Abstract
Osteoporosis and cardiovascular disease often coexist. Vertebral fractures incidentally imaged in the course of routine care might be able to contribute to the prediction of cardiovascular events. Following a case-cohort design, 5,679 patients undergoing chest CT were followed for a median duration of 4.4 years. Cases were defined as patients who subsequently developed a cardiovascular event (n = 493). The presence and severity of vertebral fractures, as well as aortic, coronary and valvular calcifications on CT were investigated. Cases were more likely to be male (69 vs 60 %) and older (66 vs 61 years old). Prevalent vertebral fractures conferred an elevated risk of cardiovascular events after adjustment for age and gender [hazard ratio (HR) of 1.28, 95 % confidence interval (CI) 1.07 to 1.54]. This effect remained moderate after correction for cardiovascular calcifications (HR 1.20, CI 0.99–1.44). However, in terms of discrimination, vertebral fractures did not have substantial incremental prognostic value after correction (C-index was 0.683 vs 0.682 for models with and without vertebral fractures respectively). Prevalent vertebral fractures on routine clinical chest CT are related to future cardiovascular events but do not have additional prognostic value to models that already include age, gender and cardiovascular calcifications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Symptomatic cardiovascular disease (CVD) and osteoporosis are major, preventable and growing health burdens [1, 2]. Calcifications in the coronary arteries, aorta and on the cardiac valves are strong predictors for cardiovascular events. As predictors they are independent of other traditional risk factors [3–5]. There is evidence linking osteoporotic osseous demineralization to arterial calcifications—sometimes called the calcification paradox—although the mechanism is poorly understood [6]. Overlapping risk factors including age, smoking, inactivity, estrogen deficiency and chronic inflammation have been suggested as possible explanations [7], along with shared endogenous calcium metabolism and bone regulatory molecules and pathways [8]. There is evidence that elevated levels of homocysteine and a deficiency in vitamin B12 contribute to osteoporosis [9–12] and to cardiovascular disease, particularly stroke [13, 14]. Furthermore injury to the cervical spine can contribute to (vertebrobasilar) stroke [15–17].
Both CVD and osteoporosis are best managed preventatively to avoid events such as stroke, myocardial infarction and hip fractures [18]. This approach that necessitates timely diagnosis. Interestingly statins, which are commonly prescribed for CVD prevention, may have a positive effect on bone mineralization [19] and prevent vertebral fractures [20]. Conversely, bisphosphonates, prescribed to slow bone demineralization, may inhibit arterial calcification [21].
The increasingly widespread use of computed tomography (CT) [22] in routine care is providing new avenues for early identification of patients at risk [23]. Opportunistic risk stratification could serve to complement established approaches. Using information contained in available diagnostic imaging (performed for other conditions) does not require additional health care resources and imposes no additional burden or risk on patients. Prevalent vertebral fractures incidentally detected on routine clinical CT may contribute to CVD risk stratification. Prevalent (often asymptomatic) vertebral fractures are a relatively common finding on CT [24–27] and have been associated with all-cause mortality [28]. Together with low bone mineral density, they are also associated with CVD and cardiovascular mortality, especially in populations such as diabetics or patients who recently suffered a coronary event [29–35]. Conversely, cardiovascular calcifications on CT have also been associated with osteoporosis and fractures [36–38]. It remains unknown whether vertebral fractures are independently predictive of future CVD in routine clinical populations after taking account of other, equally accessible, cardiovascular calcifications on CT imaging.
The aim of this study was to determine whether vertebral fractures were predictive of future cardiovascular events and to quantify the incremental prognostic value, if any, of vertebral fractures for predicting CVD events, after accounting for other established risk factors, in a cohort of patients who underwent routine chest CT.
Materials and methods
PROVIDI
The present research was conducted in the context of the (Prognostic Value of unrequested Information on Diagnostic Imaging) PROVIDI study. This multicenter study aims to establish the prognostic value of unrequested findings on chest CT in routine clinical care and was described in detail elsewhere [39]. Briefly, it includes adult patients of whom chest CTs were acquired in participating Dutch hospitals between 2002 and 2005 (Fig. 1). Patients with primary lung cancer or distant metastatic disease of other origin were excluded due to their a priori poor prognosis and the attendant low likelihood that unrequested findings would alter their management, but otherwise all patients were included. The institutional ethics committees of the University Medical Center Utrecht approved the PROVIDI study and waived the need for written informed consent (decision number 06/193).
