Abstract
The aim of this study was to assess the impact of cardiovascular risk factors and plaque inflammation on the progression of atherosclerosis as assessed by positron emission tomography/computed tomography (PET/CT) imaging with 18F-radiolabled fluorodeoxyglucose (18F-FDG). This study was designed as a retrospective cohort study. Patients who received a 18F-FDG PET/CT scan and follow-up scan 9–24 months later without systemic inflammation or steroid medication were eligible for the study. 18F-FDG PET/CT included a full diagnostic contrast enhanced CT scan. Cardiovascular risk factors and medication were documented. Calcified plaque volume, lumen area and 18F-FDG uptake, quantified by the target-to-background ratio (TBR), were measured in the carotid arteries, aorta and iliac arteries. Influence of cardiovascular risk factors and vessel wall inflammation on atherosclerotic disease progression was analyzed. Ninety-four patients underwent baseline and follow-up whole body 18F-FDG PET/CT (mean follow-up time 14.5 ± 3.5 months). Annualized calcified plaque volume increased by 15.4 % (p < 0.0001), carotid and aortic lumen area decreased by 10.5 % (p < 0.0001) and 1.7 % (p = 0.045). There was no significant difference in 18F-FDG uptake at baseline and follow-up (mean TBR 1.44 ± 0.18 vs. 1.42 ± 0.19, p = 0.18). Multiple linear regression analysis identified hypertension as an independent predictor for total, aortic and iliac calcified plaque volume progression (all p < 0.04). Carotid lumen reduction was predicted by hypercholesterolemia (p = 0.008) while aortic lumen reduction was associated with BMI and mean 18F-FDG uptake (p ≤ 0.005). Furthermore we observed a dose response relationship between the number of cardiovascular risk factors and calcified plaque volume progression in the aorta (p = 0.03). Findings from this study provide data on the natural history of atherosclerotic disease burden in multiple vascular beds and emphasize the value of morphological and physiologic information provided by 18F-FDG PET/CT imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atherosclerosis is a systemic vascular disease characterized by intimal plaque formation [1]. Plaque rupture or erosion can lead to thromboembolic events such as myocardial infarction and stroke, which are an important cause of morbidity and mortality in both developing and industrialized countries [2, 3]. Non-invasive imaging of atherosclerosis has become increasingly popular as it has been shown that several markers, such as intima media thickness measurements by ultrasound [4], identification of carotid plaque hemorrhage by magnetic resonance imaging [5] as well as assessment of coronary calcified plaque burden [6], luminal stenosis [7] and increased pericardial fat volume [8] by computed tomography (CT) are associated with cerebro- or cardiovascular events. However most of these imaging modalities are limited as they provide primarily morphological information of one vascular bed and physiological information is not available. Positron emission tomography/computed tomography (PET/CT) imaging with 18F-radiolabled fluorodeoxyglucose (18F-FDG) is able to derive physiological and morphological information of various vascular beds non-invasively and it has previously been shown by our group that 18F-FDG uptake is a strong predictor of future cardio- or cerebrovascular events in cancer patients [9].
Therefore 18F-FDG PET/CT has become increasingly popular to study the physiology of the atherosclerotic disease process [9–12] and several studies are under way which use PET/CT prospectively to identify PET/CT derived markers which might help to identify patients at risk for subsequent events [13]. Interestingly, to date little data exists that describes the natural progression of functional and morphological variables of atherosclerosis as derived by 18F-FDG PET/CT as well as potentially influencing factors.
The aims of this study were (1) to assess the natural history of calcified plaque burden, vascular luminal area and 18F-FDG-uptake and (2) to assess the impact of cardiovascular risk factors and plaque inflammation on atherosclerotic disease progression or regression.
