Abstract
Objectives
The objective of this study was to evaluate whether baseline 18F-fluorodeoxyglucose (FDG) uptake is associated with carotid plaque progression.
Methods
A total of 156 subjects with carotid plaque were enrolled and underwent carotid magnetic resonance imaging (MRI) (at baseline and the 12-month follow-up) and positron emission tomography-computed tomography (PET-CT) (baseline). Carotid plaque progression was evaluated by two indices (the incidence of plaque progression and percentage of plaque increase) with three-dimensional (3D) imaging, while the 18F-FDG uptake was evaluated by the 18F-FDG uptake levels and 18F-FDG uptake velocity. The association between plaque progression and 18F-FDG uptake was investigated by the trend test and multivariate logistic regression analysis.
Results
Of the 156 subjects, 80 (51.3%) showed carotid plaque progression during the 12-month follow-up. Firstly, no association was found between 18F-FDG uptake levels and plaque progression. Secondly, significant differences in the incidence of plaque progression were observed among the groups with different uptake velocities, showing a significant decreasing trend ranging from high to intermediate to low (p = 0.002, trend test). After adjusting for covariates, an adequate prediction of the 18F-FDG uptake velocity for the incidence of plaque progression was revealed (OR = 0.682, p < 0.05). In addition, no association was found between the 18F-FDG uptake velocity and the percentage of plaque increase in the subjects with plaque progression (p = 0.757, trend test).
Conclusions
Our findings suggest 18F-FDG uptake velocity is independently associated with the incidence of carotid plaque progression. Additionally, the 18F-FDG uptake velocity, as another important parameter of PET-CT, warrants further study in future clinical research.
Key Points
• The18F-FDG uptake levels were not associated with the carotid plaque progression.
• The18F-FDG uptake velocity could predict the incidence of carotid plaque progression.
• The18F-FDG uptake velocity with related factors warrants more attention in future clinical research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the leading causes of mortality and morbidity worldwide, and carotid atherosclerotic plaque is a primary etiological factor associated with it [1]. Studies on the intrinsic mechanisms of atherosclerosis have confirmed that plaque formation is a chronic inflammatory process and that inflammation may play a key role in plaque progression [2].
Making use of the advantages of positron emission tomography-computed tomography (PET-CT), i.e., the combination of metabolic and structural imaging, a few studies have investigated the occurrence and development of atherosclerotic plaques by detecting the uptake of fluorodeoxyglucose (FDG) [3], a glucose analog that is retained within cells at a rate proportional to that of glucose-dependent metabolic reactions, which are markedly increased in immune cells compared with vascular cells. These studies revealed a significant increase in FDG uptake levels in atherosclerotic plaques [4], characteristic FDG uptake levels in unstable plaques [5], differences in FDG uptake levels in various plaque components [5], and changes in FDG uptake levels in response to therapeutic interventions [6]. The above evidence suggests that FDG uptake is closely correlated with plaque-related factors, including inflammation and macrophage infiltration in the vascular wall, risk factors for atherosclerosis, and plaque burden [7].
Prediction and timely intervention in cases of carotid atherosclerotic plaque progression are the most effective strategies for the prevention and treatment of acute cardiovascular and cerebrovascular events. The predictive value of plaque-related factors (intraplaque hemorrhage, calcification, hypertension, lipid composition, and serological index) in plaque progression has been unanimously confirmed in recent years [8, 9]. However, whether FDG uptake is as powerful as plaque-related factors in predicting plaque progression has not been determined. Although a precedent study predicted carotid plaque progression for 24 months using FDG uptake, the results were based on the differences in FDG uptake levels obtained from two PET-CT examinations performed at baseline and at the follow-up 6 months later [10]. To further explore the direct relationship between FDG uptake and carotid plaque progression and to avoid two PET-CT scans, we hypothesized that FDG uptake at baseline is associated with carotid plaque status and is also a reliable predictor of carotid plaque progression.
In this longitudinal follow-up study, we observed the progression of carotid plaques using 3D imaging (at baseline and the 12-month follow-up) and detected the 18F-FDG uptake of plaque (baseline) and explored the association between plaque progression (the incidence of plaque progression and the percentage of plaque increase) and 18F-FDG uptake (18F-FDG uptake level and 18F-FDG uptake velocity).
