Abstract
The impact of pulmonary embolism on right ventricular (RV) performance can be evaluated by echocardiography, however, the relationship between pulmonary vascular involvement and RV burden is controversial. To assess the effect of clot burden on RV performance we studied 85 patients (aged 53 ± 17 years, 39 female) with confirmed PE by multislice computed tomography (CT) and echocardiography within 24 h of diagnosis. A CT score ranging from 1 to 20 points according to the pulmonary arteries involved was used. RV function was evaluated with fractional area change (FAC), with dysfunction present when FAC < 40 %. Tissue Doppler RV systolic (s’) velocities and myocardial performance index (MPI) were obtained, as well as pulmonary artery pressure (PAP). Mean CT score was 9.4 ± 6.7. Only 31 out of 85 patients (37 %) presented with RV dysfunction, with FAC measuring 27.8 ± 7.2 % in patients with dysfunction compared to 47.8 ± 4.4 for those with preserved RV function (p < 0.05). RV dysfunction was associated to older age, higher CT scores, increased pulmonary pressures and MPI and decreased s’ (p < 0.001). An inverse correlation with CT clot burden was found for FAC (r = −0.57), whereas a direct correlation was seen for PAP (r = 0.51) and MPI (0.32). No correlation was observed for tissue Doppler velocities. In patients with acute PE, the effect of clot burden on RV performance is better expressed FAC than tissue Doppler indexes; the increase in pulmonary pressure is proportional to the magnitude of obstruction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Right ventricular (RV) dysfunction is associated with high morbidity and mortality after pulmonary thromboembolism. Severity of dysfunction is influenced not only by the degree of pulmonary obstruction, but also by the previous cardiovascular status and circulating vasoconstrictive mediators. Right ventricular performance is essentially evaluated by echocardiography, since it is portable, non-invasive and repeatable, not requiring radiation or contrast administration; on the other hand, multidetector chest tomography (CT), the method of choice for PE diagnosis, may also be used to evaluate the extent of pulmonary vascular obstruction. Studies comparing severity of clot load with RV burden assessed by echocardiography, however, report controversial results [1–3], ranging from a good correlation to poor agreement between the severity of obstruction and RV performance. Some limitations concern the retrospective nature of the studies, inclusion of a small number of patients, and a relatively heterogeneous methodology to investigate RV dysfunction. Due to the complexity of RV morphology, evaluation of RV performance by echocardiography is challenging, and either assessed qualitatively by visual estimation of RV contractility (RV dilatation and septal flattening) or semi quantitatively with various techniques, including tissue Doppler myocardial velocities and RV fractional area change (FAC). Because most studies comparing CT to echo were at best qualitative, we attempted to study the relationship between clot burden and RV performance with a more accurate methodology.
Objective
To assess the influence of severity of pulmonary obstruction on RV function in patients with acute PE.
Methods
Patients
The group consisted of patients with clinical suspicion of acute PE (dyspnea and chest pain for the past week with clinical risk factors) from 2007 to 2011. Patients with a history of previous PE or pulmonary chronic diseases were not included in the study. Additionally, the previous echocardiographic examination, when available, was reviewed to compare with current RV function. Diagnosis was confirmed by multidetector chest computed tomography (CT), undertaken after results of estimated glomerular filtration rate. Patients with estimated glomerular filtration rate <30 mL/min did not undertake CT and were thus not included in the study. All patients underwent echocardiography and BNP measurements up to 24 h of diagnosis. Patients with left ventricular ejection fraction <0.55 and significant arrhythmia (frequent premature beats or atrial fibrillation) were excluded. Poor echocardiographic window and indeterminate CT readings precluding confident interpretation of the study were also criteria of exclusion. Patients were treated with intravenous heparin or fractioned heparin at the discretion of the attending physician, whereas thrombolytic therapy was used only in case of hemodynamic instability (systolic blood pressure <90 mmHg, signs of poor peripheral perfusion). The study was approved by the Ethics Committee of the institution, and patients gave the written informed consent to participate.