Materials and methods
Patient selection and follow-up
This study was designed as a retrospective cohort study. Institutional review board (IRB) approval was waived given the retrospective study design. Patients of at least 40 years of age who were referred to our institution for a diagnostic, contrast enhanced 18F-FDG PET/CT scan between 2011 and 2012 and a follow-up contrast enhanced 18F-FDG PET/CT scan 9–24 months later were eligible for the study. Patients with prior or ongoing steroid medication, inflammation of unknown origin, sepsis, vasculitis or ongoing radiation were excluded from the study. Cardiovascular risk factors as well as relevant medications and other important clinical information were recorded during the first 18F-FDG PET/CT scan in our institution by chart review. Hypertension was defined as a repeatedly elevated systolic pressure of ≥140 mmHg or a diastolic pressure of ≥90 mmHg. Diabetes was defined as a fasting plasma glucose level of ≥126 mg/dL or a plasma glucose level in a 2 h oral glucose tolerance test of ≥200 mg/dL. Hypercholesterolemia was defined as LDL levels >160 mg/dL or total cholesterol levels above 240 mg/dL. Body mass index was defined as the body mass divided by the square of the height. A positive family history is defined as a parent and/or sibling with a history of treated angina, myocardial infarction, percutaneous coronary catheter interventional procedure, coronary artery bypass grafting, stroke or sudden cardiac death before 55 years in men or 65 years in women. Coronary artery disease was defined as stenosis of more than 50 % in coronary angiography, status post revascularization, myocardial infarction or unstable angina. A cardiac event was defined as myocardial infarction or unstable angina. A cerebrovascular event was defined as ischemic stroke, transient ischemic attack or amaurosis fugax.
Imaging technique
All 18F-FDG PET/CT examinations were performed on a Gemini PET/CT scanner (Philips Healthcare, Hamburg, Germany). The system consists of a germanium oxyorthosilicate fullring PET scanner and a 2-detector-row CT scanner. Patients fasted for at least 6 h before the scan to ensure a serum glucose level below 130 mg/dL. Before the scan 20 mg of furosemide, 20 mg of butylscopolamine bromide and 5 MBq/kg of body weight 18F-FDG (mean dose 240 MBq, range 200–480 MBq) were administered intravenously. Afterwards patients rested for 45 min. Transmission data was acquired 60 min after injection of 18F-FDG by means of a diagnostic contrast enhanced (Ultravist 370, Bayer-Schering AG, Berlin, Germany) venous phase CT-scan extending from the base of the skull to the proximal thighs using the following parameters: tube current 195 mAs, tube potential 120 kV, matrix 512 × 512, slice thickness 6 mm, increment 5 mm/s, rotation time 0.5 s, pitch factor 1.5. The CT-scan was performed during breath hold. After CT examination PET data were acquired in 3-dimensional mode with a 144 × 144 matrix. The emission scan duration per bed position was 2.5 min. Following scatter and decay correction PET data were reconstructed iteratively with and without attenuation correction and axial, coronal and sagittal reformats were produced.
Image analysis
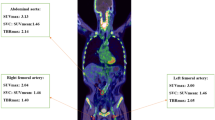
All 18F-FDG PET/CT data were read by a board certified nuclear medicine physician and board certified radiologist with more than 10 years experience in vascular imaging in consensus decision, who were blinded to all clinical patient data and to the time point of the examination. Maximal standardized uptake values (SUV) for 18F-FDG were measured in three cross-sections of each of the following main arterial segments: both common carotid arteries, thoracic aorta, abdominal aorta and both iliac arteries. To measure SUV, circular regions of interest (ROIs) were placed over the carotid and iliac vessels to fully cover the arterial wall. In larger vessels, ROIs with a diameter of 1 cm were slid along the circumference of the arterial wall to locate and measure the maximal SUV of the segment. Measurements were performed over the whole vessel axis. All measurements were performed on axial 18F-FDG PET/CT images as previously described [9, 14].
For blood-pool SUV measurements, three 1-cm-diameter ROIs were placed in the mid lumen of the inferior vena cava, the superior vena cava, and the right atrium. Mean blood-pool activity was calculated from these measurements. Measurements are illustrated in Fig. 1.
