Abstract
Introduction
Radiotherapy, like most anticancer treatments, achieves its therapeutic effect by inducing different types of cell death in tumors.
Cell death markers and imaging modalities
To evaluate treatment efficacy a variety of routine anatomical imaging modalities is used. However, changes in tumor physiology, metabolism and proliferation often precede these volumetric changes. Therefore, reliable biomarkers and imaging modalities that could assess treatment response more rapidly or even predict tumor responsiveness to treatment in an early phase would be very useful to identify responders and/or avoid ineffective, toxic therapies. A better understanding of cell death mechanisms following irradiation is essential for the development of such tools.
Cell death and available assays
In this review the most prominent types of radiation-induced cell death are discussed. In addition, the currently available assays to detect apoptosis, necrosis, mitotic catastrophe, autophagy and senescence in vitro and, if applicable, in vivo, are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Cell death
Cell death is an essential biological process for eliminating abundant and unwanted cells during embryonic development, growth, differentiation and maintenance of tissue homeostasis. Deregulation of cell death pathways is associated with various diseases. Failure to eliminate cells that have been exposed to mutagenic agents may contribute to the development of cancer and resistance to anticancer therapy. In fact, the capacity to evade (apoptotic) cell death has been defined as one of the hallmarks of cancer [1].
It has become clear in recent years that cell death comprises a large number of different types of cellular demise (Table 1). According to a recent classification at least eight different types of cell death can be defined [2]. These can be divided into apoptotic and non-apoptotic. The major types are discussed in this review.
1.1.1 Apoptotic cell death
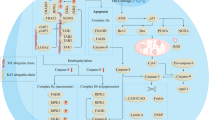
Apoptosis is defined by morphological criteria and by the requirement for active participation of the dying cell (Table 1). It can be induced by a great variety of stimuli, including growth factor deprivation, cell detachment (also known as anoikis), cytostatic drugs and ionizing radiation. Such stimuli may use unique “private induction pathways” to engage the apoptotic machinery. However, the molecular events in the execution phase of apoptosis are shared and impinge on the caspase family of proteases. Caspases are synthesized as pro-enzymes and activated by proteolysis. The private induction pathway activates the inducer caspases; these in turn cleave and activate the effector caspases, whose proteolytic action on specific cellular components brings about the typical apoptotic morphology. Inducer caspases are activated by multimerization on a scaffold protein, whereupon induced proximity allows for cross-cleavage and activation. Two major pathways for inducer caspase activation can be distinguished: the intrinsic pathway in which caspase-9 multimerizes at the Apaf-1 scaffold downstream from mitochondria, and the extrinsic pathway in which caspase-8 or caspase-10 multimerize at the cytoplasmic tail of death receptors [3].
Most apoptotic stimuli, including radiation depend on the intrinsic mitochondrial pathway in which induced permeability of the mitochondrial outer membrane permits the release of pro-apoptotic factors into the cytosol. Of these, cytochrome c binds to the scaffold protein Apaf-1 and allows recruitment and activation of the inducer caspase-9, forming the apoptosome complex. In addition, Smac/Diablo alleviates blockade of caspase-9 and effector caspases by the inhibitor of apoptosis proteins. The mitochondria also release other factors that contribute to morphological changes [4].
The Bcl-2 protein family mediates mitochondrial permeability and is therefore the key regulator of most apoptotic pathways [5]. The presence of Bcl-2 homology (BH) domains is their common characteristic. The family consists of the anti-apoptotic Bcl-2 group, the pro-apoptotic Bax group and the pro-apoptotic BH3 domain-only group. Family members can heterodimerize through interaction of the BH3 domain α helix with a groove formed by BH1 and BH2 domains. Death-induction involves collaboration between Bax type proteins and BH3 domain-only family members [6]. The BH3 domain-only group has many members that differ in responsiveness to stimuli and in the pathways they regulate. They are controlled at the transcriptional and/or post-translational level [5]. For instance, Noxa and Puma are transcriptionally upregulated by p53 and implicated in DNA damage pathways, Bmf binds to the cytoskeleton and is activated upon cell detachment, Bad is sequestered in the cytosol and released upon dephosphorylation induced by growth factor deprivation, while Bid is activated by proteolysis, downstream from death receptors. The Bax group contains the ubiquitous Bax and Bak and the ovary-specific Bok. Of these, Bak is constitutively associated with mitochondria. Studies on Bax and Bak knock out cells have recently illuminated how pro-apoptotic Bcl-2 family members operate [6, 7]. BH3 domain-only proteins require at least one Bax type partner to induce cell death. They induce a conformational change in Bax/Bak and its assembly in the mitochondrial membrane into homomultimers with presumed channel forming properties [7–9]. Inhibitory Bcl-2 family members such as Bcl-2 and Bcl-XL sequester BH3-domain only proteins, disallowing them to anticipate in pro-apoptotic complex formation [10]. A refinement of this model suggests that some BH3 domain-only proteins specialize in complexing with inhibitory Bcl-2 family members, while others preferentially interact with and activate Bax or Bak [11].
