Abstract
Speckle-tracking left ventricular global longitudinal strain (GLS) assessment may provide substantial prognostic information for hypertrophic cardiomyopathy (HCM) patients. Reference values for GLS have been recently published. We aimed to evaluate the prognostic value of standardized reference values for GLS in HCM patients. An analysis of HCM clinic patients who underwent GLS was performed. GLS was defined as normal (more negative or equal to −16 %) and abnormal (less negative than −16 %) based on recently published reference values. Patients were followed for a composite of events including heart failure hospitalization, sustained ventricular arrhythmia, and all-cause death. The power of GLS to predict outcomes was assessed relative to traditional clinical and echocardiographic variables present in HCM. 79 HCM patients were followed for a median of 22 months (interquartile range 9–30 months) after imaging. During follow-up, 15 patients (19 %) met the primary outcome. Abnormal GLS was the only echocardiographic variable independently predictive of the primary outcome [multivariate Hazard ratio 5.05 (95 % confidence interval 1.09–23.4, p = 0.038)]. When combined with traditional clinical variables, abnormal GLS remained independently predictive of the primary outcome [multivariate Hazard ratio 5.31 (95 % confidence interval 1.18–24, p = 0.030)]. In a model including the strongest clinical and echocardiographic predictors of the primary outcome, abnormal GLS demonstrated significant incremental benefit for risk stratification [net reclassification improvement 0.75 (95 % confidence interval 0.21–1.23, p < 0.0001)]. Abnormal GLS is an independent predictor of adverse outcomes in HCM patients. Standardized use of GLS may provide significant incremental value over traditional variables for risk stratification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most prevalent inherited cardiovascular disease, affecting approximately 1 in 500 people worldwide [1]. HCM is a heterogeneous and unpredictable disease that can be associated with significant cardiovascular morbidity and mortality, most commonly related to heart failure and ventricular arrhythmias [2–7]. Current methods of risk stratification for patients with HCM are imperfect, relying on a combination of clinical and imaging variables [1, 8]. Although standard two-dimensional transthoracic echocardiography (TTE) adequately characterizes structural and functional parameters in HCM, the prognostic utility of routine TTE is limited.
Speckle-tracking echocardiography has emerged as a quantitative technique for accurate evaluation of regional myocardial function through analysis of the motion of speckles identified on routine TTE. Speckle-tracking left ventricular global longitudinal strain (GLS) incorporates strain measurements from all myocardial segments into a single measure of myocardial function [9] and may predict adverse cardiovascular events in HCM patients [10, 11]. To date, however, the prognostic utility of GLS has been limited by the lack of standardized reference ranges. We hypothesized that abnormal GLS, as defined by recently published reference values [12], would predict adverse cardiovascular events in a cohort of HCM patients compared to traditional clinical and echocardiographic variables. To address this hypothesis, we incorporated GLS values into HCM clinical risk prediction in a retrospective analysis.
Methods
Established outpatient HCM clinic patients were maintained in a database at the Emory HCM Clinic. GLS analysis was included as part of the routine TTE evaluation beginning in August 2009. Patients were required to have at least one follow-up visit or hospitalization ≥30 days after index GLS assessment to be eligible for the study. Demographics, clinical characteristics, TTE measures, and long-term outcomes were assessed retrospectively as detailed below. The Emory University Institutional Review Board approved all aspects of this study prior to the initiation of formal data analysis.
Study population
All subjects underwent outpatient evaluation and GLS at the Emory HCM clinic between August 2009 and May 2013. HCM was defined as the presence of a non-dilated left ventricle with wall thickness of at least 15 mm in at least one myocardial segment in the absence of any associated cardiac or systemic disease. Traditional clinical variables of interest included demographics (age and sex), functional assessment (New York Heart Association [NYHA] class), pertinent history (family history of sudden cardiac death, history of arrhythmias, and history of unexplained syncope [no identifiable neurologic or cardiovascular cause]), and diagnostic testing (exercise treadmill test for blood pressure response, standard 12-lead electrocardiography, and 24 h Holter monitoring for arrhythmias). Blood pressure response to exercise was assessed using a standard or modified Bruce protocol with an abnormal blood pressure response defined as less than 20 mmHg increase from rest to peak exercise. Arrhythmias included atrial fibrillation and non-sustained ventricular tachycardia (NSVT; defined as ≥3 beats at ≥120 beats per minute). Prior or interval septal reduction therapy (SRT; including surgical myectomy and transcatheter alcohol septal ablation) was also recorded.
