurrent initiation of the MET in different organs is implausible, the pattern reflects anatomical aspects, characteristics of each seed TC, and the duration of a successful TCD [1, 2, 67]. Finally, number 6 of Fig. 7 illustrates multifocal MET in one organ. Size of the foci and location support a sequential initiation by different TCs, which has been known since decades [47]. Modern in vivo magnetic resonance imaging and videomicroscopy have allowed tracking of solitary TC and the development of MET and provide increasing evidence for this model [34, 35, 68, 69].
17 Future prospects
Although the proposed model is uncomplicated, in view of the philosophy of science, it explains many observations, in part by logical conclusions from a tremendous volume of empirical data. However, the model seems to contradict what we know of the complexity and diversity of BC pathogenesis. This model of MET initiation agrees with our understanding of cancer induction. We know that after the doubling of the first TC, further growth is independent of the cause of initiation which may have occurred among others by radiation, viruses, chemicals, or genes. The holistic description leads in almost the same manner to a uniform MET process, although the reductionist’s genetic approach to the mind-boggling complexity and magnitude of signaling pathways rejects such a simplification. Nonetheless, merely the complexity of the MET process alone is an argument for the hypothesis, because the complexity cannot have been evolutionarily developed differentially for each of our arbitrarily classified hundred or more solid cancers. Furthermore, the generalization can be shown with comparable data for all cancers. The dysregulation of important pathways relevant for tissue generation and organ repair likely produces the diversity observed among cancers [70]. The hypothesis that distant MET do not metastasize is astonishing and can be explained by the suggestion that TCs can “go and grow” only once, even though the biological reason for this is not understood (up to now).
Models are important if they can explain and challenge observations and therefore rearrange research priorities through refutable hypotheses. The benefits of screening, as well as the chances and limits of adjuvant and palliative treatments, can be argued with this model. The limits of post-treatment care or quality assessment due to early MET initiation should be thoroughly considered. The necessary discussion of these far-reaching aspects exceeds the limits of this paper. Nevertheless, the discussion of alternative hypotheses and resulting research directions would be enlightening with perhaps a reconsidering of clinical research priorities. Finally, the importance of cancer registries must be mentioned. It is a challenge to analyze, compare, and interpret the data of so many different cancers. Yet, the accomplishment of such allows for cancer registries to become an important infrastructure for quality assessment and for support of clinical research and the health care of cancer patients.
Abbreviations
- BC:
-
Breast cancer
- MET:
-
(Distant) metastasization
- pLN:
-
(Positive) lymph node
- PT:
-
Primary tumor
- TC(D):
-
Tumor cell (dissemination)
- VD(T):
-
Volume doubling (time)
References
Chambers, A. F., Groom, A. C., & MacDonald, I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews. Cancer, 2(8), 563–572. doi:10.1038/nrc865.
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature Reviews. Cancer, 3(6), 453–458. doi:10.1038/nrc1098.
Munich Cancer Registry. http://www.Tumorregister-muenchen.De/facts/specific_analysis.Php.
Curado, M., Edwards, B., Shin, H., Storm, H., Ferlay, J., Heanue, M., et al. (2007). Cancer incidence in five continents, vol. Ix. Lyon: IARC Scientific Publication No.160.
Nguyen, D. X., Bos, P. D., & Massague, J. (2009). Metastasis: from dissemination to organ-specific colonization. Nature Reviews. Cancer, 9(4), 274–284. doi:10.1038/nrc2622.
Weinberg, R. (2007). The biology of cancer. New York: Garland Science.
Sugarbaker, E. V., Cohen, A. M., & Ketcham, A. S. (1971). Do metastases metastasize? Annals of Surgery, 174(2), 161–166.
Tait, C. R., Dodwell, D., & Horgan, K. (2004). Do metastases metastasize? The Journal of Pathology, 203(1), 515–518. doi:10.1002/path.1544.
Sobin, L., Gospodarowicz, M., & Wittekind, C. (Eds.). (2009). Uicc: TNM classification of malignant tumors (7th ed.). Wiley-Blackwell: New York.
Leong, S. P., Cady, B., Jablons, D. M., Garcia-Aguilar, J., Reintgen, D., Jakub, J., et al. (2006). Clinical patterns of metastasis. Cancer and Metastasis Reviews, 25(2), 221–232. doi:10.1007/s10555-006-8502-8.
Sarfati, D., Blakely, T., & Pearce, N. (2010). Measuring cancer survival in populations: relative survival vs cancer-specific survival. International Journal of Epidemiology, 39(2), 598–610. doi:10.1093/ije/dyp392.