Echocardiography
Patients underwent a comprehensive echocardiography (Vivid 7, GE Medical Systems, Milwaukee, WI) with bidimensional measurements of left ventricular diameters and ejection fraction according to ASE guidelines [4]. Left and right ventricular diastolic diameters were measured from the apical 4-chamber view to obtain RV diameters and right/left ventricular ratio. Parasternal, apical and subcostal views were used to subjectively evaluate RV systolic contractility. Right ventricular FAC was obtained from apical 4-chamber view and given as maximal RV area during end-diastole minus area in end-systole divided by maximal area during diastole all multiplied by 100. Systolic (s’) myocardial velocities were obtained from the RV annulus from the apical 4-chamber view, with the cursor set as parallel as possible to the wall to minimize angle limitation and used to assess RV function. Color flow mapping was used to help position the pulsed-wave Doppler sample-size at the correct place. Tissue Doppler time intervals were used to calculate myocardial performance index (MPI), given as the ratio of the RV isovolumic contraction time (ICT) + isovolumic relaxation time (IRT) divided by RV ejection time (ET), as follows: RV MPI = (ICT + IRT)/ET [5]. Patients were divided into 2 groups according to the presence of RV dysfunction by FAC, with RV dysfunction present when FAC < 40 % [6]. Additionally, RV dysfunction was also judged to be present in the event of RV hypokinesia associated with RV dilatation (RV/LV ratio ≥1) and abnormal septal movement (flattening). Tricuspid regurgitation jet was obtained and pulmonary artery systolic pressure (PASP) given as the velocity gradient added to right atrial pressure estimated from inferior vena cava diameter and collapsibility. The average of three cycles was used for analysis. Patients were asked to breathe slowly or to maintain apnea for a few seconds, and Doppler measurements recorded, whenever possible, at end-expiration. Data was digitally stored and subsequently analyzed.
Multidetector computed tomography
Multislice thoracic angiotomography was performed in a 64 slices scanner (Aquilion, Toshiba Medical Systems, Tochiki-ken, Japan). After the monophasic injection of 100 mL of contrast material [iobitridol (Henetix 300; Guerbet, Rio de Janeiro, Brazil), 300 mg of iodine per milliliter] and 40 mL of saline, at a rate of 4.0 mL/s, as the contrast material was detected in the pulmonary artery trunk (contrast material bolus tracking with a threshold of 180 HU) the images were acquired in the caudocranial direction (1 × 1 mm). For each lung, the main, lobar, segmental, and subsegmental arteries were examined for pulmonary embolism. Reformatted sagittal and coronal views (3 × 3 mm) were also sent to the picture archiving and communication system (PACS). Pulmonary embolism diagnosis consisted of direct visualization of occlusive or subocclusive endoluminal thrombus (central filling defect completely or partially outlined by contrast agent) or complete occlusion by thrombus in normal-sized or enlarged pulmonary vessels. Definition of CT obstruction index was the following: the arterial tree was regarded as having ten major segmental branches in the each lung. The presence of a filling defect or obstruction in any one of these branches, regardless of the degree of obstruction, scored 1 point. Emboli in the most proximal arterial level received a total score equal to the number of segmental arteries arising distally, according to predetermined anatomic subdivisions: maximum score of 3 for the upper lobe arteries, 2 for the middle lobe and the lingual arteries, 5 for the lower lobe arteries; a score of 7 points was given for the intermediate arteries and 10 for the main pulmonary arteries. A single filling defect extending into more than one anatomic location was scored for each location up to, but not exceeding, the maximum designated for each region. The maximum score was 10 for each lung, and the maximum possible CT obstruction score was 20 for both lungs. The percentage of vascular obstruction was given as the patient score divided by the maximal total score and by multiplying the result by 100. The imaging studies were analyzed by two radiologists in consensus, and any disagreement was resolved by a third investigator. Examiners were unaware of results of echocardiography and vice versa.
Statistics
Continuous variables were presented as mean ± SD, if normally distributed, whereas categorical variables as observed frequencies and percentages. Groups were tested with Student’s t test for continuous variables and Chi-square or Fisher’s exact test for categorical variables, as appropriate. Correlation of variables with CT was undertaken with Pearson correlation coefficient and to clinical parameters using the Spearman rank test. Analysis was performed with SPSS statistical software (version 17.0; SPSS, Inc, Chicago, Ill) and significance was determined at the 0.05 level. Echocardiographic measurements (RV diameters and area and TD velocities) were repeated after 3 months by the same and a distinct observer in 20 randomly selected patients to assess intra and interobserver variability analysis, and tested by intraclass correlation coefficient. Interobserver variability of CT score was checked in 10 randomly selected patients with intraclass correlation coefficient.