Since vertebral fracture assessment requires multiplanar reconstruction and hence thin CT slices, only patients from three out of eight participating centers were included. The excluded hospitals did not routinely store the thin slices during the study period. The cohort for the present study consisted of 5,679 patients, 3,315 from two tertiary centers and 2,364 from one peripheral hospital (Fig. 1). Since the exclusion of the other five participating centers was not plausibly related to either the determinants nor the outcome (missing completely at random) no attempt was made to correct or adjust for the excluded centers.
Case-cohort study population
Following the case-cohort design, a random sample, i.e. (‘subcohort’), was taken from this study population (N = 1,151, 20 %) [31]. The case-cohort design reduces the cost of classic cohort design through the use of a subcohort. This is a completely random sample from the entire cohort at baseline and is usually a small fraction of the full cohort (typically below 10 % [46]). Cases identified during follow-up are added to the subcohort to form the case-cohort dataset. In this way all the available cases are included in the analysis. Since the number of cases is almost universally the group limiting the precision of the results (i.e. they are the smallest group) it is desirable to include them all. Excluding a portion of the non-cases has little impact on the precision since the group of non-cases in a cohort is so much larger than the group of cases. Since the subcohort is randomly sampled the majority of non-cases not included in the subcohort are missing completely at random. Excluding them in this way does not bias the results (since it is random) and since there are still more non-cases than cases it has a neglible impact on the precision of the results. Expensive and time consuming covariates, such as vertebral fracture and cardiovascular calcification assessment thus only need to be determined in the subcohort and the additional cases identified during follow-up. Cases were defined as all patients from the full study population who experienced a cardiovascular event during follow-up.
Fatal and non-fatal CVD events were obtained through linkage of subjects with the Dutch National Death Registry and the National Registry of Hospital Discharge Diagnoses from baseline to January 2008. Database linkage was performed with a validated probabilistic method [40]. In these national databases, cause of death and the indications for hospitalization are coded by physicians according to the International Classification of Diseases [41, 42]. All events were classified using the 9th (discharge diagnoses) and 10th (cause of death) revision of the International Classification of Diseases. Hypertensive disease (codes 401–405), ischemic heart disease (codes 410–414), heart failure (code 428), peripheral vascular (codes 440–448), cerebrovascular disease (codes 430–438), or other heart disease (code 429) were included as cardiovascular events. Cardiovascular death prevailed over hospital admissions, so that when a cardiovascular cause of death was listed, that endpoint code and failure time was used. In cases of multiple valid hospital admissions the first to occur was used. When study subjects had more than one chest CT during follow-up, only the first eligible CT was evaluated. CTs were made with a range of different systems from different vendors and were stored at a maximum slice thickness of 3-mm.
The full case cohort dataset consisted of the randomly sampled subcohort and additional cardiovascular cases outside the subcohort.
Vertebral measurements
Chest CT scans were scored for vertebral fractures. The reader was blinded for baseline patient characteristics and outcome status. CT scoring was performed at a research workstation (iX Viewer; Image Sciences Institute). Semiquantitative Vertebral Fracture Assessment (VFA) similar to that widely applied elsewhere [43] was used to identify and classify vertebral fractures. This method identifies fractures according to the height loss of the vertebral body (viewed sagittally), with adjacent normal unfractured vertebrae providing comparison here. The vertebrae were assessed on sagittal reconstructions around the mid-sagittal point, with the rater free to adjust the window level, orientation and slice thickness of the reconstruction as desired. This method has been shown to be reliable for the identification of fractures and their severity [44]. The three fracture grades are: mild grade 1 fractures (height loss 20–25 %), moderate grade 2 fractures (25–40 %) and severe grade 3 fractures with height loss of more than 40 % (Fig. 2). Deformities that seemed non-fractural in origin (e.g. Schmorl’s nodes, strongly scoliotic deformity or congenital anomalies) were not counted as fractures (Fig. 2).