Benson, J. R., & della Rovere, G. Q. (2007). Management of the axilla in women with breast cancer. The Lancet Oncology, 8(4), 331–348. doi:10.1016/S1470-2045(07)70103-1.
Michaelson, J. S., Silverstein, M., Wyatt, J., Weber, G., Moore, R., Halpern, E., et al. (2002). Predicting the survival of patients with breast carcinoma using tumor size. Cancer, 95(4), 713–723. doi:10.1002/cncr.10742.
Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., et al. (2000). Molecular portraits of human breast tumours. Nature, 406(6797), 747–752. doi:10.1038/35021093.
Sotiriou, C., & Pusztai, L. (2009). Gene-expression signatures in breast cancer. The New England Journal of Medicine, 360(8), 790–800. doi:10.1056/NEJMra0801289.
Bernards, R., & Weinberg, R. A. (2002). A progression puzzle. Nature, 418(6900), 823. doi:10.1038/418823a.
Balic, M., Lin, H., Young, L., Hawes, D., Giuliano, A., McNamara, G., et al. (2006). Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clinical Cancer Research, 12(19), 5615–5621. doi:10.1158/1078-0432.CCR-06-0169.
Clarke, M. F., & Fuller, M. (2006). Stem cells and cancer: two faces of eve. Cell, 124(6), 1111–1115. doi:10.1016/j.cell.2006.03.011.
Chojniak, R., & Younes, R. N. (2003). Pulmonary metastases tumor doubling time: assessment by computed tomography. American Journal of Clinical Oncology, 26(4), 374–377. doi:10.1097/01.COC.0000026481.38654.52.
Finlay, I. G., Meek, D., Brunton, F., & McArdle, C. S. (1988). Growth rate of hepatic metastases in colorectal carcinoma. The British Journal of Surgery, 75(7), 641–644.
Peer, P. G., van Dijck, J. A., Hendriks, J. H., Holland, R., & Verbeek, A. L. (1993). Age-dependent growth rate of primary breast cancer. Cancer, 71(11), 3547–3551.
Kuroishi, T., Tominaga, S., Morimoto, T., Tashiro, H., Itoh, S., Watanabe, H., et al. (1990). Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Japanese Journal of Cancer Research, 81(5), 454–462.
Richards, M. A., Westcombe, A. M., Love, S. B., Littlejohns, P., & Ramirez, A. J. (1999). Influence of delay on survival in patients with breast cancer: a systematic review. Lancet, 353(9159), 1119–1126. doi:S0140673699021431.
Engel, J., Eckel, R., Kerr, J., Schmidt, M., Furstenberger, G., Richter, R., et al. (2003). The process of metastasisation for breast cancer. European Journal of Cancer, 39(12), 1794–1806. doi:S0959804903004222.
Aguirre-Ghiso, J. A. (2007). Models, mechanisms and clinical evidence for cancer dormancy. Nature Reviews. Cancer, 7(11), 834–846. doi:10.1038/nrc2256.
Naumov, G. N., Folkman, J., Straume, O., & Akslen, L. A. (2008). Tumor-vascular interactions and tumor dormancy. APMIS, 116(7–8), 569–585. doi:10.1111/j.1600-0463.2008.01213.x.
Husemann, Y., Geigl, J. B., Schubert, F., Musiani, P., Meyer, M., Burghart, E., et al. (2008). Systemic spread is an early step in breast cancer. Cancer Cell, 13(1), 58–68. doi:10.1016/j.ccr.2007.12.003.
Meng, S., Tripathy, D., Frenkel, E. P., Shete, S., Naftalis, E. Z., Huth, J. F., et al. (2004). Circulating tumor cells in patients with breast cancer dormancy. Clinical Cancer Research, 10(24), 8152–8162. doi:10.1158/1078-0432.CCR-04-1110.
Cristofanilli, M., Budd, G. T., Ellis, M. J., Stopeck, A., Matera, J., Miller, M. C., et al. (2004). Circulating tumor cells, disease progression, and survival in metastatic breast cancer. The New England Journal of Medicine, 351(8), 781–791. doi:10.1056/NEJMoa040766.
Pachmann, K., Camara, O., Kavallaris, A., Krauspe, S., Malarski, N., Gajda, M., et al. (2008). Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. Journal of Clinical Oncology, 26(8), 1208–1215. doi:10.1200/JCO.2007.13.6523.
Townson, J. L., & Chambers, A. F. (2006). Dormancy of solitary metastatic cells. Cell Cycle, 5(16), 1744–1750. doi:2864.
Wikman, H., Vessella, R., & Pantel, K. (2008). Cancer micrometastasis and tumour dormancy. APMIS, 116(7–8), 754–770. doi:10.1111/j.1600-0463.2008.01033.x.