Echocardiography
Standard two-dimensional and Doppler TTE was performed on a GE Vivid 7 and E9 (General Electric, Milwaukee, WI). Traditional echocardiographic variables included measures of chamber quantification, markers of dynamic obstruction, spectral Doppler assessment of outflow tract gradients and diastolic parameters, and color Doppler assessment of mitral valve regurgitation. Specifically, left ventricular (LV) ejection fraction and left atrial volume index (LAVI) were assessed by the Simpson biplane method of discs. Maximal LV wall thickness was measured in the basal, mid, and apical short axis views at end-diastole. Systolic anterior motion of the mitral valve was assessed by M-mode in the parasternal long axis at the level of the mitral valve leaflets. Spectral Doppler assessment included pulsed wave and continuous wave Doppler evaluation in the LV mid-cavity and outflow tract, pulsed wave Doppler evaluation of early mitral inflow (E wave) and tissue Doppler assessment of the lateral mitral annulus (e′ wave). Diastolic function and LV filling pressures were characterized by the ratio of the E wave to the e′ wave (E/e′).
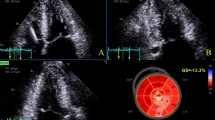
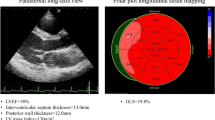
Speckle-tracking GLS analysis was performed on-line on apical long-axis, apical 4-chamber, and apical 2-chamber views (GE EchoPAC, General Electric, Milwaukee, WI). The frame rate for analysis was between 60 and 80 frames/s. The timing of aortic valve closure was indicated on the apical 3-chamber view. Peak longitudinal strain values were measured for each of the 17-segment American Heart Association model and GLS was presented as the average strain value of all evaluable segments. GLS was dichotomized based on published reference values [12], with normal GLS defined as more negative or equal to −16 %, and abnormal defined as values less negative than −16 % (Fig. 1).
Typical global longitudinal strain (GLS) results. Demonstration of normal GLS (equal to or more negative than −16 %; a) and abnormal GLS (less negative than −16 %; b). Longitudinal strain values were obtained for each of the 17 AHA segments from the apical long-axis (“LAX”), apical 4-chamber (“A4C”), and apical 2-chamber (“A2C”) views. GLS (shown as “GLPSS” and “GLPS” in a and b, respectively) was defined as the average value of all evaluable segments. AHA American Heart Association
Outcomes
The primary composite outcome included heart failure hospitalization, sustained ventricular arrhythmia, and all-cause death during follow-up. Heart failure hospitalization was defined as hospital admission for worsening dyspnea and congestive findings such as pulmonary and peripheral edema treated with intravenous diuretic therapy. Sustained ventricular arrhythmias included appropriate implantable cardioverter–defibrillator shocks, greater than 30 s of ventricular tachycardia on Holter monitoring, or resuscitated pulseless ventricular tachycardia or ventricular fibrillation cardiac arrest.
Statistics
Categorical variables are presented as proportions and continuous variables are presented as the mean ± standard deviation (unless otherwise stated). Categorical variables were analyzed by the Chi square test. Normally distributed continuous variables were assessed with the Student t test and non-normally distributed continuous variables were assessed with the Mann–Whitney U test. The difference in event-free survival according to normal and abnormal GLS was evaluated by Kaplan–Meier survival analysis and compared by the Mantel–Cox log-rank test. Traditional clinical variables, echocardiographic variables, and GLS were assessed by univariate Cox regression analyses to identify predictors of the primary composite outcome. Independent predictors of the primary outcome were analyzed by backward conditional multivariate Cox regression analyses separately for traditional echocardiographic variables plus GLS and traditional clinical variables plus GLS to evaluate the predictive power of GLS in both contexts. A predictive model was created that combined all clinical and echocardiographic variables associated with p ≤ 0.15 on univariate analysis. Model goodness-of-fit was tested with the Hosmer–Lemeshow test. The incremental benefit of incorporating GLS was assessed by analysis of the increase in C-statistic and continuous net reclassification improvement based on our multivariate model before and after the addition of GLS [13]. Bootstrapping with 1000 samples was then conducted for all of our multivariate analyses to assess internal validity. Inter-observer variability was assessed with Cohen’s kappa coefficient for categorical variables and intra-class correlation for continuous variables. Statistical analyses were performed with SPSS version 20 (Chicago, Illinois) and SAS version 9.3 (Cary, North Carolina). A p value of <0.05 was considered significant.