Methods
Study subjects
Subjects with 10% to 40% carotid stenosis on at least one side were recruited into this study at the Affiliated Hospital of Jiangsu University. Carotid stenosis was determined with duplex sonography according to the Strandness criteria [11]. The side with greater stenosis was defined as the index artery. Additional criteria had to be met: all subjects enrolled had refused statin therapy (serious side effects of statins) but volunteered for a standardized diet and exercise program; subjects were asymptomatic for 6 months before recruitment; subjects voluntarily accepted the follow-up plan of this study, including carotid MRI (at baseline and follow-up in 12 months), PET-CT data (baseline), and monthly surveys (lifestyle, diet, demographic information, and biomarkers). In addition, subjects with any of the following conditions were excluded: (1) carotid endarterectomy (CEA) before or during the current study on the index carotid artery; (2) previous sessions of radiation therapy; (3) contraindications for MRI; (4) significant changes in lifestyle or diet during follow-up; (5) failure to maintain blood glucose below 9.0 mmol/L before PET-CT examination; and (6) failure to follow diet and exercise plans (follow-up every 3 months). Of the 194 subjects available, 7 subjects were excluded because of marginal image quality, insufficient plaque size (3 subjects), absence of the common carotid bifurcation in the field of view (5 subjects), significant changes in lifestyle or diet (17 subjects), or failure to follow-up (6 subjects). Therefore, carotid MRI (baseline and follow-up 12 months) and PET-CT (baseline) data for 156 subjects were obtained (see details in Online Supplemental Material). The study protocol gained the approval of the institutional ethics committee, and informed consent was obtained from all participants. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The general information for these 156 subjects is shown in Table 1.
Acquisition and analysis of PET-CT images
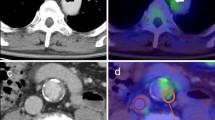
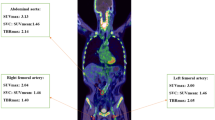
Imaging protocols for 18FDG-PET-CT have been described in detail in previous publications [12]. 18FDG-PET-CT scans were performed using a Siemens Biograph mCT scanner. Subjects fasted for at least 6 h prior to infusion of 200 MBq of 18F-FDG (5.5 mCi). Before PET scanning, a low-dose CT was performed for the attenuation of correction, anatomic coregistration, and fusion of PET-CT images (multiple times). The maximum standard uptake values (SUV) of 18F-FDG were measured in an axial orientation along each carotid artery from 1 cm up to 3 cm below the bifurcation, which was performed by manually drawing the circular regions of interest that encompassed the vessel wall. The target-to-background ratio (TBR) was calculated from the ratio of the maximal arterial standardized uptake values and mean venous background activity within the superior vena cava (correction for aorta) and the jugular vein (correction for carotids). After the 18F-FDG infusion, the PET images of the subjects were collected every 10 min until the 18F-FDG uptake levels entered the plateau stage [13] (Fig. 1).
Acquisition of MRI and analysis on 3D images
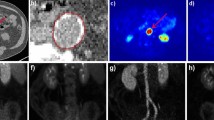
MR examinations were scheduled for more than 24 h after PET-CT scan. The images were acquired using a Siemens Magnetom Trio 3-T scanner with an 8-channel head coil. A standardized carotid artery MRI protocol was adopted to acquire high-resolution, cross-sectional images. All images were obtained with a field-of-view = 16 × 16 cm; TR = 2150 ms; TE = 12.5 ms; echo train length = 10; matrix size = 280 × 280; flip angle = 90°; number of excitations = 2; and section thickness of 0.8 mm with no section gap (spatial resolution before zero-fill interpolation, 0.625 × 0.625 × 0.8 mm). Imaging coverage was 16 mm. To minimize motion and increase intersubject reproducibility during positioning, a thin pillow was placed at the base of the coil surrounding the sides and back of the head. The total acquisition time was approximately 12 min and 20 s. No scanner hardware or software changes were made during the study period (baseline and follow-up). The 2D DICOM images were imported into Mimics software (Materialise Corporation). After setting the spatial position coordinates, the signal threshold curve was delineated from the maximum to the minimum signal (air). Then, the corresponding masks were assigned to the blood vessel wall. Based on the reconstruction of these masks, volume and 3D images of the carotid artery were obtained. Additionally, a comparison of the baseline and follow-up images was made using 3-matic software (Materialise Corporation) [14]. The wall thickness analysis tool was used to show any differences between groups (Fig. 2).