Unlike all other apoptotic stimuli, death receptors can activate effector caspases and induce apoptosis independent of the mitochondria via the extrinsic pathway. Death receptors are members of the tumor necrosis factor (TNF) receptor superfamily and activate inducer caspase-8/10 at the death domain in their cytoplasmic tail. TNF-related apoptosis-inducing ligand (TRAIL) receptors and CD95 do this by a common principle. Upon ligand binding, preformed receptor trimers recruit Fas-associated protein with death domain (FADD), which in turn recruits caspase-8/10. Within this death-inducing signaling complex (DISC), caspase-8/10 are activated by multimerization. They are subsequently released into the cytosol where they cleave and activate effector caspases. Death receptors also connect to the mitochondrial pathway for caspase activation, which is regarded as an amplification loop for effector caspase activation. Upon cleavage by caspase-8/10, the carboxy-terminal fragment of BH3-only protein Bid (tBid) relocates to mitochondria where it induces membrane permeability via Bax or Bak. This releases mediators such as cytochrome c and Smac/Diablo that allow activation of caspase-9 and effector caspases.
Although radiation-induced apoptosis is caspase-dependent [12–14], it is not well understood how radiation activates the caspase cascade. The observation that Bcl-2 is able to block radiation-induced cytochrome c release and apoptosis [14–16], demonstrates the involvement of a mitochondria-dependent mechanism in this response. This notion is further supported by the fact that p53, which is activated by DNA-damaging agents like ionizing radiation, is a direct transcriptional regulator of both Bcl-2, Bax, Puma, Noxa and Bid [17–19] and can act as an apoptogenic factor at the mitochondrial membrane [20]. There are also other direct links between p53 and death receptors: the CD95 and TRAIL receptor-2 (DR5) genes contain a p53 response element and both receptors (and/or their respective ligands) can be upregulated in response to radiation and chemotherapeutic drugs [21–23], causing activation of the initiator caspase 8.
1.1.2 Non-apoptotic cell death
Non-apoptotic cell death includes necrosis, mitotic cell death (mitotic catastrophe), autophagy and senescence. These will be shortly discussed (Table 1).
Necrosis
Necrosis is usually the cellular response to severe and massive toxic insults associated with infection, inflammation or ischemia, such as defective membrane potential, cellular energy depletion, nutrient starvation, damage to membrane lipids or loss of function of ion channels/pumps. A major difference with apoptotic cell death which is energy-dependent, is the depletion of the adenosine triphosphate pool and production associated with necrosis. Conditions of increased intracellular calcium or nicotinamide adenine dinucleotide depletion by excessive activation of Poly adenosine diphosphate-ribose polymerase following DNA damage are believed to be a cause of necrotic cell death as well [24]. Necrotic morphology is characterized by increased cytoplasmic vacuolation, organelle degeneration, damage to membrane lipids with cell swelling and rupture, and induction of inflammation due to the release of cellular contents. Although necrosis is usually considered a passive and uncontrolled process, recent data indicate that this mode of cell death is partially under cellular control [25].
Mitotic catastrophe
Mitotic catastrophe refers to the process when cells attempt to divide without proper repair of DNA damage. This aberrant mitosis is associated with giant, multinucleated cells and uncondensed chromosomes, distinguishing mitotic catastrophe morphologically from other types of cell death [26]. It is the most prominent mode of cell death after radiation in solid tumors and may occur at long intervals after exposure depending on the proliferation rate and DNA repair capacity of the irradiated cells [27, 28]. An essential element of the cellular response to radiation involves cell cycle arrest allowing the damaged DNA to be repaired before replication and mitosis occur [29]. When the damage is too extensive and repair fails, cells may undergo apoptosis. Frequently, however, these checkpoints are defective and cells may enter mitosis prematurely before DNA repair is completed. Because in a large proportion of tumors, cell cycle checkpoints are compromised and apoptotic pathways frequently suppressed, tumor cells preferentially execute this mitotic mode of cell death after treatment with DNA damaging regimens.
Autophagy
Autophagy is an evolutionary conserved physiological mechanism responsible for the routine degradation and turnover of (non-essential) cytoplasmic components by lysosomal hydrolases [30]. Autophagy can be activated under various stress conditions such as starvation, hypoxia, hyperthermia and oxidative stress. In response to radiation autophagy has been shown to be associated with the development of acidic vesicular organelles [31]. Furthermore, activation of autophagy by inhibiting mammalian target of rapamycin signaling may contribute to the cellular response to radiation [32]. Defective autophagy has been implicated in a variety of diseases including vacuolar myopathies, neurodegenerative disorders and cancer [33, 34]. Morphologically, autophagy is characterized by the appearance of a large number of cytosolic vacuole-like structures (autophagic vesicles). It remains unclear how autophagy is involved in tumor cell growth and whether inhibition of this mode of cell death modulates the response to anticancer treatment. Cross-talk between autophagic cell death and apoptosis has been described at the level of mitochondria, estrogen receptor and lysosomes [35].