Results
Of 104 HCM patients with GLS imaging, 25 did not have ≥30 days of follow-up. Thus, 79 patients were included in the final analysis. Overall, 70 patients (88 %) had asymmetric septal hypertrophy, 6 patients (8 %) had concentric LV hypertrophy, and 3 patients (4 %) had isolated apical hypertrophy. All 17 myocardial segments were analyzed in 73 (92 %) of the 79 patients, with an average of 15.3 segments evaluated in the 6 other patients. Of 1,343 potential segments available for analysis, only 10 segments (0.7 %) were non-evaluable due to poor image quality.
Traditional clinical and echocardiographic variables and GLS classification
Baseline patient characteristics stratified by GLS classification are presented in Table 1. Patients with abnormal GLS had significantly higher LV wall thicknesses, LAVI, and E/e′ on average compared to patients with normal GLS. Otherwise, there were no significant baseline differences for those with abnormal versus normal GLS. At baseline, 7 patients (9 %) had a prior history of SRT. At a median follow-up of 112 days (interquartile range 26–803 days), 10 patients (12 %) underwent subsequent SRT. Ultimately, 17 patients (21 %) had any SRT by the end of study. There were no significant differences in GLS dependent on prior SRT (p = 0.12), SRT in follow-up (p = 0.17), or any SRT by study end (p = 0.89).
Traditional clinical and echocardiographic variables and outcome status
At a median follow-up of 22 months (interquartile range 9–30 months), 15 patients (19 %) met the primary composite outcome. The individual components of the primary outcome stratified by abnormal GLS are listed in Table 2. The average time to event was 11 months (5 months for heart failure hospitalization, 18 months for sustained ventricular arrhythmia, and 13 months for all-cause death). Regarding baseline clinical and echocardiographic characteristics, more patients experiencing the primary outcome had a history of NSVT (33 vs 6 %, p = 0.003) and abnormal GLS (87 vs 48 %, p = 0.006); average LV wall thickness was significantly larger (26 ± 7 vs 22 ± 7 mm, p = 0.042) and GLS was significantly less negative (−11.5 ± 3.0 vs −15.0 ± 4.2 %, p = 0.004) in those who met the primary outcome. Otherwise, there were no significant differences in baseline clinical or echocardiographic variables between patients meeting the primary outcome and those not. Kaplan–Meier survival analysis stratified by normal and abnormal GLS is depicted in Fig. 2.
Primary composite outcome Kaplan–Meier survival analysis for patients with normal and abnormal global longitudinal strain (GLS). Primary composite outcome = heart failure hospitalization, sustained ventricular arrhythmia, and all-cause death. Normal GLS is equal to or more negative than −16 % (≤−16 %) and abnormal GLS is less negative than −16 % (>−16 %)
Outcome prediction
Echocardiographic predictors of the primary composite outcome are shown in Table 3. The only independent echocardiographic predictor of the primary outcome was GLS. Clinical predictors of the primary outcome and GLS are shown in Table 4. A history of NSVT and abnormal GLS were both independently associated with the primary outcome. The clinical and echocardiographic variables included in our predictive model are shown in Table 5. In this model, both a history of NSVT and abnormal GLS were again independent predictors of the primary outcome. The C-statistic of our predictive model was 0.76 prior to inclusion of GLS, and increased to 0.81 with the addition of GLS (p = 0.10). The incremental value of GLS for risk stratification of our primary outcome was demonstrated by net reclassification improvement of 0.75 [95 % confidence interval (CI) 0.21–1.23, p < 0.0001], primarily driven by correct upward risk reclassification of patients with events, as demonstrated in Fig. 3.