Clinical examination of related serum indicators
(See details in Online Supplemental Material)
Statistical analysis
Data were presented as the mean ± SD for continuous variables and as n (%) for categorical variables. The intragroup correlation coefficient (ICC) was used to compare the 2D and 3D imaging of the carotid atherosclerotic plaques, and the images were analyzed by three independent trained observers blinded to any subject characteristics (Zhang, Xu, and Zhu). The status of the 18F-FDG uptake was classified as “progression” or “no progression” by binary taxonomy of the unordered categorical variables. A trend test was used to reveal the surface relationships between plaque progression and 18F-FDG uptake velocity. One-way ANOVA, chi-square test, and paired t test were used to screen for multiple factors in plaque progression at baseline and follow-up at 12 months. Before the logistic regression analysis, the collinearity of the continuous variables was verified using multiple linear regression (variance inflation factor (VIF)). Then, the relationship between the 18F-FDG uptake velocity and plaque progression was verified using multivariate logistic regression. In addition, one-way ANOVA was used to compare intergroup differences, and Pearson’s analysis was also used in this study. All statistical analyses were conducted using commercially available SPSS 20.0 software (SPSS Inc.) and R 2.11.0 software (R Foundation for Statistical Computing). P < 0.05 was considered to indicate statistical significance. GraphPad Prism 5 (GraphPad Software, Inc.) was utilized to create statistical graphs.
Results
Plaque progression detected by 3D imaging
Repeated measurements showed that the ICC of 3D imaging (ICC, 0.921) was significantly higher than that of the four other methods (see details in Online Supplementary Material). In addition, regardless of the boundaries between the plaque and vascular intima, the absolute value of plaque progression could be accurately determined by the differences between the two sets of 3D imaging data (values obtained at follow-up minus the values at baseline). In this follow-up study involving 156 participants, no plaque rupture was found. Plaque progression was found in 80 patients (51.3%) from baseline to the 12-month follow-up, while the rest of the participants (48.7%) showed no progress. In participants exhibiting plaque progression, the plaque volume at the 12-month follow-up was approximately 15.7% larger than that at baseline (Fig. 3).
Characteristics of 18F-FDG uptake in atherosclerotic plaques
Significant 18F-FDG uptake was shown in atherosclerotic plaques, as evidenced by the local concentration of 18F-FDG in the vascular wall of the diseased segments. These plaques showed different 18F-FDG uptake levels, with the highest uptake levels reaching 3.0, while the lowest levels reached only 1.4. In addition, significant differences in the 18F-FDG uptake velocity were also found, with 24 subjects reaching peak values in only 15 min (group A), 32 subjects in 25 min (group B), 28 subjects in 35 min (group C), 48 subjects in 45 min (group D), and, in the slowest group, 24 subjects in 55 min (group E). Furthermore, there was no correlation between the 18F-FDG uptake levels and 18F-FDG uptake velocity in any subjects (r = 0.103, p = 0.637).
Association between plaque progression and the characteristics of 18F-FDG uptake
Binary taxonomy of the unordered categorical variables (18F-FDG uptake levels) using the statuses “progression” and “no progression” was performed. The results showed that each 18F-FDG uptake level presented a random distribution in either “progression” or “no progression,” and there was no significant difference in 18F-FDG uptake levels between them (Fig. 4a). In contrast, a clear relationship was found between the 18F-FDG uptake velocity and plaque progression, and there were significant differences in the incidence of plaque progression among the five groups (22/24 vs. 26/32 vs. 20/28 vs. 8/48 vs. 4/24), which showed a significant decreasing trend ranging from high to intermediate to low (p = 0.002, trend test) (Fig. 4b). However, there was no pronounced trend in the percentage of plaque increase (p = 0.757, trend test) (Fig. 4c). These results suggested that the velocity but not the levels of 18F-FDG uptake might be related to the incidence of plaque progression but not to the percentage of plaque increase.