Senescence
Senescence is usually defined as a state of permanent loss of proliferative capacity. Two types of senescence have been described: replicative senescence which is associated with aging and results from a progressive shortening of telomeres during cell division, and accelerated senescence which can be induced by DNA damage, oncogenic mutation and treatment with telomere-shortening agents [36]. Because senescent cells have undergone irreversible growth arrest and lost clonogenic potential, they may constitute an important component of the tumor response to therapy [37]. The induction of senescence is accompanied by specific cellular morphological changes such as increased granularity in enlarged and flattened cells. A number of genes involved in cell cycle regulation have also been implicated in senescence, including p53, p21, Rb and p16.
1.2 Markers and monitoring of cell death
1.2.1 Apoptosis
For quantitative in vitro analysis of apoptosis, a number of assays are currently available. These include nuclear staining and cytometric DNA-fragmentation assays, terminal uridine deoxynucleotidyl transferase nick end labeling (TUNEL) staining, DNA laddering on sodium dodecyl-polyacrylamide gel electrophoresis, detection of subG1/G0 cell population, Annexin V staining, caspase-activity assays and detection of changes in mitochondrial membrane potential (Table 1). Some of these assays can be used for ex vivo detection of apoptosis in tumor tissue biopsies before, during and after treatment. Many studies have indeed been undertaken to established a correlation between the level of apoptosis prior to, and the tumor response following irradiation. The results of these studies, however, have been conflicting. While some studies have shown that a high apoptotic index correlates with good prognosis, others show the reverse [38]. Both methodological differences and intra-/inter-tumor variations among these studies complicate interpretation of the results [39]. Moreover, since apoptosis is controlled by a large number of positive and negative regulators, focusing on the apoptotic index alone will certainly not lead to the identification of a solid predictor for treatment outcome. For example, the combination of mutated p53 and overexpression of Bcl-2 was identified as a highly significant unfavorable prognostic factor for local control and disease-free, disease-specific and overall survival in 83 patients with transitional cell cancer of the bladder treated by radiotherapy [40]. Thus, in addition to classical prognostic factors, apoptosis may be helpful in predicting tumor response to therapy. Obviously, larger and prospective randomized studies are needed to establish the role of such tumor biological parameters in predicting the natural history of a tumor and its radiosensitivity.
1.2.2 In vivo imaging of apoptosis
Our increased knowledge on the processes that regulate apoptosis has identified several targets that can be used as specific cell death markers, including membrane alterations, mitochondrial permeabilization and cytochrome c release, caspase activation, DNA degradation and nuclear fragmentation. One of the early events during the apoptotic program is the externalization of phosphatidyl serine (PS) from the cytoplasmic to the extracellular leaflet of the plasma membrane lipid bilayer (Fig. 1). This redistribution of PS results from coordinated translocase, floppase and scramblase activities, and precedes most of the other events of the apoptotic cascade. When exposed PS serves as a binding site for Annexin V.
Annexin V is a member of a larger family of at least 13 calcium and phospholipid binding proteins [41]. It is widely expressed in eukaryotic cells where it is mainly confined to the cytosolic side of the plasma membrane. In addition, Annexin V can be found at low plasma concentrations in healthy humans [42]. The protein consists of 319 amino acids forming a single planar cyclic polypeptide chain with a total molecular weight of 35.8 kDa. Originally, Annexin V was implicated in blood coagulation as it inhibits prothrombin activation and prevents thrombus formation. It also displays anti-inflammatory properties through its inhibitory effect on phospholipase A2 activation, preventing the release of arachidonic acid [43]. Finally, Annexin V has been shown to act as an inhibitor of protein kinase C. Most of these biological functions of Annexin V can be explained by its high calcium-dependent binding affinity for negatively charged phospholipids, in particular for PS with nanomolar affinity [44]. Normal, quiescent cells can harbor up to 5,000 PS-binding sites for Annexin V [45], while this number can increase 1,000-fold in activated thrombocytes and endothelial cells. Importantly, in apoptotic tumor cells as many as 6–14 × 103/cell PS-binding sites can be found [46].
Based on these observations, Annexin V is regarded as a useful marker for PS-exposing cells when labeled with detectable tags. Indeed, fluorescent residues like fluorescein isothiocyanate are now routinely used for cell-sorting purposes by fluorescence-activated cell sorting and cytological and histological identification of apoptotic cells.