Birkeland, S. A., & Storm, H. H. (2002). Risk for tumor and other disease transmission by transplantation: a population-based study of unrecognized malignancies and other diseases in organ donors. Transplantation, 74(10), 1409–1413. doi:10.1097/01.TP.0000034717.19606.B5.
Logan, P. T., Fernandes, B. F., Di Cesare, S., Marshall, J. C., Maloney, S. C., & Burnier, M. N., Jr. (2008). Single-cell tumor dormancy model of uveal melanoma. Clinical & Experimental Metastasis, 25(5), 509–516. doi:10.1007/s10585-008-9158-2.
Heyn, C., Ronald, J. A., Ramadan, S. S., Snir, J. A., Barry, A. M., MacKenzie, L. T., et al. (2006). In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magnetic Resonance in Medicine, 56(5), 1001–1010. doi:10.1002/mrm.21029.
Hölzel, D., Eckel, R., & Engel, J. (2009). [Colorectal cancer metastasis: frequency, prognosis, and consequences]. Chirurg, 80(4), 331–340. doi:10.1007/s00104-008-1603-x.
Kim, M. Y., Oskarsson, T., Acharyya, S., Nguyen, D. X., Zhang, X. H., Norton, L., et al. (2009). Tumor self-seeding by circulating cancer cells. Cell, 139(7), 1315–1326. doi:10.1016/j.cell.2009.11.025.
Fisher, B., Anderson, S., Fisher, E. R., Redmond, C., Wickerham, D. L., Wolmark, N., et al. (1991). Significance of ipsilateral breast tumour recurrence after lumpectomy. Lancet, 338(8763), 327–331.
Fisher, B., & Anderson, S. J. (2010). The breast cancer alternative hypothesis: is there evidence to justify replacing it? Journal of Clinical Oncology, 28(3), 366–374. doi:10.1200/JCO.2009.26.8292.
Chambers, A. F., Naumov, G. N., Vantyghem, S. A., & Tuck, A. B. (2000). Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Research, 2(6), 400–407.
Glaves, D., Huben, R. P., & Weiss, L. (1988). Haematogenous dissemination of cells from human renal adenocarcinomas. British Journal of Cancer, 57(1), 32–35.
van de Vijver, M. J., He, Y. D., van’t Veer, L. J., Dai, H., Hart, A. A., Voskuil, D. W., et al. (2002). A gene-expression signature as a predictor of survival in breast cancer. The New England Journal of Medicine, 347(25), 1999–2009. doi:10.1056/NEJMoa021967.
Bos, P. D., Zhang, X. H., Nadal, C., Shu, W., Gomis, R. R., Nguyen, D. X., et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature, 459(7249), 1005–1009. doi:10.1038/nature08021.
Liu, R., Wang, X., Chen, G. Y., Dalerba, P., Gurney, A., Hoey, T., et al. (2007). The prognostic role of a gene signature from tumorigenic breast-cancer cells. The New England Journal of Medicine, 356(3), 217–226. doi:10.1056/NEJMoa063994.
Kang, Y., Siegel, P. M., Shu, W., Drobnjak, M., Kakonen, S. M., Cordon-Cardo, C., et al. (2003). A multigenic program mediating breast cancer metastasis to bone. Cancer Cell, 3(6), 537–549. doi:S1535610803001326.
Fisher, B. (1980). Laboratory and clinical research in breast cancer—a personal adventure: the David A. Karnofsky memorial lecture. Cancer Research, 40(11), 3863–3874.
Talmadge, J. E., Wolman, S. R., & Fidler, I. J. (1982). Evidence for the clonal origin of spontaneous metastases. Science, 217(4557), 361–363.
Fidler, I. J. (1973). The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. European Journal of Cancer, 9(3), 223–227.
Klein, C. A. (2009). Parallel progression of primary tumours and metastases. Nature Reviews. Cancer, 9(4), 302–312. doi:10.1038/nrc2627.
Pantel, K., & Brakenhoff, R. H. (2004). Dissecting the metastatic cascade. Nature Reviews. Cancer, 4(6), 448–456. doi:10.1038/nrc1370.
Palmieri, D., Chambers, A. F., Felding-Habermann, B., Huang, S., & Steeg, P. S. (2007). The biology of metastasis to a sanctuary site. Clinical Cancer Research, 13(6), 1656–1662. doi:10.1158/1078-0432.CCR-06-2659.
Mehta, M. P., Tsao, M. N., Whelan, T. J., Morris, D. E., Hayman, J. A., Flickinger, J. C., et al. (2005). The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. International Journal of Radiation Oncology, Biology, Physics, 63(1), 37–46. doi:10.1016/j.ijrobp.2005.05.023.