Relationships between the 18F-FDG uptake velocity and plaque progression. a There was no relationship between the 18F-FDG uptake levels and “progression”. b The trend test showed decreasing incidence of plaque progression ranging from most to least in groups with different uptake velocities. c There was no intergroup difference in the percentage of plaque increase in the subjects with plaque progression
Predicting the incidence of plaque progression with the 18F-FDG uptake velocity
To further investigate the relationship between the incidence of plaque progression and the 18F-FDG uptake velocity, possible factors associated with plaque progression were included. First, demographic information, such as age, sex, and blood pressure, were collected. Second, as plaque progression is a chronic inflammatory process, biological markers of inflammation were also included. Third, characteristics of the plaque that were proven to be closely related to plaque progression were considered. Fourth, to ensure a comprehensive screening, certain related factors reported in previous studies were also included in this study. Finally, of the twenty-seven factors, twelve were selected by screening: LDL, cholesterol, IL-6, IL-1β, TNF-α, intraplaque hemorrhage, calcification, lipid-rich necrotic core, PLA2, sVCAM, PAI-1, and sE-selectin (Table 2).
The collinearity of the continuous variables was excluded by multivariate linear regression (all variance inflation factor (VIF) values were < 5.0). Then, logistic regression analyses, in which the incidence of plaque progression was predicted by the 18F-FDG uptake velocity, were conducted with adjustments for those twelve indicators. An adequate prediction of the 18F-FDG uptake velocity (OR = 0.682, p < 0.05) was revealed, as well as the other five covariates (Table 3). Furthermore, receiver operating curve (ROC) analyses revealed the capacity of 18F-FDG uptake velocity at baseline in predicting the onset of plaque progression (p < 0.001; area under the ROC [AUC], 0.845 (95% CI [0.788; 0.901])). According to the result of the best Youden Index, the corresponding cut-off value in this sample was 40 min with 79.8% sensitivity and 85.0% specificity (Fig. 5).
In addition, the associations between the 18F-FDG uptake velocity and these meaningful factors at baseline were also analyzed. The results showed that three of these five factors (intraplaque hemorrhage, calcification, and IL-1β) were closely related to the 18F-FDG uptake velocity, which suggested the possible underlying causes of the incidence of plaque progression predicted by the 18F-FDG uptake velocity (Fig. 6).
Relationships between the 18F-FDG uptake velocity and the risk factors. a Significant differences in uptake velocity were found in intraplaque hemorrhage (+) vs. intraplaque hemorrhage (−). b Significant differences in uptake velocity were found in calcification (+) vs. calcification (−). c No correlation was found in the uptake velocity and the levels of low density lipoprotein. d Significant correlations were found in the uptake velocity and the levels of IL-1β. e No correlation was found in the uptake velocity and the levels of sVCAM. *p < 0.05
Discussion
To the best of our knowledge, the present study is the first to predict plaque progression in patients with carotid atherosclerosis by detecting 18F-FDG uptake at baseline, thereby strongly complementing plaque-related research. We detected 18F-FDG metabolism in the carotid plaques at baseline using PET-CT and followed up plaque progression for 12 months with MRI examinations. Our results confirmed that (1) the incidence of plaque progression was associated with rapid 18F-FDG uptake velocity at baseline, (2) the 18F-FDG uptake velocity may be a comprehensive manifestation of plaque status, which can be a reliable predictor of the incidence of plaque progression, and (3) the 18F-FDG uptake velocity, as an another important parameter of PET-CT, warrants more attention in future clinical research.
The 18F-FDG uptake velocity is a new index that attracts increasing attention from researchers who have been concerned with 18F-FDG uptake levels. Unlike the 18F-FDG uptake levels, which represent the ultimate level of metabolic uptake, the 18F-FDG uptake velocity, another important indicator of FDG uptake that reveals dynamic glucose metabolism in local tissues, reflects the metabolic characteristics of the lesion or tissue [13]. In previous studies, American scholars evaluated 18F-FDG uptake velocity in rats and chickens by multiple successive injections of 18F-FDG at different time points, revealing brain regions that become active during behavior [15]. In contrast to this detection method, the measurement method adopted in vitro in this paper might be more convenient and did not require multiple injections. Notably, some factors that might affect the 18F-FDG uptake velocity (e.g., age, blood flow, and blood pressure) were not fully corrected for in this study. Although the time for the 18F-FDG in the blood to reach the lesion or tissue site may be affected by the age, blood flow, and blood pressure of the individual, which may have also affected the 18F-FDG uptake velocity, the resulting differences may be only a few seconds or tens of seconds and may be ignored in light of the observation intervals of 10 min.