More recently, in vivo detection of apoptosis has become feasible with the development of radiolabeled Annexin V-based PS-targeting molecules. Recombinant Annexin V coupled to the bifunctional chelator molecule hydrazinonicotinamide (HYNIC) and radiolabeled with 99mTc is currently the most widely studied and extensively used tracer for in vivo visualization and quantification of apoptosis. Initial studies focused on a variety of experimental models, including CD95/Fas-induced hepatic apoptosis [47]. Anti-CD95/Fas antibody-treated mice demonstrated a three-fold rise in hepatic uptake of Annexin V above control, identified both by imaging and scintillation well counting. The increase in hepatic uptake in anti-CD95/Fas antibody-treated mice correlated to histological evidence of fulminant hepatic apoptosis. In patients, 99mTc-HYNIC-Annexin V has successfully been applied in studies on myocardial ischemia [48], transplantation medicine [49] and hypoxic brain injury [50]. In oncology, 99mTc-HYNIC-Annexin V is increasingly being employed to monitor and predict the response to anti-cancer therapy. In one of the first clinical studies the safety and feasibility of 99mTc-Annexin V (TAV) for imaging chemotherapy-induced apoptosis in various malignancies immediately after the first course of chemotherapy was evaluated [51]. Fifteen patients presenting with lung cancer, lymphoma or breast cancer underwent 99mTc-Annexin V scintigraphy before and within 3 days after their first course of chemotherapy. Tumor response was evaluated by computed tomography (CT) and positron emission tomography (PET) scans, 3 months after completing treatment. In all cases, no tracer uptake was observed before treatment. However, 24–48 h after the first course of chemotherapy, seven patients who showed 99mTc-Annexin V uptake at tumor sites, suggesting apoptosis, had a complete or partial response. Conversely, six of the eight patients who showed no significant post-treatment tumor uptake exhibited progressive disease. Despite the lack of tracer uptake after treatment, the two patients with breast cancer had a partial response. Overall survival and progression-free survival were significantly related to tracer uptake in treated lung cancers and lymphomas. These data suggest that early 99mTc-Annexin V tumor uptake may be a predictor of response to treatment in patients with late stage lung cancer and lymphoma.
It should be noted, however, that (tumor) cells dying by necrosis also bind Annexin V. This is explained by the fact that PS in the inner leaflet becomes accessible for Annexin V binding as soon as the plasma membrane is ruptured and becomes permeable during necrosis. This limits the specificity of PS targeting by Annexin V ligands and hampers the in vivo discrimination between apoptotic and necrotic cell death. Therefore, Haas et al. [52] focused on a tumor entity known to be extremely radio- and chemosensitive and to respond by extensive induction of apoptosis [53, 54]. In a series of 11 low grade follicular lymphoma patients 99mTc-Annexin V scintigraphy (TAVS) was performed before and 24 h after the last fraction of 2 × 2 Gy involved field radiotherapy. Fine-needle aspiration cytology of the target lymphoma was performed on five consecutive days to verify the apoptotic nature of the response. Baseline tracer uptake was absent in six and weak in five patients. Optimal timing for cytological apoptosis assessment was found to be between 24–48 h after treatment. In all but one patient the post-treatment TAV uptake matched the post-treatment cytology and correlated with the type and onset of the clinical response (two partial and eight complete remissions). These results indicate that the increase in TAV uptake after low dose radiotherapy correlated with the induction of apoptosis and predicted outcome. In a subsequent study of 38 patients with different tumor types (non-Hodgkin lymphoma (NHL) n = 31; non-small cell lung carcinoma (NSCLC) n = 4; head and neck squamous cell carcinomas n = 3) treated with various modalities (radiotherapy n = 26; cisplatin-based chemotherapy n = 4; cisplatin-based concurrent chemoradiotherapy n = 3), TAVS was acquired before and within 48 h after the start of anti-cancer therapy [55, 56]. The changes in TAV tumor uptake (ΔU) were visually and quantitatively evaluated and compared with response to treatment according to Response Evaluation Criteria In Solid Tumors (RECIST) criteria (Fig. 2). A statistically highly significant correlation (p < 0.0001) was found between ΔU and treatment outcome for both visual and quantitative analysis (see updated analysis in Fig. 3). Recently, the prognostic significance of TAVS during chemotherapy in lung cancer was evaluated. In 16 consecutive chemotherapy-naive patients with advanced stage NSCLC scheduled for cisplatin-based chemotherapy, TAVS was performed before and within 48 h after the start of therapy. Chemotherapy-induced changes in tumor Annexin V uptake, calculated as maximum count per pixel and expressed as percentage to baseline value, were compared with treatment response determined according to RECIST. A significant correlation (r 2 = 0.86; p = 0.0001) was found between Annexin V metabolic changes and treatment outcome. All patients with notably increased Annexin V tumor uptake showed complete or partial response. Less prominently increased or decreased uptake correlated with stable or progressive disease.