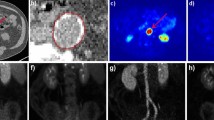
18F-FDG PET/CT images of an inflamed, calcified atherosclerotic lesion. a and b show different calcified lesions in the aortic arch with (arrows) and without increased 18F-FDG-uptake (chevrons). Vessel wall calcifications were identified on the contrast enhanced CT scans by Hounsfield units ranging from 400 to 2000. To measure standard uptake values (SUV), circular regions of interest (ROIs) were placed over the carotid and iliac vessels to fully cover the arterial wall. In larger vessels, ROIs with a diameter of 1 cm were slid along the circumference of the arterial wall to locate and measure the maximal SUV of the segment. c and d show cross-sections of the abdominal aorta and illustrate measurements of SUV in the blood-pool (red circle) and vessel wall (orange circle) that were used for calculations of target to background ratios (TBR) according to the following formula: TBRabdominal−aorta = SUVabdominal−aorta/SUVblood−pool
Target-to-background ratio (TBR) was calculated for each arterial segment as the ratio of maximal SUV in the segment and blood-pool SUV. Mean TBR values were calculated for carotid arteries, thoracic aorta, abdominal aorta, aorta and iliac arteries as well as all segments combined.
Lumen area measurements were performed manually on axial CT images with fixed window settings optimized for vascular CT. Mean aortic lumen area was measured at 20 consecutive locations of the thoracic aorta starting at the level of the tracheal bifurcation. Mean carotid lumen area was measured at 10 consecutive locations starting one slice below the level of the carotid bifurcation. Vessel wall calcifications were identified on the contrast enhanced CT scans by Hounsfield units ranging from 400 to 2000. Measurements were obtained in every vascular segment separately. Agatston scores for the carotid arteries, thoracic aorta, abdominal aorta, aorta and iliac arteries as well as the total Agatston score for all territories were used for further analysis [15]. Pericardial fat represents a composite of epicardial and paracardial fat and was defined as all voxels between the aortic root and the diaphragm with −30 to −200 Hounsfield units (Fig. 2) [14, 16, 17]. Both Agatston Score and pericardial fat volume parameters were assessed using the volume analysis tool of the cardiac workstation (Leonardo, Siemens AG, Healthcare Sector, Erlangen, Germany).
Measurement of pericardial fat. Pericardial fat was defined as a composite of epicardial and paracardial fat and measured as the combined volume of all mediastinal voxels between the aortic root and the diaphragm with −30 to −200 Hounsfield units. a shows a axial slice right above the diaphragm, b the result of the semiautomatic segmentation of pericardial fat (yellow area)
Endpoints
Endpoints for the assessment of atherosclerosis progression included the annualized decrease in carotid and aortic lumen areas, annualized increase in carotid, aortic, iliac and total Agatston score as well as annualized increase in carotid, aortic, iliac and mean TBR values as calculated from the measurements obtained at baseline and follow-up 18F-FDG PET/CT.
Statistical analysis
Categorical variables are presented as absolute and relative frequencies. Continuous variables are presented as mean and standard deviation. All changes (both absolute and percentages) are presented as annualized rates. Normal distribution of the data was assessed by histogram analysis and visual inspection. The one-sample t test was used for the comparison of annual change to zero. For between-group comparison unpaired Student t test was used. For the assessment of metric variables univariate regression analysis was performed. Predictors of atherosclerosis progression were assessed using multiple linear regression analysis with traditional risk factors (smoking, diabetes, hypertension, hypercholesterolemia, BMI and family history of CVD) and 18F-FDG PET/CT data (TBR, calcified wall volume and pericardial fat volume) included in the model. Furthermore patients were stratified and analyzed according to categories with low cardiovascular risk (no traditional risk factors), intermediate risk (1–2 risk factors) and high risk (3–6 risk factors). A p value of <0.05 was chosen to indicate statistical significance. Statistical analysis was performed using SPSS for Windows, Version 19.0 (IBM, Chicago, IL, USA).