Modern imaging techniques, including MRI (T1, T2, T2*, proton density imaging (PDI), susceptibility-weighted imaging (SWI), and even magnetic resonance spectroscopy (MRS)) [16,17,18], ultrasound (US) [19], CT angiography [20], optical coherence tomography (OCT) [21], and PET-CT [22], as powerful image postprocessing techniques, have allowed the identification of atherosclerotic plaques. However, the detection and imaging of the most significant and intuitive manifestation of plaque progression, bulging of the lumen, and increased plaque size still rely on the indirect 2D measurements (the average wall thickness and average wall area) [23, 24]. Although the 3D imaging method based on voxel automatic segmentation used in the present study had shortcomings similar in nature to those of previous 2D protocols because the atherosclerotic plaque itself possesses an irregular three-dimensional structure, such as the inability to distinguish between the boundaries of the plaque and the vessel wall, this methodology still presented specific advantages, as confirmed by the results of this study (the ICC determined by 3D imaging was significantly higher than that determined by the 2D measurements). In fact, the application of 3D imaging to study atherosclerotic plaques may allow the acquisition of more valuable data, such as the effects of 3D plaque morphology on hemodynamics and the effects of 3D morphology on plaque stability, which merit further exploration.
The first finding in this study was that there was no relationship between 18F-FDG uptake levels and carotid plaque progression. Uptake levels merely represented the maximum uptaking capacity in plaque, so essentially, it was a cross-sectional indicator of instantaneity. This might also be the reason why plaque progression was predicted with 18F-FDG uptake levels by two PET-CT scans in a previous report [10]. The most important finding in this study was the confirmed relationship between the 18F-FDG uptake velocity and plaque progression, which was actually affected by various related factors. Our results suggested that 18F-FDG uptake velocity might be a comprehensive manifestation of plaque status in the period of evolution to platform stage. In addition to these significant risk factors, the intrinsic relationship between the 18F-FDG uptake velocity and plaque progression still requires further investigation, especially in patients who do not carry those high-risk factors but still present significant plaque progression. Importantly, the results of this study suggested that there is no relationship between the percentage of plaque increase and the 18F-FDG uptake velocity; however, this finding might be observed because the 18F-FDG uptake velocity in this study was not a continuous variable (our observation time was set at 10-min intervals). This association might be explored in future studies by the dynamic detection of direct indices in the peripheral blood of experimental animals. In addition, the application of various specific contrast agents of PET-CT will further clarify the pathological mechanism of atherosclerotic plaque [25].
We acknowledge the presence of several limitations in our study: (i) Larger-scale studies are needed to replicate these observations and demonstrate their generalizability to broader patient populations. (ii) Although covariates at baseline were adjusted for in the logistic regression, there might still be some undetected covariates that may further affect plaque progression during the follow-up period of 12 months. (iii) Some technical factors (e.g., tracer circulation time, image acquisition and postprocessing scheme) may be sources of bias. (iv) Although we attempted to control for selection bias with strict inclusion/exclusion and multivariate analysis, our results might be subject to inevitable bias of a single-center study and the possibility of missing confounding factors. Despite these limitations, the current study is important, as it directly investigated for the first time the possibility of using the 18F-FDG uptake velocity in atherosclerotic plaques as an independent predictor of plaque progression.