Results
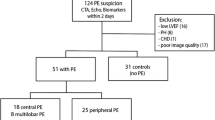
From 101 patients with clinical suspicion of PE and CT confirmation, 9 were excluded due to inadequate images (7 echocardiographic and 2 CT), 3 because of systolic heart failure, 2 due to arrhythmia and 2 patients refused to participate, so a total of 85 patients completed all the examinations. There were 39 female, and mean age was 53 ± 17 years. Main predisposing factors were deep venous thrombosis (28 %), malignancy (24 %), surgical procedure (20 %) and long distance flight (8 %). Only 3 patients were hemodynamically unstable at admission, and were treated with thrombolytic therapy along with systemic anticoagulation. None of the patients was submitted to surgical or catheter embolectomy.
Assessment of CT
The two patients excluded were due to poor contrast enhancement and respiratory motion artifacts. The volume of contrast agent ranged from 60 to 70 mL. Assessment of the CT score added 1–3 min to regular CT evaluation. Average CT pulmonary vessels obstruction score was 9.4 ± 6.7, with most patients (54 %) considered to have minor (<50 % clot obstruction) PE by CT. Maximal CT scores (20 points) was seen in 19 (22 %) of the patients; in three of these patients RV function was normal or nearly normal; CT findings showed multiple obstructions to segmentar and subsegmentar arteries, with no involvement of main pulmonary arteries. Mean CT score was higher (13.7 ± 6.5 points) in patients with decreased RV function, whereas a preserved function was associated with lower scores (6.9 ± 5.5 points).
Interobserver variability
The intraclass correlation coefficient was 0.988 for interobserver variability (95 % CI: 0.954, 0.997) for CT measurements, showing high agreement between measurements.
Echocardiography
Except for an increased PASP (40 ± 12 mmHg), mean values for echocardiographic RV variables were within normal range (Table 1). Tricuspid regurgitation was present and measurement of gradient feasible in 56 (66 %) patients. Tricuspid regurgitation was mostly mild (45 patients). Comparison of severity of CT clot burden with echocardiographic variables showed an inverse correlation for FAC (r = −0.57, Fig. 1), and a direct correlation for PASP (r = 0.51). A poor correlation was observed for MPI (r = 0.32) and CT scores, and no significant correlation was found for tissue Doppler s’ velocities.
According to FAC measurements, most patients had normal RV performance, with only 31 out of 85 patients (37 %) presenting with impaired function. Using semiquantitative estimation, the prevalence of RV dysfunction was even lower (25 patients, 29 %). Prior echocardiographic examinations were available for 15 out of these 25 patients (60 %) presenting with echocardiographic RV dysfunction. The previous echocardiogram disclosed a normal RV function in all. Patients with decreased RV function had lower tissue Doppler s’ velocity, RV MPI and RV/LV ratio. These patients were also older, and had higher CT scores (Table 1). Tricuspid regurgitation was usual in patients with RV dysfunction (93 %), but less common in patients with normal RV (66 %), thus precluding estimation of pulmonary artery pressure (PAP) in all patients.
Inter and intraobserver variability
The intraclass correlation coefficient was 0.98 (intraobserver) and 0.96 (interobserver) for s’ wave velocity, and 0.90 (intraobserver) and 0.90 (interobserver) for RV area measurements.
Discussion
Pulmonary thromboembolism carries a significant risk of morbidity and mortality, mainly resulting from RV dysfunction. Acutely increased pulmonary vascular resistance results in RV pressure overload, increasing both RV size and pressure and decreasing right coronary perfusion pressure, resulting in RV failure. We assessed the relationship of the severity of RV dysfunction using echocardiography and the extent of mechanical pulmonary obstruction. Controversial results [1–3] have been reported comparing the severity of pulmonary vascular obstruction and RV performance, ranging from a good correlation between the severity of obstruction and RV performance [3] to poor agreement with RV function [1]. Because most studies comparing CT to echocardiography were at best qualitative, and suffered from methodological issues (retrospective or included a limited number of patients), we postulated that a better association could be provided for clot burden and RV function if more detailed echocardiographic methodology was used and the influence of cardiovascular status was reduced. Accordingly, we only included patients with preserved LV ejection fraction in our study.