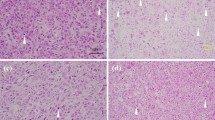
A In vivo 99mTc-annexin V scintigraphy. An example of baseline (a, c, e) and 24 h post-treatment scans (d, f). Diagnostic CT (a, b) of a patient with NHL is shown. This patient demonstrated a complete response after low dose (2 × 2 Gy) radiotherapy on follow-up CT scan (b). TAVS (c, d) and SPECT/CT fusion images (e, f) show precise localization of increased tracer uptake. B In vivo 99mTc-annexin V scintigraphy. An example of baseline (a, c, e) and 72 h post-treatment scans (d, f). Diagnostic CT (a, b) of a patient with HNSCC is shown. This patient demonstrated a complete response after cisplatin-based chemo-radiotherapy on follow-up CT scan (b). TAVS (c, d) and SPECT/CT fusion images (e, f) show precise localization of increased tracer uptake. C In vivo 99mTc-annexin V scintigraphy. An example of a baseline (a, c, e) and 48 h post-treatment scan (d, f). Diagnostic CT (a, b) of a patient with NSCLC is shown. This patient demonstrated a partial response after cisplatin-based chemotherapy on follow-up CT scan (b). TAVS (c, d) and SPECT/CT fusion images (e, f) show precise localization of increased tracer uptake. Figure 2A is reprinted from: Radiotherapy and Oncology 72(3), 333-339 (2004) Kartachova M, Haas RLM, Valdés Olmos RA, Hoebers FJP, van Zandwijk N, Verheij M. In vivo imaging of apoptosis by 99m-Annexin V scintigraphy: visual analysis in relation to treatment response, with permission from Elsevier. Figures 2B and 2C are reprinted from: Current Medical Imaging Reviews 1, 221-228 (2005) Kartachova M, Verheij M, van Eck B, Hoefnagel K, Valdés Olmos R. Methodological aspects and applications of in vivo imaging of apoptosis in oncology: an illustrative review, with permission from Bentham Science Publishers Ltd
Schematic correlation between changes in TAV tumor uptake (ΔU) and clinical response after treatment for NSCLC (n = 15; cisplatin-based chemotherapy), NHL (n = 27; low dose 2 × 2 Gy radiotherapy) or HNSCC (n = 5; cisplatin-based chemoradiotherapy). ΔU was determined according to a semi-quantitative grading scale. Spearman rank test showed a significant correlation. PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response; see also [52, 55, 56]
Collectively, these studies indicate that TAVS might be useful as a predictive test for treatment response early during the course of treatment. It may therefore offer the possibility to identify those patients who will respond to treatment avoiding toxic and ineffective regimens for those patients who will resist therapy. Despite these promising results, the evaluation of TAVS data is complicated by a low tumor-to-background ratio and the limited spatial resolution of the gamma camera. To overcome these limitations, co-registration of functional (single-photon emission computed tomography; SPECT) and anatomical information (CT, MRI) can be applied [57, 58] (Fig. 2). This provides a detailed localization of the areas of increased tracer uptake within anatomical structures, and allows not only to compare tumor uptake on pre- and post-treatment scans on corresponding levels, but also to discriminate areas of pathological tracer accumulation from structures with enhanced physiological uptake, such as the bone marrow and salivary glands.
In addition to Annexin V scintigraphy, other approaches for in vivo imaging of apoptotic cell death are being developed. Perhaps the most promising strategy involves targeting of activated caspases because of the higher degree of specificity as compared to the PS-targeting approach of Annexin V which also detects necrotic cell death [59]. Finally, the use of technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) is worth mentioning because this tracer accumulates within mitochondria depending on the Bcl-2 status. In several studies it has been shown that absent or reduced early 99mTc-MIBI uptake in tumors is associated with a Bcl-2-mediated resistance to chemo- and radiotherapy [60].
1.2.3 Necrosis
In tumor tissue samples necrotic cells are routinely identified by established morphological criteria. Particularly in hypoxic areas this type of cell death is abundantly present. No specific imaging modalities are available for in vivo monitoring of necrosis. As mentioned above, Annexin V scintigraphy does not discriminate between apoptotic and necrotic cell death. Fluorodeoxyglucose-18 PET is increasingly used to monitor response to treatment in tumors [61], but tracer accumulation does not occur in necrotic areas.
1.2.4 Mitotic catastrophe
The formation of multinucleated cells indicative of mitotic catastrophe can de identified in tissue samples by standard techniques. No specific in vitro or in vivo assays are available for this mode of cell death.
1.2.5 Autophagy
The detection of autophagy is limited to in vitro and ex vivo settings and mainly relies on morphological assays to detect autophagic vesicles. Transmission electron microscopy is currently the standard method to monitor autophagy in tissue. In addition, immunohistochemical staining of autophagy-specific biomarkers such as microtubule-associated protein 1 light chain 3 appeared to be a valuable technique to detect autophagosome formation in tissue. Furthermore, demonstration of granular cytoplasmic ubiquitin inclusions by immunohistochemistry may be an attractive technique to measure autophagic cell death in some human diseases such as neurodegenerative disorders, heart failure and atherosclerosis [62]. Other approaches developed to monitor autophagy include flow cytometric analysis of acridine orange uptake and measurement of cytosolic lactate dehydrogenase contents. To date there are no imaging modalities to monitor autophagy in vivo.