Results
Patient population
Baseline characteristics of the patients are reported in Table 1. We identified 163 patients with a contrast enhanced 18F-FDG PET/CT scan and a follow-up scan 9–24 months later between 2011 and 2012. Thirty-one patients received ongoing radiation therapy, in 21 patients the 18F-FDG PET/CT scan was performed due to suspected vasculitis or inflammation of unknown origin and 17 patients received steroid medication. Thus 94 patients with baseline and follow-up contrast-enhanced 18F-FDG PET/CT were included in the analysis. The mean (median) follow-up time was 14.5 ± 3.5 months (14.0 months); range 9.0–24.0 months. The study population consisted mainly of female patients (64.9 %) with a mean age of 62.5 years. The indication for 18F-FDG PET/CT was cancer staging or suspected cancer in all patients. Patient suffered predominantly from colon/rectal cancer (20.2 %), cervical/ovarian cancer (20.2 %) or breast cancer (19.1 %). The study population included few diabetics (8.5 %) and many smokers (44.7 %). The prevalence of hypertension was 30.9 % and a positive family history of cardiovascular disease was present in 43.6 %. Although 36.2 % all patients had known hypercholesterolemia only 8.5 % received statin medication. Few patients had a history of coronary artery disease (9.6 %), previous myocardial infarction (3.2 %) or transient ischemic attacks (3.2. %). At baseline or during follow-up 40.4 % of all patients received chemotherapy.
Baseline 18F-FDG PET/CT data
Overall 564 vessel segments of 94 patients were assessed at baseline. Mean TBR for all vessel segments was 1.44 ± 0.18 (range 1.08–1.88). High TBR values were observed in the abdominal aorta and thoracic aorta [1.61 ± 0.27 (range 1.16–2.76) and 1.58 ± 0.23 (range 1.17–2.35), respectively] while lower TBR values were measured in the carotid and iliac arteries [1.26 ± 0.20 (range 0.73–1.68) and 1.35 ± 0.23 (range 0.95–2.10)]. Average Agatston score was 89.7 ± 276.5 in the carotid arteries, 520.0 ± 1223.2 in the thoracic aorta, 1629.0 ± 2823.1 in the abdominal aorta and 822.9 ± 1316.3 in the iliac arteries. Mean aortic and carotid lumen area were 497.7 ± 99.4 mm2 (range 318.3–787.1 mm2) and 54.0 ± 17.7 mm2 (range 25.7–106.6 mm2), respectively. Average pericardial fat volume was 130.7 ± 65.6 cm3 (range 27.4–373.3 cm3).
Progression of 18F-FDG PET/CT parameters
Average time between baseline and follow-up 18F-FDG PET/CT was 14.6 ± 3.6 months (range 9.0–24.0 months). In the carotid arteries TBR values were significantly lower at follow-up with an annualized difference of −3.9 % (p = 0.006). There were trends towards lower values at follow-up for all vascular territories but these were not significant. When annualized TBR differences were compared among carotid arteries, aorta and iliac arteries no significant difference was observed (all p ≥ 0.069).
We observed a significant increase in annualized total Agatston score of 15.4 % (469.9 ± 931.4; p < 0.0001). Calcifications advanced in all vascular territories (all p ≤ 0.04) but most pronounced in the thoracic and abdominal aorta with an annualized difference of 23.4 and 16.3 % (p < 0.0001) as illustrated in Fig. 3. Over the follow-up period carotid artery and aortic lumen showed a significant reduction of −10.5 and −1.7 % (all p ≤ 0.005), respectively. Pericardial fat increased significantly with an annualized difference of 3.1 % (p = 0.026). An overview of 18F-FDG PET/CT derived parameters at baseline and follow-up is provided in Table 2.
Predictors of disease progression
Univariate analysis
Patients with hypertension had significant more increase in overall calcified wall volume than patients without hypertension (969.27 ± 1482.62 vs. 247.14 ± 368.21; p = 0.0001). This difference was mainly driven by increased calcifications in the thoracic aorta (282.41 ± 650.49 vs. 42.27 ± 106.13; p = 0.005) and iliac arteries (196.19 ± 450.38 vs. −0.39 ± 405.84; p = 0.04). Reduction of carotid lumen area was more pronounced in subjects with hypercholesterolemia (−2.66 ± 8.01 vs. 7.70 ± 8.91; p = 0.008). In unifactorial linear regression analysis BMI significantly predicted aortic lumen reduction (R2 = 0.05; p = 0.03). Other analyzed parameters were not significant and are shown in Online Resource 1 and 2.