Reclassification of outcome events and non-events based a predictive model with the incorporation of abnormal global longitudinal strain (GLS). The baseline model included atrial fibrillation, NSVT on Holter monitoring, left atrial volume index, LV outflow gradient, and maximum LV wall thickness. NRIEVENT represents the net percentage of patients with an event that were correctly assigned a higher predicted risk after the addition of abnormal GLS to the model. NRINON-EVENT represents the net percentage of patients without an event that were correctly assigned a lower predicted risk after the addition of abnormal GLS to the model. NRITOTAL represents the sum of NRIEVENT and NRINON-EVENT. KM Kaplan–Meier, LV left ventricle, NRI net reclassification improvement, NSVT non-sustained ventricular tachycardia
Reproducibility analysis
Inter-observer variability for GLS was assessed by 2 independent operators (G.H. and M.P.) in 20 randomly selected patients. The intra-class correlation coefficient for GLS was 0.95 (p < 0.0001) with excellent agreement between readers for classification of normal and abnormal GLS (Cohen’s kappa coefficient 0.89, p < 0.0001).
Discussion
In this study evaluating the incremental benefit of standard speckle-tracking GLS measurements in an HCM population, we observed two primary and significant findings. First, we found abnormal GLS, independent of traditional clinical and echocardiographic markers, predicts a composite endpoint inclusive of heart failure and sustained ventricular arrhythmias. Second, the incorporation of GLS in a routine clinical HCM TTE protocol demonstrated excellent inter-observer consistency, which further supports routine measurement of GLS as both feasible and practical [9, 14, 15]. In aggregate, our findings suggest that the use of this quantitative myocardial imaging modality may be a valuable addition to risk stratification algorithms for HCM patients.
Several clinical and echocardiographic variables have been previously implicated for risk prediction in HCM patients, including NYHA functional class [2, 5–7, 16], atrial fibrillation [2, 6, 7], sustained or non-sustained ventricular arrhythmia [5, 6], severity of LVOT obstruction [4, 6, 7], severity of LV hypertrophy [3, 7, 16], and extent of left atrial enlargement [6]. However, no clinical or echocardiographic variable has demonstrated consistent prognostic power across these studies. GLS has also previously been associated with increased risk of major adverse cardiac events in HCM patients, but these were relatively small studies that lacked the implementation of standardized reference ranges for abnormal GLS [10, 11, 17, 18]. Moreover, previous GLS studies did not adjust for traditional HCM risk factors [10, 11]. Assessing the prognostic value of abnormal GLS, independent of previously accepted clinical and echocardiographic risk markers, was the primary purpose of the current study.
Our data highlight GLS as a potentially valuable subclinical biomarker for patients with HCM. An explanation for the improved predictive value of GLS may be that abnormal GLS is a better surrogate of the underlying myofibril disarray and myocardial fibrosis present in HCM compared to previously reported HCM risk factors. It has been shown that myocardial segments with abnormal GLS in HCM patients correlate with replacement fibrosis on cardiac magnetic resonance (CMR) and computed tomography imaging [19–25], as well as interstitial fibrosis present within surgical histopathology specimens post-myectomy [26, 27]. In addition, GLS has demonstrated utility for differentiating HCM from other causes of increased LV wall thickness [28, 29], delineating septal and apical hypertrophy patterns [30], and identification of subclinical myocardial dysfunction in HCM gene positive patients [31]. In regards to SRT, GLS does not normalize after surgical myectomy [32] or transcatheter alcohol septal ablation [33], which also supports the proposition that abnormal GLS is more representative of the permanent pathologic cellular changes present in HCM rather than clinically relevant severe LV hypertrophy.