1.2.6 Senescence
Senescent cells in culture are identified by their inability to undergo DNA synthesis. Biochemically, senescence is accompanied by the induction of senescence-associated β-galactosidase (SA-beta-gal), which can be detected by histochemical staining of cells using the artificial substrate X-gal. [63]. The presence of the SA-beta-gal biomarker is independent of DNA synthesis and generally distinguishes senescent cells from quiescent cells. The method to detect SA-beta-gal is single cell-based assay, which can identify senescent cells even in heterogeneous tumor cell populations. In vivo, the permanent loss of proliferative capacity may be derived from changes in PET imaging with 18F-FDG or 18F-FLT during treatment.
2 Conclusions
The cellular response to radiation is complex and may lead to different biological outcomes. Understanding the underlying molecular mechanisms of the various types of cell death is essential for the identification of useful biomarkers and the development of imaging modalities to monitor and predict tumor response to radiation. This becomes even more important with the recent introduction of biological response modifiers that target the radiation response at different levels. 99mTc-Annexin V scintigraphy represents an apposite example of a non-invasive technique that allows visualization and quantification of radiation induced cell death in vivo. Clearly, additional and more specific tests are needed for accurate patient selection to ensure optimal treatment and limit side effects.
References
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100, 57–70.
Kroemer, G., El-Deiry, W. S., Golstein, P., Peter, M. E., Vaux, D., Vandenabeele, P., et al. (2005). Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death. Cell Death and Differentiation, 12, 1463–1467.
Hengartner, M. O. (2000). The biochemistry of apoptosis. Nature, 407, 770–776.
Wang, X. (2001). The expanding role of mitochondria in apoptosis. Genes & Development, 15, 2922–2233.
Puthalakath, H., & Strasser, A. (2002). Keeping killers on a tight leash: Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death and Differentiation, 9, 505–512.
Zong, W. X., Lindsten, T., Ross, A. J., MacGregor, G. R., & Thompson, C. B. (2001). BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes & Development, 15, 1481–1486.
Wei, M. C., Lindsten, T., Mootha, V. K., Weiler, S., Gross, A., Ashiya, M., et al. (2000). tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes & Development, 14, 2060–2071.
Desagher, S., Osen-Sand, A., Nichols, A., Eskes, R., Montessuit, S., Lauper, S., et al. (1999). Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. Journal of Cell Biology, 144, 891–901.
Antonsson, B., Montessuit, S., Sanchez, B., & Martinou, J. C. (2001). Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. Journal of Biological Chemistry, 276, 11615–11623.
Cheng, E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T., et al. (2001). BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Molecular Cell, 8, 705–711.
Letai, A., Bassik, M. C., Walensky, L. D., Sorcinelli, M. D., Weiler, S., & Korsmeyer, S. J. (2002). Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell, 2, 183–192.
Datta, R., Kojima, H., Banach, D., Bump, N. J., Talanian, R. V., Alnemri, E. S., et al. (1997). Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. Journal of Biological Chemistry, 272, 1965–1969.
Kataoka, T., Schröter, M., Hahne, M., Schneider, P., Irmler, M., Thome, M., et al. (1998). FLIP prevents apoptosis induced by death receptors but not by perforin/granzyme B, chemotherapeutic drugs, and gamma irradiation. Journal of Immunology, 161, 3936–3942.
Tepper, A. D., de Vries, E., van Blitterswijk, W. J., & Borst, J. (1999). Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. Journal of Clinical Investigation, 103, 971–978.
Sentman, C. L., Shutter, C. L., Hockenberry, D., Kanagaa, O., & Korsmeyer, S. J. (1991). Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell, 67, 879–888.
Strasser, A., Harris, A. W., Jacks, T., & Cory, S. (1994). DNA damage can induce apoptosis in proliferating lymphoid cells via p53-independent mechanisms inhibitable by Bcl-2. Cell, 79, 189–192.
Miyashita, T., Kralewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Liebermann, D. A., et al. (1994). Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene, 9, 1799–1805.
Miyashita, T., & Reed, J. C. (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell, 80, 293–299.
Fei, P., Bernhard, E. J., & El-Deiry, W. S. (2002). Tissue-specific induction of p53 targets in vivo. Cancer Research, 62, 7316–7327.
Mihara, M., Erster, S., Zaika, A., Petrenko, O., Chittenden, T., Pancoska, P., et al. (2003). p53 has a direct apoptogenic role at the mitochondria. Molecular Cell, 11, 577–590.
Friesen, C., Herr, I., Krammer, P. H., & Debatin, K. M. (1996). Involvement of the CD95 (APO-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Medicine, 2, 574–577.
Müller, M., Strand, S., Hug, H., Heinemann, E. M., Walczak, H., Hofmann, W. J., et al. (1997). Drug-induced apoptosis in hepatoma cells is mediated by the CD95 (APO-1/Fas) receptor/ligand system and involves activation of wild-type p53. Journal of Clinical Investigation, 99, 403–413.
Belka, C., Schmid, B., Marini, P., Durand, E., Rudner, J., Faltin, H., et al. (2001). Sensitization of resistant lymphoma cells to irradiation-induced apoptosis by the death ligand TRAIL. Oncogene, 20, 2190–2196.