The results of this study demonstrate that most usual echocardiographic parameters evaluating RV did not demonstrate a good correlation with clot burden. Severity of pulmonary obstruction correlated best to quantitative parameters such as FAC and pulmonary systolic pressure, with patients with more severe vascular obstruction showing lower FAC and higher pulmonary pressures. Though a simple estimate of RV function, FAC has shown a good correlation with magnetic resonance measured RV ejection fraction [7]. In most cases, patients with extensive obstruction of the pulmonary vasculature (>50 % of total circulation) presented with RV failure. The correlation of clot burden and RV dysfunction using FAC was suboptimal, however, even after excluding the influence of prior cardiovascular status [8]. In contrast, in a few patients, a preserved RV function was observed. Because venous thromboembolism is a dynamic process, some degree of thrombus resolution with pulmonary artery patency and improvement of RV function might have occurred [9], especially considering that echocardiography was undertaken up to 24 h of after CT diagnosis. In patients with PE treated with anticoagulation alone, serial pulmonary angiograms and perfusion lung scans suggest that resolution of PE occurs in up to 10 % of the patients in 24 h [10]. Alternatively, a few patients had minimal pulmonary obstruction on CT, but poor RV function on echocardiography. A number of explanations can be proposed to support that; the release of pulmonary vasoconstrictors (thromboxane, serotonin, endothelin) during PE, together with hypoxia, might contribute to progressive increase in pulmonary vascular resistance resulting in RV dysfunction [8, 11], implying that mechanical obstruction alone may not account for all the negative effects of a PE. Additionally, this discrepancy could be due to recurrent thrombosis: though spontaneous thrombolysis may develop in up to 50 % of the patients with PE, recurrent thrombosis may also ensue, even after treatment initialization [12], resulting in further RV impairment. Finally, in a few patients, the RV insult may persist even after there is partial pulmonary artery recanalization. A limited correlation between RV function and the extent of perfusion defects has been shown by Miller, even in patients without prior cardiopulmonary disease [13].
Regarding tissue Doppler variables, both tissue Doppler s’ velocities, an estimate of RV longitudinal function, and RV MPI, an index that incorporates systolic and diastolic function, correlated weakly to the severity of vascular obstruction. Despite the extensive data using tissue Doppler velocities for RV assessment [14, 15], this analysis can be influenced by age, loading conditions and the presence of significant tricuspid regurgitation [16]. In a few patients with large PE and RV dysfunction (demonstrated both qualitatively and by FAC), RV myocardial s’ velocities were not decreased if significant tricuspid regurgitation was present. Additionally, the RV free wall contains predominantly transverse fibers [17], which are sufficient to eject blood in the low resistance pulmonary vascular bed. Kind et al. [18], using magnetic resonance to assess patients with pulmonary hypertension, have shown a stronger correlation with RV ejection fraction and fractional transverse distance change, especially at mid RV level (r = 0.70). On the other hand, in that study, correlation for TAPSE (a measure of longitudinal function) and RVEF was weaker (r = 0.21). It is thus suggested that measures of transverse function (including FAC) should be used as assessment of pump function in these patients. Furthermore, the interventricular septum is an important architectural component of the RV, and paradoxical leftward septal bowing found in PE increases the end-systolic dimensions and, as a consequence, affects FAC, but not S’ wave, since the latter only accounts for movement of the basal RV free wall.
Concerning MPI, this variable has been shown a good correlation both with RV function and pulmonary hypertension [5]. However, MPI may be increased in patients with pulmonary hypertension independently of the presence of RV dysfunction [19]. It is likely that the rise in pulmonary resistance will prolong RV isovolumic contraction time and therefore MPI in most patients with PE, even if RV dysfunction does not follow. We have observed MPI to be increased in most patients with PE, so it could not discriminate RV dysfunction for the distinct CT scores.
In contrast, pulmonary pressure was significantly increased and well correlated with CT scan, in agreement with previous studies [20]. Elevated PAPs occur in about half of patients with acute PE, dropping progressively during treatment [21].