There are important clinical implications of our findings. First, after adjusting for traditional markers of HCM disease progression and pathogenesis, abnormal GLS retained independent association with a primary outcome consisting of heart failure, sustained ventricular arrhythmias, and all-cause death in this cohort of HCM patients. These findings suggest that the incorporation of GLS with standardized reference values into the evaluation of HCM patients may be useful for risk stratification. Second, the addition of GLS into a routine clinical TTE protocol was feasible from both the practical and economic aspects of a busy clinical echocardiography lab. Simplistic acquisition and interpretation of GLS cumulatively adds less than 5 min to the evaluation of a HCM patient [34]. Moreover, the elimination of the need for potentially costly complementary imaging, such as CMR, may render GLS a more cost-effective imaging modality, provided decreased financial burden comes without the expense of inadequate prognostic utility. Future direct comparisons between CMR and GLS in HCM represent an important area of further study, with particular potential implications for patients with contraindications or limited access to CMR imaging. Whether GLS impacts specific clinical algorithms and assists medical decision-making (i.e. identifying HCM patients at high-risk for ventricular arrhythmias) will require carefully designed prospective and longitudinal trials.
Limitations
Several limitations of this study are noteworthy. First, the small sample size limited the overall power of this study, as well as the interpretation of multivariate analyses. However, we believe the demonstration that abnormal GLS was already highly predictive of the primary outcome in a modestly sized cohort also represents an overall strength of the study. Second, because of the retrospective nature of this study, we cannot be sure other unidentified confounders present in the study cohort affected the primary outcome. Third, this was a single center study utilizing only GE equipment and a single cardiac sonographer, which may limit applicability to other centers with different equipment and personnel. However, meta-analytic data has shown that inter-vendor differences do not cause significant variability in GLS values [12] and our inter-observer analysis demonstrated excellent reproducibility. Lastly, our primary composite outcome included heart failure hospitalization, sustained ventricular arrhythmia, and all-cause death, which may appear as a heterogeneous combination. However, heart failure and sustained ventricular arrhythmias are the most common causes of morbidity and mortality in HCM patients [2–7] and similar composites have been reported in prior research evaluating GLS in HCM patients [10, 11, 18]. Future prospective study incorporating large patient populations will be needed for validation of this initial and provocative data.
Conclusions
Abnormal speckle-tracking GLS is independently associated with adverse cardiovascular outcomes in patients with HCM. The feasibility, high reproducibility, and validated reference values of normal GLS may support more routine use of this modality in the TTE examination of HCM patients.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- GLS:
-
Global longitudinal strain
- HCM:
-
Hypertrophic cardiomyopathy
- LAVI:
-
Left atrial volume index
- LV:
-
Left ventricle
- NSVT:
-
Non-sustained ventricular tachycardia
- NYHA:
-
New York Heart Association
- SRT:
-
Septal reduction therapy
- TTE:
-
Transthoracic echocardiography
References
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011) 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 58(25):2703–2738. doi:10.1016/j.jacc.2011.10.825
Cecchi F, Olivotto I, Montereggi A, Santoro G, Dolara A, Maron BJ (1995) Hypertrophic cardiomyopathy in Tuscany: clinical course and outcome in an unselected regional population. J Am Coll Cardiol 26(6):1529–1536. doi:10.1016/0735-1097(95)00353-3
Decker JA, Rossano JW, Smith EO, Cannon B, Clunie SK, Gates C, Jefferies JL, Kim JJ, Price JF, Dreyer WJ, Towbin JA, Denfield SW (2009) Risk factors and mode of death in isolated hypertrophic cardiomyopathy in children. J Am Coll Cardiol 54(3):250–254. doi:10.1016/j.jacc.2009.03.051