Zong, W. X., Ditsworth, D., Bauer, D. E., Wang, Z. Q., & Thompson, C. B. (2004). Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes & Development, 18, 1272–1282.
Sun, X., Li, Y., Li, W., Zhang, B., Wang, A. J., Sun, J., et al. (2006). Selective induction of necrotic cell death in cancer cells by beta-lapachone through activation of DNA damage response pathway. Cell Cycle, 5, 2029–2035.
Takai, H., Tominaga, K., Motoyama, N., Minamishima, Y. A., Nagahama, H., Tsukiyama, T., et al. (2000). Aberrant cell cycle checkpoint function and early embryonic death in Chk1(−/−) mice. Genes & Development, 14, 1439–1447.
Ianzini, F., & Mackey, M. A. (1998). Delayed DNA damage associated with mitotic catastrophe following X-irradiation of HeLa S3 cells. Mutagenesis, 13, 337–344.
Blank, M., Lerenthal, Y., Mittelman, L., & Shiloh, Y. (2006). Condensin I recruitment and uneven chromatin condensation precede mitotic cell death in response to DNA damage. Journal of Cell Biology, 74, 195–206.
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B., & Craig, R. (1991). Participation of p53 protein in the cellular response to DNA damage. Cancer Research, 51, 6304–6311.
Wang, C. W., & Klionsky, D. J. (2003). The molecular mechanism of autophagy. Molecular Medicine, 9, 65–76.
Paglin, S., Hollister, T., Delohery, T., Hackett, N., McMahill, M., Sphicas, E., et al. (2001). A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Research, 61, 439–444.
Paglin, S., & Yahalom, J. (2006). Pathways that regulate autophagy and their role in mediating tumor response to treatment. Autophagy, 2, 291–293.
Levine, B., & Klionsky, D. J. (2004). Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Developmental Cell, 6, 463–747.
Degenhardt, K., Mathew, R., Beaudoin, B., Bray, K., Anderson, D., Chen, G., et al. (2006). Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell, 10, 51–64.
Kelekar, A. (2005). Autophagy. Annals of the New York Academy of Sciences, 1066, 259–271.
Campisi, J. (2000). Cancer, aging and cellular senescence. In Vivo, 14, 183–188.
Roninson, I. B. (2003). Tumor cell senescence in cancer treatment. Cancer Research, 63, 2705–2715.
Verheij, M., van Blitterswijk, W. J., & Bartelink, H. (1998). Radiation-induced apoptosis: The ceramide-SAPK signaling pathway and clinical aspects. Acta Oncológica, 37, 575–581.
Chapman, J. D., & Anderson, P. R. (1999). Predicting and overcoming the radioresistance of individual tumors. International Journal of Radiation Oncology, Biology, Physics, 44, 477–479.
Ong, F., Moonen, L. M. F., Gallee, M. P. W., ten Bosch, C., Zerp, S. F., Hart, A. A. M., et al. (2001). Prognostic factors in transitional cell cancer of the bladder: An emerging role for Bcl-2 and p53. Radiotherapy and Oncology, 61, 169–175.
Gerke, V., & Moss, S. E. (2002). Annexins: From structure to function. Physiological Reviews, 82, 331–371.
Andree, H. A., Stuart, M. C., Hermens, W. T., Reutelingsperger, C. P., Hemker, H. C., Frederik, P. M., et al. (1992). Clustering of lipid-bound annexin V may explain its anticoagulant effect. Journal of Biological Chemistry, 267, 17907–17912.
Ahn, N. G., Teller, D. C., Bienkowski, M. J., McMullen, B. A., Lipkin, E. W., & de Haen, C. (1988). Sedimentation equilibrium analysis of five lipocortin-related phospholipase A2 inhibitors from human placenta. Evidence against a mechanistically relevant association between enzyme and inhibitor. Journal of Biological Chemistry, 263, 18657–18663.
van Heerde, W. L., de Groot, P. G., & Reutelingsperger, C. P. (1995). The complexity of the phospholipid binding protein Annexin V. Thrombosis and Haemostasis, 73, 172–179.
Sun, J., Bird, P., & Salem, H. H. (1993). Interaction of annexin V and platelets: Effects on platelet function and protein S binding. Thrombosis Research, 69, 289–296.
Sugimura, M., Donato, R., Kakkar, V. V., & Scully, M. F. (1994). Annexin V as a probe of the contribution of anionic phospholipids to the procoagulant activity of tumour cell surfaces. Blood Coagulation & Fibrinolysis, 5, 365–373.
Blankenberg, F. G., Katsikis, P. D., Tait, J. F., Davis, R. E., Naumovski, L., Ohtsuki, K., et al. (1999). Imaging of apoptosis (programmed cell death) with 99mTc annexin V. Journal of Nuclear Medicine, 40, 184–191.