Severity of clot burden can be assessed semiquantitatively, using various proposed scoring systems mainly reflecting the distribution of clots, and formulated by tabulating the presence and degree of arterial obstruction of clot in individual branches of pulmonary artery [1, 3], or fully quantitatively, via clot volume quantification. Though examining smaller vessels is technically more demanding, obstruction at subsegmentar branches was systematically searched for, and subsegmentar obstruction given the same score as to a proximal vessel. However, obstruction of subsegmentar vessels was not commonly observed, most likely due to the difficulty in assessing contrast opacification inside such small vessels. Although we had a substantial number of low CT scores, with approximately half of the study population having <50 % of pulmonary vascular obstruction, that was comprised mainly of segmentar vessels. On the other hand, we felt we could not ignore subsegmentar vessel obstruction for score analysis, as patients fulfilled all inclusion criteria for the study. For practical reasons, we chose to use a simpler score based on Miller index (easier and repeatable).
Neither CT score nor RV function were remarkably compromised in our population, indicating that most patients had minor PE. CT is widely available and systematically undertaken at the least clinical suspicion of PE in our service, resulting in an excessive routine diagnosis of small PE. In addition, in previous studies with a higher prevalence of RV dysfunction, the methodology used for assessment of RV function was relatively heterogeneous; for instance, pulmonary hypertension was considered an echocardiographic sign of RV dysfunction in various studies [13, 21, 22]. It is likely that elevated pulmonary pressures are predominantly found mainly in patients with chronic pulmonary hypertension and no evidence of RV dysfunction. Since the pulmonary circulation is a low resistance system, the RV tolerates poorly acute elevations in afterload, hence, in acute PE, pulmonary pressures are not as elevated as in chronic PH, where adaptive changes maintain stroke volume and higher pulmonary pressures.
Limitations
The RV has a complex morphology, and most two dimensional echocardiographic methods are at best used as determinants of RV dysfunction, in a qualitative (by visual estimation of RV contractility and indirectly by septal flattening and RV dilatation) or semiquantitative manner. However, the advantages of two dimensional echocardiography (non-invasive, repeatable and portable, not requiring the use of radiation or contrast) make this examination essential for RV function assessment. Is likely that three-dimensional echocardiography would make a better approach to study the RV, but this alternative is still under development. Additionally, though echocardiogram was undertaken within 24 h of CT, changes in vascular obstruction and lung perfusion may have occurred within that period, influencing hemodynamics and RV function, and this might have contributed to some disparity in the results. Patients with prior chronic pulmonary diseases, including obstructive sleep apnea, might present with a higher prevalence of RV dysfunction; these patients were not included in the study, based on clinical history and physical examination, along with previous echocardiographic data. It is possible, however, that we missed patients without a clinical history of obstructive sleep apnea, but most likely in this situation the echocardiographic changes would be less proeminent.
Conclusion
The severity of vascular pulmonary obstruction in pulmonary embolism can be expressed by quantitative assessment of RV function, with FAC a better estimation than longitudinal function measurements.
References
Qanadli SD, El Hajjam M, Vieillard-Baron A, Joseph T, Mesurolle B, Oliva VL, Barre O, Bruckert F, Dubourg O, Lacombe P (2001) New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol 6:1415–1420. doi:10.2214/ajr.176.6.1761415
Bankier AA, Janata K, Fleischmann D, Kreuzer S, Mallek R, Frossard M, Domanovits H, Herold CJ (1997) Severity assessment of acute pulmonary embolism with spiral CT: evaluation of two modified angiographic scores and comparison with clinical data. J Thorac Imaging 2:150–158. doi:10.1097/00005382-199704000-00012
Mastora I, Remy-Jardin M, Masson P, Galland E, Delannoy V, Bauchart JJ, Remy J (2003) Severity of acute pulmonary embolism: evaluation of a new spiral CT angiographic score in correlation with echocardiographic data. Eur Radiol 1:29–35. doi:10.1007/s00330-002-1515-y