Kofflard MJ, Ten Cate FJ, van der Lee C, van Domburg RT (2003) Hypertrophic cardiomyopathy in a large community-based population: clinical outcome and identification of risk factors for sudden cardiac death and clinical deterioration. J Am Coll Cardiol 41(6):987–993
Kyriakidis M, Triposkiadis F, Anastasakis A, Theopistou A, Tocta R, Barbetseas J, Gialafos J (1998) Hypertrophic cardiomyopathy in Greece: clinical course and outcome. Chest 114(4):1091–1096
Li M, Wang QB, Cheng K (2007) Long term follow-up results of 199 patients with hypertrophic cardiomyopathy. Zhonghua xin xue guan bing za zhi 35(11):988–991
Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM (1999) Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. J Am Med Assoc 281(7):650–655
O’Mahony C, Tome-Esteban M, Lambiase PD, Pantazis A, Dickie S, McKenna WJ, Elliott PM (2013) A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart (Br Card Soc) 99(8):534–541. doi:10.1136/heartjnl-2012-303271
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smulevitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 24(3):277–313. doi:10.1016/j.echo.2011.01.015
Funabashi N, Takaoka H, Horie S, Ozawa K, Takahashi M, Yajima R, Saito M, Fujiwara K, Tani A, Kamata T, Kanaeda A, Uehara M, Kataoka A, Kobayashi Y (2013) Risk stratification using myocardial peak longitudinal-strain on speckle-tracking transthoracic-echocardiogram to predict major adverse cardiac events in non ischemic hypertrophic-cardiomyopathy subjects confirmed by MDCT. Int J Cardiol 168(4):4586–4589. doi:10.1016/j.ijcard.2013.06.056
Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J (2012) Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 13(7):617–623. doi:10.1093/ejechocard/jer318
Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH (2013) Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 26(2):185–191. doi:10.1016/j.echo.2012.10.008
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27(2):157–172; discussion 207–112. doi:10.1002/sim.2929
Kalogeropoulos AP, Georgiopoulou VV, Gheorghiade M, Butler J (2012) Echocardiographic evaluation of left ventricular structure and function: new modalities and potential applications in clinical trials. J Cardiac Fail 18(2):159–172. doi:10.1016/j.cardfail.2011.10.019
Oxborough D, George K, Birch KM (2012) Intraobserver reliability of two-dimensional ultrasound derived strain imaging in the assessment of the left ventricle, right ventricle, and left atrium of healthy human hearts. Echocardiography 29(7):793–802. doi:10.1111/j.1540-8175.2012.01698.x
Adabag AS, Casey SA, Kuskowski MA, Zenovich AG, Maron BJ (2005) Spectrum and prognostic significance of arrhythmias on ambulatory Holter electrocardiogram in hypertrophic cardiomyopathy. J Am Coll Cardiol 45(5):697–704. doi:10.1016/j.jacc.2004.11.043
Debonnaire P, Thijssen J, Leong DP, Joyce E, Katsanos S, Hoogslag GE, Schalij MJ, Atsma DE, Bax JJ, Delgado V, Marsan NA (2014) Global longitudinal strain and left atrial volume index improve prediction of appropriate implantable cardioverter defibrillator therapy in hypertrophic cardiomyopathy patients. Int J Cardiovasc Imaging 30(3):549–558. doi:10.1007/s10554-014-0378-z
Paraskevaidis IA, Farmakis D, Papadopoulos C, Ikonomidis I, Parissis J, Rigopoulos A, Iliodromitis EK, Kremastinos DT (2009) Two-dimensional strain analysis in patients with hypertrophic cardiomyopathy and normal systolic function: a 12-month follow-up study. Am Heart J 158(3):444–450. doi:10.1016/j.ahj.2009.06.013
Chang SA, Lee SC, Choe YH, Hahn HJ, Jang SY, Park SJ, Choi JO, Park SW, Oh JK (2012) Effects of hypertrophy and fibrosis on regional and global functional heterogeneity in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 28(Suppl 2):133–140. doi:10.1007/s10554-012-0141-2
Popovic ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY (2008) Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 21(12):1299–1305. doi:10.1016/j.echo.2008.09.011
Prinz C, van Buuren F, Faber L, Bitter T, Bogunovic N, Burchert W, Horstkotte D (2012) Myocardial fibrosis is associated with biventricular dysfunction in patients with hypertrophic cardiomyopathy. Echocardiography 29(4):438–444. doi:10.1111/j.1540-8175.2011.01588.x
Urbano-Moral JA, Rowin EJ, Maron MS, Crean A, Pandian NG (2014) Investigation of global and regional myocardial mechanics with 3-dimensional speckle tracking echocardiography and relations to hypertrophy and fibrosis in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 7(1):11–19. doi:10.1161/circimaging.113.000842