Multivariate analysis
In a multivariate linear regression analysis of traditional risk factors (smoking, diabetes, hypertension, hypercholesterolemia, BMI and family history of CVD) and 18F-FDG PET/CT data (TBR, calcified wall volume and pericardial fat volume) hypertension was the only independent predictor of total calcified wall volume progression (R2 = 0.12, p = 0.028). When vascular territories were analyzed separately, hypertension predicted progression of calcifications in the thoracic aorta, aorta and iliac arteries (p ≤ 0.039). In the thoracic aorta a positive family history of CVD contributed to the model. Smoking significantly predicted progression of mean TBR (R2 = 0.08, p = 0.006) but not TBR values in the separate vascular territories. Carotid lumen reduction was predicted by hypercholesterolemia (R2 = 0.08, p = 0.008) while aortic lumen reduction was associated with BMI and mean TBR (R2 = 0.14, p ≤ 0.005). A summary of the different models is shown in Table 3.
Analysis of risk categories
We observed a trend to higher calcified wall volume in patients with more risk factors at both baseline and follow-up in all analyzed vascular territories (Fig. 4). These differences were significant in the abdominal aorta and aorta when low risk and moderate risk patients were compared (all p ≤ 0.01), in the iliac arteries when moderate risk and high risk patients were compared (p ≤ 0.02) and in the abdominal aorta, aorta and iliac arteries when low risk and high rik patients were compared (all p ≤ 0.004). Furthermore, vascular territories also experienced a significant different progression in calcified wall volume between baseline and follow-up depending on risk category (Fig. 5). The annualized difference in calcified wall volume in the abdominal aorta and aorta were significantly higher in the moderate and high risk group when compared to the low risk group (312.80 ± 805.91 and 319.14 ± 563.16 vs. 27.05 ± 72.78; p = 0.008 and 0.03 for abdominal aorta; 450.52 ± 1085.02 and 405.37 ± 627.08 vs. 77.27 ± 173.26; p = 0.01 and p = 0.03 for aorta, respectively). We did not observe a significant difference in the annualized difference of calcified plaque volume in any vascular territory between the moderate and high risk group (p ≥ 0.15, Fig. 5). Furthermore we detected a smaller aortic lumen in the high risk population compared to the low risk population at both baseline (436.37 ± 74.43 vs. 530.10 ± 96.92 mm2; p = 0.007) and follow-up (411.45 ± 84.01 vs. 527.04 ± 106.05 mm2; p = 0.003), annualized change in aortic lumen was not significantly different for both groups (p = 0.06). There was no difference in TBR and carotid lumen among risk categories.
Calcified plaque volumes in different vascular territories at baseline and follow-up stratified by cardiovascular risk category. Patients were stratified as category 1 with low risk for cardiovascular events (no traditional risk factors), category 2 with intermediate risk (1–2 risk factors) or category 3 with high risk (3–6 risk factors). Risk factors included smoking, diabetes, hypertension, hypercholesterolemia, body mass index and family history of cardiovascular disease. There was a trend towards higher calcified plaque volume with increasing number of risk factors. This trend was significant in the abdominal aorta when category 1 was compared to category 2 (a) and category 3 (b). The same was true for the aorta (c and d). In the iliac arteries there was a significant difference when category 3 was compared to category 1 (e) and category 2 (f)
Annualized difference in calcified wall volume in different vascular beds stratified by cardiovascular risk category. Patients were stratified as category 1 with low risk for cardiovascular events (no traditional risk factors), category 2 with intermediate risk (1–2 risk factors) or category 3 with high risk (3–6 risk factors). Risk factors included smoking, diabetes, hypertension, hypercholesterolemia, body mass index and family history of cardiovascular disease. There was a trend towards increased progression of calcified plaque volume in all territories. This trend reached statistical significance in the abdominal aorta when category 1 was compared to categories 2 (a) and 3 (b). The same was true for the aorta (c and d)
Discussion
This study provides data on the natural history and influencing factors of atherosclerosis disease progression in multiple vascular beds as derived by 18F-FDG PET/CT including calcified plaque burden carotid and aortic lumen as well as vessel wall inflammation.