Min JK, Spencer KT, Furlong KT, DeCara JM, Sugeng L, Ward RP, Lang RM (2005) Clinical features of complications from transesophageal echocardiography: a single-center case series of 10,000 consecutive examinations. J Am Soc Echocardiogr 9:925–929. doi:10.1016/j.echo.2005.01.034
Zimbarra Cabrita I, Ruisanchez C, Dawson D, Grapsa J, North B, Howard LS, Pinto FJ, Nihoyannopoulos P, Gibbs JS (2010) Right ventricular function in patients with pulmonary hypertension; the value of myocardial performance index measured by tissue Doppler imaging. Eur J Echocardiogr 8:719–724. doi:10.1093/ejechocard/jeq051
Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, Wustmann K, Steck H, Eigenmann C, Schwerzmann M, Seiler C (2011) Right ventricular systolic function assessment: rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr 11:871–880. doi:10.1093/ejechocard/jer138
Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD (2007) Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography 5:452–456. doi:10.1111/j.1540-8175.2007.00424.x
Stratmann G, Gregory GA (2003) Neurogenic and humoral vasoconstriction in acute pulmonary thromboembolism. Anesth Analg 2:341–354. doi:10.1213/01.ane.0000068983.18131.f0
Menendez R, Nauffal D, Cremades MJ (1998) Prognostic factors in restoration of pulmonary flow after submassive pulmonary embolism: a multiple regression analysis. Eur Respir J 3:560–564. doi:10.1183/09031936.98.11030560
Levine M, Hirsh J, Weitz J, Cruickshank M, Neemeh J, Turpie AG, Gent M (1990) A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest 6:1473–1479. doi:10.1378/chest.98.6.1473
Smulders YM (2000) Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res 1:23–33. doi:10.1016/s0008-6363(00)00168-1
Siragusa S, Cosmi B, Piovella F, Hirsh J, Ginsberg JS (1996) Low-molecular-weight heparins and unfractionated heparin in the treatment of patients with acute venous thromboembolism: results of a meta-analysis. Am J Med 3:269–277. doi:10.1016/S0002-9343(97)89484-3
Miller RL, Das S, Anandarangam T, Leibowitz DW, Alderson PO, Thomashow B, Homma S (1998) Association between right ventricular function and perfusion abnormalities in hemodynamically stable patients with acute pulmonary embolism. Chest 3:665–670. doi:10.1378/chest.113.3.665
Smarz K, Zaborska B, Jaxa-Chamiec T, Maciejewski P, Budaj A (2012) Right ventricular dysfunction and exercise capacity after inferior (posterior) wall acute myocardial infarction. Am J Cardiol 6:784–789. doi:10.1016/j.amjcard.2012.05.007
Rodrigues AC, Cordovil A, Monaco C, Guimaraes L, Cury A, Naccarato GA, Lira-Filho E, Fischer CH, Vieira ML, Morhy S (2013) Right ventricular assessment by tissue-Doppler echocardiography in acute pulmonary embolism. Arq Bras Cardiol 6:524–530. doi:10.5935/abc.20130099
Hsiao SH, Lin SK, Wang WC, Yang SH, Gin PL, Liu CP (2006) Severe tricuspid regurgitation shows significant impact in the relationship among peak systolic tricuspid annular velocity, tricuspid annular plane systolic excursion, and right ventricular ejection fraction. J Am Soc Echocardiogr 7:902–910. doi:10.1016/j.echo.2006.01.014
Torrent-Guasp FF, Whimster WF, Redmann K (1997) A silicone rubber mould of the heart. Technol Health Care 1–2:13–20
Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk-Noordegraaf A (2010) Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson 35. doi:10.1186/1532-429X-12-35
Hsiao SH, Chang SM, Lee CY, Yang SH, Lin SK, Chiou KR (2006) Usefulness of tissue Doppler parameters for identifying pulmonary embolism in patients with signs of pulmonary hypertension. Am J Cardiol 5:685–690. doi:10.1016/j.amjcard.2006.03.053
McIntyre KM, Sasahara AA (1971) The hemodynamic response to pulmonary embolism in patients without prior cardiopulmonary disease. Am J Cardiol 3:288–294. doi:10.1016/0002-9149(71)90116-0
Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L (1997) Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J 3:479–487. doi:10.1177/875647939701300620
Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, Conti A, Agnelli G, Berni G (2000) Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 24:2817–2822. doi:10.1161/01.CIR.101.24.2817
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, A.C., Guimaraes, L., Guimaraes, J.F. et al. Relationship of clot burden and echocardiographic severity of right ventricular dysfunction after acute pulmonary embolism. Int J Cardiovasc Imaging 31, 509–515 (2015). https://doi.org/10.1007/s10554-014-0576-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-014-0576-8