Hofstra, L., Liem, I. H., Dumont, E. A., Boersma, H. H., van Heerde, W. L., Doevendans, P. A., et al. (2000). Visualisation of cell death in vivo in patients with acute myocardial infarction. Lancet, 356, 209–212.
Blankenberg, F. G., Robbins, R. C., Stoot, J. H., Vriens, P. W., Berry, G. J., Tait, J. F., et al. (2000). Radionuclide imaging of acute lung transplant rejection with annexin V. Chest, 117, 834–840.
Lorberboym, M., Blankenberg, F. G., Sadeh, M., & Lampl, Y. (2006). In vivo imaging of apoptosis in patients with acute stroke: Correlation with blood–brain barrier permeability. Brain Research, 1103, 13–19.
Belhocine, T., Steinmetz, N., Hustinx, R., Bartsch, P., Jerusalem, G., Seidel, L., et al. (2002). Increased uptake of the apoptosis-imaging agent (99m)Tc recombinant human Annexin V in human tumors after one course of chemotherapy as a predictor of tumor response and patient prognosis. Clinical Cancer Research, 8, 2766–2774.
Haas, R., de Jong, D., Valdés Olmos, R. A., Zerp, S. F., van den Heuvel, I., Bartelink, H., et al. (2004). In vivo imaging of radiation-induced apoptosis by 99mTc-annexin-V scintigraphy in follicular lymphoma patients. International Journal of Radiation Oncology, Biology, Physics, 59, 782–787.
Dubray, B., Breton, C., Delic, J., Klijanienko, J., Maciorowski, Z., Vielh, P., et al. (1997). In vitro radiation-induced apoptosis and tumour response to radiotherapy: A prospective study in patients with non-Hodgkin lymphomas treated by low-dose irradiation. International Journal of Radiation Biology, 72, 759–760.
Verheij, M., & Bartelink, H. (2000). Radiation-induced apoptosis. Cell and Tissue Research, 301, 133–142.
Kartachova, M., Haas, R. L. M., Valdés Olmos, R. A., Hoebers, F. J. P., van Zandwijk, N., & Verheij, M. (2004). In vivo imaging of apoptosis by 99m-annexin V scintigraphy: Visual analysis in relation to treatment response. Radiotherapy and Oncology, 72, 333–339.
Kartachova, M., van Zandwijk, N., Burgers, S., van Tinteren, H., Verheij, M., & Valdes Olmos, R. A. (2007). Prognostic significance of 99mTc Hynic-rh-Annexin V scintigraphy during platinum-based chemotherapy in advanced lung cancer. Journal of Clinical Oncology, 25, 2534–2539.
Kartachova, M., Verheij, M., van Eck, B., Hoefnagel, K., & Valdés Olmos, R. (2005). Methodological aspects and applications of in vivo imaging of apoptosis in oncology: An illustrative review. Current Medical Imaging Review, 1, 221–228.
Kartachova, M., Valdes Olmos, R. A., Haas, R. L. M., Hoebers, F. J. P., van den Brekel, M. W., van Zandwijk, N., et al. (2006). Mapping of treatment-induced apoptosis in normal structures: 99mTc hynic-rh-annexin V SPECT and CT image fusion. European Journal of Nuclear Medicine and Molecular Imaging, 33, 893–899.
Faust, A., Wagner, S., Law, M. P., Hermann, S., Schnockel, U., Keul, P., et al. (2007). The nonpeptidyl caspase binding radioligand (S)-1-(4-(2-[18F]fluoroethoxy)-benzyl)-5-[1-(2-methoxymethylpyrrolidinyl)sulfonyl]isatin [18F]CbR) as potential positron emission tomography-compatible apoptosis imaging agent. Quarterly Journal of Nuclear Medicine and Molecular Imaging, 51, 67–73.
Del Vecchio, S., Zannetti, A., Aloj, L., Caraco, C., Ciarmiello, A., & Salvatore, M. (2003). Inhibition of early 99mTc-MIBI uptake by Bcl-2 anti-apoptotic protein overexpression in untreated breast carcinoma. European Journal of Nuclear Medicine and Molecular Imaging, 30, 879–887.
Borst, G. R., Belderbos, J. S., Boellaard, R., Comans, E. F., De Jaeger, K., Lammertsma, A. A., et al. (2005). Standardised FDG uptake: A prognostic factor for inoperable non-small cell lung cancer. European Journal of Cancer, 41, 1533–1541.
Martinet, W., De Meyer, G. R., Andries, L., Herman, A. G., & Kockx, M. M. (2006). Detection of autophagy in tissue by standard immunohistochemistry: Possibilities and limitations. Autophagy, 2, 55–57.
Itahana, K., Campisi, J., & Dimri, G. P. (2007). Methods to detect biomarkers of cellular senescence: The senescence-associated beta-galactosidase assay. Methods in Molecular Biology, 371, 21–31.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verheij, M. Clinical biomarkers and imaging for radiotherapy-induced cell death. Cancer Metastasis Rev 27, 471–480 (2008). https://doi.org/10.1007/s10555-008-9131-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-008-9131-1