Over a mean follow-up period of 15 months we observed a significant increase in calcified wall volume by 15.4 % and reduction in aortic and carotid lumen area by 1.7 % and 10.5 %, respectively, while there was a trend towards lower TBR values in most vascular territories.
We identified several factors that were associated with accelerated disease progression. More specifically, hypertension and family history of CVD predicted progression of atherosclerotic calcifications whereas smoking predicted progression of vascular inflammation. Carotid lumen reduction was associated with hypercholesterolemia and aortic lumen reduction was predicted by BMI and mean TBR, suggesting that vascular inflammation was associated with an accelerated progression of atherosclerosis in the aorta. Furthermore patients with a higher number of traditional cardiovascular risk factors had a higher calcified plaque burden at baseline and follow-up as well as increased progression of calcified wall volume in the abdominal aorta and aorta.
These results are in line with earlier studies in coronary arteries evaluating the effect of various cardiovascular risk factors on the prevalence, extent and progression on calcified plaque burden. Coronary calcifications were linked to hypertension, hypercholesterolemia, diabetes and smoking [18–21].
However, data on the influence of these risk factors on other vascular beds was limited and restricted to certain patient populations. In patients with a history of ischemic stroke or transient ischemic attack, newly detectable calcifications and annual calcification growth in carotid arteries have been found to be associated with age, hypertension and smoking [22]. Carotid atherosclerosis and disease progression has been associated with a variety of cardiovascular risk factors including hypercholesterolemia in both younger and older cohorts [23–25]. Aortic wall thickening has been used to evaluate the progression of aortic atherosclerosis [26, 27]. However, we were unable to identify reports that study the natural history of aortic calcifications.
Ectopic fat deposits like pericardial fat, abdominal visceral fat, intramuscular or intrahepatic fat release various bioactive substances that influence insulin resistance, glucose and lipid metabolism and inflammation, which all contribute to cardiovascular risk [28]. Pericardial fat releases cytokines like leptin, resistin, tumor necrosis factor-α, interleukin-6, visfatin, and chemerin and has been associated with progression of coronary atherosclerosis as detected by coronary artery calcium scoring [28–30]. We did not observe any effect of the amount of paracardial fat on atherosclerotic disease extent or progression in other vascular territories, which is compatible with a locally limited paracrine and proinflammatory activity of pericardial fat [31].
Interestingly we did not observe an association between vessel wall inflammation and progression of arterial wall calcifications. Atherosclerotic plaque formation is a sequential process with inflammation as the predominant mechanism in early plaque stages. During this inflammation 18F-FDG peaks and erosion of the fibrous cap may lead to thromboembolic events [9, 32]. The inflammatory process also initiates calcium metabolism with formation of early calcium deposits. While this process might be visualized by hydroxyapatite-specific 18F-sodiumfluoride PET imaging, early calcium deposits might not be visualized by CT imaging until their density reaches a certain threshold. Mineralization process may exceed inflammatory activity within atherosclerotic lesions with decreasing 18F-FDG uptake but ongoing increase in calcified wall volume [32, 33]. Given the relative large amount of arterial wall calcification it might be speculated that most atherosclerotic lesions in this patient population were not at the state of maximum inflammation at the time of baseline imaging or that active inflammation is not the driving force of ongoing calcification in later plaque stages. Other markers of atherosclerosis progression like reduction in lumen area might be more suitable to assess the impact on inflammation. This hypothesis is underlined by the observed significant reduction in aortic lumen area which was predicted by baseline TBR. Other promising markers to assess atherosclerotic disease progression/regression would be wall volume and volume of soft plaque. However the imaging protocol in this study was not optimized for vascular imaging and therefore these parameters could not be obtained with satisfying accuracy.
We observed smaller TBR values in smaller vessels. This might in part be explained by partial volume effects. In addition, the arterial wall thickness is substantially smaller in smaller vessels which might also contribute to smaller TBR values. Last but not least the plaque burden might differ across vessel wall territories and might also contribute to the observed differences.