Aoyama, H., Shirato, H., Tago, M., Nakagawa, K., Toyoda, T., Hatano, K., et al. (2006). Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA, 295(21), 2483–2491. doi:10.1001/jama.295.21.2483.
Holmgren, L., O’Reilly, M. S., & Folkman, J. (1995). Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Natural Medicines, 1(2), 149–153.
Steeg, P. S. (2006). Tumor metastasis: mechanistic insights and clinical challenges. Natural Medicines, 12(8), 895–904. doi:10.1038/nm1469.
Adjuvant tamoxifen in the management of operable breast cancer: the Scottish Trial. Report from the Breast Cancer Trials Committee, Scottish Cancer Trials Office (MRC) Edinburgh (1987). Lancet, 2(8552), 171–175. doi:S0140-6736(87)90762-8.
de Boer, M., van Deurzen, C. H., van Dijck, J. A., Borm, G. F., van Diest, P. J., Adang, E. M., et al. (2009). Micrometastases or isolated tumor cells and the outcome of breast cancer. The New England Journal of Medicine, 361(7), 653–663. doi:10.1056/NEJMoa0904832.
Veronesi, U., Marubini, E., Mariani, L., Valagussa, P., & Zucali, R. (1999). The dissection of internal mammary nodes does not improve the survival of breast cancer patients. 30-year results of a randomised trial. European Journal of Cancer, 35(9), 1320–1325. doi:S0959-8049(99)00133-1.
Fisher, B., Anderson, S., Bryant, J., Margolese, R. G., Deutsch, M., Fisher, E. R., et al. (2002). Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England Journal of Medicine, 347(16), 1233–1241. doi:10.1056/NEJMoa022152.
Morton, D. L., Thompson, J. F., Cochran, A. J., Mozzillo, N., Elashoff, R., Essner, R., et al. (2006). Sentinel-node biopsy or nodal observation in melanoma. The New England Journal of Medicine, 355(13), 1307–1317. doi:10.1056/NEJMoa060992.
Hartgrink, H. H., van de Velde, C. J., Putter, H., Bonenkamp, J. J., Klein Kranenbarg, E., Songun, I., et al. (2004). Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. Journal of Clinical Oncology, 22(11), 2069–2077. doi:10.1200/JCO.2004.08.026.
Adam, R., Delvart, V., Pascal, G., Valeanu, A., Castaing, D., Azoulay, D., et al. (2004). Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg, 240(4), 644–657. doi:00000658-200410000-00010. discussion 657–648.
Fisher, B., & Fisher, E. R. (1966). Transmigration of lymph nodes by tumor cells. Science, 152(727), 1397–1398.
Aoi, T., Yae, K., Nakagawa, M., Ichisaka, T., Okita, K., Takahashi, K., et al. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 321(5889), 699–702. doi:10.1126/science.1154884.
Zahl, P. H., Maehlen, J., & Welch, H. G. (2008). The natural history of invasive breast cancers detected by screening mammography. Archives of Internal Medicine, 168(21), 2311–2316. doi:10.1001/archinte.168.21.2311.
Chlebowski, R. T., Kuller, L. H., Prentice, R. L., Stefanick, M. L., Manson, J. E., Gass, M., et al. (2009). Breast cancer after use of estrogen plus progestin in postmenopausal women. The New England Journal of Medicine, 360(6), 573–587. doi:10.1056/NEJMoa0807684.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet, 1, 571–573.
Naumov, G. N., Wilson, S. M., MacDonald, I. C., Schmidt, E. E., Morris, V. L., Groom, A. C., et al. (1999). Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. Journal of Cell Science, 112(Pt 12), 1835–1842.
Kienast, Y., von Baumgarten, L., Fuhrmann, M., Klinkert, W. E., Goldbrunner, R., Herms, J., et al. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine, 16(1), 116–122. doi:10.1038/nm.2072.
Hoon, D. S., Kitago, M., Kim, J., Mori, T., Piris, A., Szyfelbein, K., et al. (2006). Molecular mechanisms of metastasis. Cancer and Metastasis Reviews, 25(2), 203–220. doi:10.1007/s10555-006-8500-x.
Acknowledgements
We thank the many doctors and clinicians who cooperate within the complex network of the Munich Cancer Registry despite the back-breaking medical bureaucracy for the daily health care. It is our duty to provide an adequate evaluation of the submitted data for outcomes, quality management and innovative treatments.
Conflict of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hölzel, D., Eckel, R., Emeny, R.T. et al. Distant metastases do not metastasize. Cancer Metastasis Rev 29, 737–750 (2010). https://doi.org/10.1007/s10555-010-9260-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-010-9260-1