Funabashi N, Kataoka A, Horie S, Ozawa K, Takaoka H, Takahashi M, Yajima R, Saito M, Umazume T, Fujiwara K, Kamata T, Uehara M, Kobayashi Y (2013) Distinguishing 320 slice CT-detected focal fibrotic lesions and non-fibrotic lesions in hypertrophic cardiomyopathy by assessment of regional myocardial strain using two dimensional speckle tracking echocardiography. Int J Cardiol 169(6):e109–e113. doi:10.1016/j.ijcard.2013.10.029
Funabashi N, Takaoka H, Horie S, Ozawa K, Daimon M, Takahashi M, Yajima R, Saito M, Fujiwara K, Tani A, Kamata T, Uehara M, Kataoka A, Kobayashi Y (2013) Regional peak longitudinal-strain by 2D speckle-tracking TTE provides useful information to distinguish fibrotic from non-fibrotic lesions in LV myocardium on cardiac MR in hypertrophic cardiomyopathy. Int J Cardiol 168(4):4520–4523. doi:10.1016/j.ijcard.2013.06.105
Yajima R, Kataoka A, Takahashi A, Uehara M, Saito M, Yamaguchi C, Lee K, Komuro I, Funabashi N (2012) Distinguishing focal fibrotic lesions and non-fibrotic lesions in hypertrophic cardiomyopathy by assessment of regional myocardial strain using two-dimensional speckle tracking echocardiography: comparison with multislice CT. Int J Cardiol 158(3):423–432. doi:10.1016/j.ijcard.2011.01.096
Almaas VM, Haugaa KH, Strom EH, Scott H, Dahl CP, Leren TP, Geiran OR, Endresen K, Edvardsen T, Aakhus S, Amlie JP (2013) Increased amount of interstitial fibrosis predicts ventricular arrhythmias, and is associated with reduced myocardial septal function in patients with obstructive hypertrophic cardiomyopathy. Europace 15(9):1319–1327. doi:10.1093/europace/eut028
Almaas VM, Haugaa KH, Strom EH, Scott H, Smith HJ, Dahl CP, Geiran OR, Endresen K, Aakhus S, Amlie JP, Edvardsen T (2013) Noninvasive assessment of myocardial fibrosis in patients with obstructive hypertrophic cardiomyopathy. Heart (Br Card Soc). doi:10.1136/heartjnl-2013-304923
Sun JP, Stewart WJ, Yang XS, Donnell RO, Leon AR, Felner JM, Thomas JD, Merlino JD (2009) Differentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol 103(3):411–415. doi:10.1016/j.amjcard.2008.09.102
Butz T, van Buuren F, Mellwig KP, Langer C, Plehn G, Meissner A, Trappe HJ, Horstkotte D, Faber L (2011) Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: a study in athletes and in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 27(1):91–100. doi:10.1007/s10554-010-9665-5
Yang H, Carasso S, Woo A, Jamorski M, Nikonova A, Wigle ED, Rakowski H (2010) Hypertrophy pattern and regional myocardial mechanics are related in septal and apical hypertrophic cardiomyopathy. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 23(10):1081–1089. doi:10.1016/j.echo.2010.06.006
Yiu KH, Atsma DE, Delgado V, Ng AC, Witkowski TG, Ewe SH, Auger D, Holman ER, van Mil AM, Breuning MH, Tse HF, Bax JJ, Schalij MJ, Marsan NA (2012) Myocardial structural alteration and systolic dysfunction in preclinical hypertrophic cardiomyopathy mutation carriers. PLoS ONE 7(5):e36115. doi:10.1371/journal.pone.0036115
Moravsky G, Bruchal-Garbicz B, Jamorski M, Ralph-Edwards A, Gruner C, Williams L, Woo A, Yang H, Laczay B, Rakowski H, Carasso S (2013) Myocardial mechanical remodeling after septal myectomy for severe obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 26(8):893–900. doi:10.1016/j.echo.2013.05.012
Sommer A, Poulsen SH, Mogensen J, Thuesen L, Egeblad H (2010) Left ventricular longitudinal systolic function after alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a long-term follow-up study focused on speckle tracking echocardiography. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol 11(10):883–888. doi:10.1093/ejechocard/jeq087
Feigenbaum H, Mastouri R, Sawada S (2012) A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J Off J Jpn Circ Soc 76(7):1550–1555
Conflict of interest
There are no conflicts of interest on the part of any authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hartlage, G.R., Kim, J.H., Strickland, P.T. et al. The prognostic value of standardized reference values for speckle-tracking global longitudinal strain in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging 31, 557–565 (2015). https://doi.org/10.1007/s10554-015-0590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-015-0590-5