The results of the current and previous studies underline the potential of 18F-FDG PET/CT for the assessment of cardiovascular disease. However, 18F-FDG PET/CT also suffers from various drawbacks including radiation exposure, high direct costs for the examination and high indirect costs associated with the evaluation of potential incidental finding that are derived during 18F-FDG PET/CT due to the whole body approach. While these drawbacks might be acceptable in research studies, they currently hamper the widespread use of 18F-FDG PET/CT in the clinical setting.
Limitations
Our cohort consisted of cancer patients that underwent 18F-FDG PET/CT imaging during routine staging. In this population other effects such as paraneoplastic phenomenas and chemotherapy might also play an important role in atherosclerosis initiation and development and may attenuate the effects of other risk factors. Since cancer type, stage and treatment varied substantially in our patient cohort and the study was not sufficiently powered to detect differences within treatment and disease groups these factors were not included within the model. However, the substantial radiation exposure of repeated full body 18F-FDG PET/CT scans [34] and strict national guidelines for reduction of radiation exposure necessitated a cohort in which 18F-FDG PET/CT is indicated for cancer evaluation.
Precise measurements of cardiovascular risk factors such as blood cholesterol levels and blood pressure were not available which prevented calculation and analysis of the Framingham or comparable risk scores.
The average follow-up period of approximately 15 months was limited which might at least in part explain why vessel wall inflammation did not change significantly during the time course of this study. However, other 18F-FDG PET/CT studies in even smaller cohorts have demonstrated that statin therapy induced changes of FDG-uptake can be observed in less than four months [35], suggesting that any change which might have occurred during the follow-up period of about 15 months was rather small and clinically not significant.
In order to accommodate for differences of the time between baseline and follow-up scan we used annualized parameters to provide estimates independent of the exact observation time. However this approach assumes a linear progression of atherosclerosis over time, which may be problematic given the different stages within the complicated disease process.
Important vascular territories such as the coronary, intracranial or calf arteries were not accessible in this study since our imaging protocol was optimized for staging of cancer patients and not for full body vascular imaging.
Images were acquired approximately 60 min after injection of 18F-FDG, as required in our cancer protocol for detection of tumors, although longer circulation times have been recommended for optimized detection of plaque inflammation [36].
In general the correlation between atherosclerotic disease progression and 18F-FDG PET/CT derived parameters as well as conventional risk factors were weak. This might in part be attributed to the retrospective design of the study and the limitations mentioned above. One might speculate that correlations would have been stronger in a prospective study with an optimized cardiovascular 18F-FDG PET/CT protocol, a hypothesis which needs to be tested in future studies.
Conclusion
In this study we were able to identify several factors, such as hypertension, vessel wall inflammation, hypercholesterolemia and BMI which were associated with accelerated atherosclerotic disease progression in distinct vascular beds. In addition we were able to provide data on the natural history of atherosclerosis disease progression outside the coronary tree as assessed by 18F-FDG PET/CT. Further 18F-FDG PET/CT studies in non-oncological patients are necessary to better elucidate the role of risk factors on plaque progression and more importantly to identify factors that are associated with the occurrence of cardio- or cerebrovascular events.