Abbreviations
- FDG:
-
Fluorodeoxyglucose
- ICC:
-
Intragroup correlation coefficient
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- PET-CT:
-
Positron emission tomography-computed tomography
- ROC:
-
Receiver operating curve
- SUV:
-
Maximum standard uptake value
- TBR:
-
Target-to-background ratio
- VIF:
-
Variance inflation factor
References
Meschia JF, Bushnell C, Boden-Albala B et al (2014) Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45:3754–3832
Huet P, Burg S, Le Guludec D, Hyafil F, Buvat I (2015) Variability and uncertainty of 18F-FDG PET imaging protocols for assessing inflammation in atherosclerosis: suggestions for improvement. J Nucl Med 56:552–559
Dweck MR, Aikawa E, Newby DE et al (2016) Noninvasive molecular imaging of disease activity in atherosclerosis. Circ Res 119:330–340
van der Valk FM, Kroon J, Potters WV et al (2014) In vivo imaging of enhanced leukocyte accumulation in atherosclerotic lesions in humans. J Am Coll Cardiol 64:1019–1029
Hyafil F, Schindler A, Sepp D et al (2016) High-risk plaque features can be detected in non-stenotic carotid plaques of patients with ischaemic stroke classified as cryptogenic using combined (18)F-FDG PET/MR imaging. Eur J Nucl Med Mol Imaging 43:270–279
Rudd JH, Narula J, Strauss HW et al (2010) Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol 55:2527–2535
Hammad B, Evans NR, Rudd JHF, Tawakol A (2017) Molecular imaging of atherosclerosis with integrated PET imaging. J Nucl Cardiol 24:938–943
Sun J, Zhao XQ, Balu N et al (2017) Carotid plaque lipid content and fibrous Cap status predict systemic CV outcomes: the MRI substudy in AIM-HIGH. JACC Cardiovasc Imaging 10:241–249
McNally JS, McLaughlin MS, Hinckley PJ et al (2015) Intraluminal thrombus, intraplaque hemorrhage, plaque thickness, and current smoking optimally predict carotid stroke. Stroke 46:84–90
Joseph P, Ishai A, Mani V et al (2017) Short-term changes in arterial inflammation predict long-term changes in atherosclerosis progression. Eur J Nucl Med Mol Imaging 44:141–150
Xu D, Hippe DS, Underhill HR et al (2014) Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging 7:366–373
Mehta NN, Torigian DA, Gelfand JM, Saboury B, Alavi A (2012) Quantification of atherosclerotic plaque activity and vascular inflammation using [18-F] fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). J Vis Exp. https://doi.org/10.3791/3777:e3777
Harris RS, Venegas JG, Wongviriyawong C et al (2011) 18F-FDG uptake rate is a biomarker of eosinophilic inflammation and airway response in asthma. J Nucl Med 52:1713–1720
Bosmans B, Famaey N, Verhoelst E, Bosmans J, Vander Sloten J (2016) A validated methodology for patient specific computational modeling of self-expandable transcatheter aortic valve implantation. J Biomech 49:2824–2830
Gold MEL, Norell MA, Budassi M, Vaska P, Schulz D (2018) Rapid (18)F-FDG uptake in brain of awake, behaving rat and anesthetized chicken has implications for behavioral PET studies in species with high metabolisms. Front Behav Neurosci 12:115
Tarkin JM, Dweck MR, Evans NR et al (2016) Imaging atherosclerosis. Circ Res 118:750–769
Zhang R, Zhou Y, Liu C et al (2017) Overestimation of susceptibility vessel sign: a predictive marker of stroke cause. Stroke 48:1993–1996
Duivenvoorden R, van Wijk D, Klimas M, Kastelein JJ, Stroes ES, Nederveen AJ (2013) Detection of liquid phase cholesteryl ester in carotid atherosclerosis by 1H-MR spectroscopy in humans. JACC Cardiovasc Imaging 6:1277–1284
Steinl DC, Kaufmann BA (2015) Ultrasound imaging for risk assessment in atherosclerosis. Int J Mol Sci 16:9749–9769
Wilson SR, Lin FY, Min JK (2011) Role of coronary artery calcium score and coronary CT angiography in the diagnosis and risk stratification of individuals with suspected coronary artery disease. Curr Cardiol Rep 13:271–279
Bouma BE, Villiger M, Otsuka K, Oh WY (2017) Intravascular optical coherence tomography [Invited]. Biomed Opt Express 8:2660–2686
Ammirati E, Moroni F, Pedrotti P et al (2014) Non-invasive imaging of vascular inflammation. Front Immunol 5:399
Luijendijk P, Lu H, Heynneman FB et al (2014) Increased carotid intima-media thickness predicts cardiovascular events in aortic coarctation. Int J Cardiol 176:776–781
Zavodni AE, Wasserman BA, McClelland RL et al (2014) Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 271:381–389
Derlin T, Richter U, Bannas P et al (2010) Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 51:862–865
Funding
This work was supported by National Natural Science Foundation of China (81871343, 81525014), Jiangsu Provincial Key Research and Development Plan (BE2017699, BE2017698, BE2018693), and Natural Science Foundation of Jiangsu Province (BK20181226, BK20171311).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jinchuan Yan.
Conflict of interest
The authors have no potential conflicts of interest relevant to this article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the institutional review board.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 726 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Liang, Y., Yang, P. et al. 18F-FDG uptake velocity but not uptake level is associated with progression of carotid plaque. Eur Radiol 30, 2403–2411 (2020). https://doi.org/10.1007/s00330-019-06535-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06535-8