References
Stary HC, Chandler AB, Dinsmore RE et al (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92:1355–1374
Naghavi M, Libby P, Falk E et al (2003) From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 108:1664–1672
Rosamond W, Flegal K, Furie K et al (2008) Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117:e25–146
den Ruijter HM, Peters SA, Groenewegen KA et al (2013) Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative. Diabetologia 56:1494–1502
Saam T, Hetterich H, Hoffmann V et al (2013) Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 62:1081–1091
Bamberg F, Sommer WH, Hoffmann V et al (2011) Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol 57:2426–2436
Chow BJ, Wells GA, Chen L et al (2010) Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J Am Coll Cardiol 55:1017–1028
Otaki Y, Rajani R, Cheng VY et al (2011) The relationship between epicardial fat volume and incident coronary artery calcium. J Cardiovasc Comput Tomogr 5:310–316
Rominger A, Saam T, Wolpers S et al (2009) 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J Nucl Med 50:1611–1620
Rudd JH, Warburton EA, Fryer TD et al (2002) Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 105:2708–2711
Tawakol A, Migrino RQ, Bashian GG et al (2006) In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 48:1818–1824
Strobl FF, Rominger A, Wolpers S et al (2013) Impact of cardiovascular risk factors on vessel wall inflammation and calcified plaque burden differs across vascular beds: a PET-CT study. Int J Cardiovasc Imaging 29:1899–1908
Falk E, Sillesen H, Muntendam P, Fuster V (2011) The high-risk plaque initiative: primary prevention of atherothrombotic events in the asymptomatic population. Curr Atheroscler Rep 13:359–366
Saam T, Rominger A, Wolpers S et al (2010) Association of inflammation of the left anterior descending coronary artery with cardiovascular risk factors, plaque burden and pericardial fat volume: a PET/CT study. Eur J Nucl Med Mol Imaging 37:1203–1212
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R (1990) Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15:827–832
Greif M, Leber AW, Saam T et al (2012) Determination of pericardial adipose tissue increases the prognostic accuracy of coronary artery calcification for future cardiovascular events. Cardiology 121:220–227
Greif M, Becker A, von Ziegler F et al (2009) Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 29:781–786
Kronmal RA, McClelland RL, Detrano R et al (2007) Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 115:2722–2730
Cassidy-Bushrow AE, Bielak LF, Sheedy PF II et al (2007) Coronary artery calcification progression is heritable. Circulation 116:25–31
Tomizawa N, Nojo T, Inoh S, Nakamura S (2015) Difference of coronary artery disease severity, extent and plaque characteristics between patients with hypertension, diabetes mellitus or dyslipidemia. Int J Cardiovasc Imaging 31:205–212
Hadamitzky M, Meyer T, Hein F et al (2012) Prognostic value of coronary computed tomographic angiography in patients with arterial hypertension. Int J Cardiovasc Imaging 28:641–650
van Gils MJ, Bodde MC, Cremers LG, Dippel DW, van der Lugt A (2013) Determinants of calcification growth in atherosclerotic carotid arteries; a serial multi-detector CT angiography study. Atherosclerosis 227:95–99
Rozie S, de Weert TT, de Monye C et al (2009) Atherosclerotic plaque volume and composition in symptomatic carotid arteries assessed with multidetector CT angiography; relationship with severity of stenosis and cardiovascular risk factors. Eur Radiol 19:2294–2301
Juonala M, Magnussen CG, Venn A et al (2010) Influence of age on associations between childhood risk factors and carotid intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study, the Childhood Determinants of Adult Health Study, the Bogalusa Heart Study, and the Muscatine Study for the International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 122:2514–2520
Chambless LE, Folsom AR, Davis V et al (2002) Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol 155:38–47
Arai Y, Hirose N, Yamamura K et al (2002) Long-term effect of lipid-lowering therapy on atherosclerosis of abdominal aorta in patients with hypercholesterolemia: noninvasive evaluation by a new image analysis program. Angiology 53:57–68
Takasu J, Masuda Y, Watanabe S et al (1994) Progression and regression of atherosclerotic findings in the descending thoracic aorta detected by enhanced computed tomography. Atherosclerosis 110:175–184
Lim S, Meigs JB (2014) Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol 34:1820–1826
Yerramasu A, Dey D, Venuraju S et al (2012) Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 220:223–230
Nakanishi R, Rajani R, Cheng VY et al (2011) Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis 218:363–368
Sacks HS, Fain JN (2007) Human epicardial adipose tissue: a review. Am Heart J 153:907–917
Cocker MS, Mc Ardle B, Spence JD et al (2012) Imaging atherosclerosis with hybrid [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol 19:1211–1225
Koenig W, Khuseyinova N (2007) Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 27:15–26
Brix G, Lechel U, Glatting G et al (2005) Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J Nucl Med 46:608–613
Tahara N, Kai H, Ishibashi M et al (2006) Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol 48:1825–1831
Rudd JH, Myers KS, Bansilal S et al (2008) Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med 49:871–878
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Holger Hetterich and Axel Rominger have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hetterich, H., Rominger, A., Walter, L. et al. Natural history of atherosclerotic disease progression as assessed by 18F-FDG PET/CT. Int J Cardiovasc Imaging 32, 49–59 (2016). https://doi.org/10.1007/s10554-015-0660-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0660-8