The presence of fracture was scored yes if there was a fracture visible. The worst fracture grade was defined as the grade of the worst fracture visible (either none, mild, moderate, or severe).
Vascular calcification measurements
CT scoring was performed at a research workstation (iX Viewer; Image Sciences Institute). The reader was blinded for patient characteristics and outcome status. Coronary artery calcifications, aortic wall calcifications, mitral valve or annulus calcifications and aortic valve calcifications were scored using a simple visual grading system [5] that has been shown to be reliable on CT in a routine setting [45]. Briefly, calcifications in the four main coronary arteries were categorized as; none, mild [1–2 focal (limited to ≤2 slices) calcifications], moderate (>2 focal calcifications or a single calcification extending for >2 slices) or severe (fully calcified coronary arteries extending over multiple segments). These were then summed (scores of 0–12).
Similarly, the number and size of calcifications in the wall of the descending aorta and ascending aorta were graded as: absent; grade 1, mild (≤3 focal calcifications); grade 2, moderate (4–5 focal calcifications or 1 calcification extending for ≥3 slices) and grade 3, severe (>5 focal calcifications or >1 calcification extending for ≥3 slices). Supra-aortic calcifications were scored as absent, present in one of the three supra-aortic arteries, or present in multiple arteries. Aortic calcifications were then also summed (0–8). Mitral valve calcifications were graded as follows: grade 0, absent; grade 1 single linear calcification; grade 2, two leaflets involved [3]. Aortic valve calcifications were categorized as absent, a single spot, a single line, linear calcifications on 2 cusps and linear calcifications on all 3 cusps (Fig. 3).
Statistical analyses
Association of vertebral fractures with future cardiovascular events
A case-cohort appropriate cox modeling was used to assess the association between prevalent vertebral fractures and future cardiovascular events [46, 47]. As discussed above, the case cohort dataset (random subcohort plus all cases outside the subcohort) is not biased and has a comparable precision to a full-cohort analysis, at a fraction of the cost. However since cases are overrepresented in the dataset (by a factor inversely proportional to the sampling fraction of the subcohort), special adjustment is required to correct for this in the analysis. To this end approaches involving weighting of the different kinds of subjects in the case-cohort dataset (non-cases randomly selected for the sub-cohort, cases randomly selected for the sub-cohort and cases added after they had been identified during follow-up) have been developed and extensively validated. These have been shown to yield reliable results and standard errors, comparable to those in a full-cohort analysis. Essentially the weighting approach uses the subcohort to estimate the distribution of the covariates in the full cohort (as if it had been measured) and then to calculate the resulting baseline hazard. The cases outside the subcohort (those identified after follow-up) are then included in the model to calculate the relevant hazard ratios (without contributing to the baseline hazard function). After crude associations were estimated in univariate cox models, the variables age and gender were then added to generate a basically adjusted model. To investigate whether the relation between prevalent vertebral fractures and future cardiovascular events was explained by cardiovascular calcifications we added these to the cox model on top age and gender for the fully adjusted model.
Added prognostic value of vertebral fractures for cardiovascular events
Finally, in addition to examining the predictive effect of vertebral fractures, the added prognostic value of vertebral fractures, on top of age, gender and cardiovascular calcifications was assessed. These other findings are easily assessable on thoracic CT and are known to be strongly predictive of cardiovascular disease. This would quantify any added value of vertebral fractures would have for (unrequested) cardiovascular risk stratification if they are additionally assessed on a thoracic CT. To assess whole-model discrimination rather than single variables within the model, a survival-appropriate concordance statistic (C-statistic) was computed for each model that was adjusted based on the performance of the model in 100 bootstrap replicates [47]. Comparing the discriminative model performance with and without vertebral fracture status gives an indication of its incremental value when distinguishing patients at a high risk of CVD from those at a lower risk of CVD. The contribution of vertebral fracture assessment was further quantified using the adjusted wald Chi squared statistic for the analysis of deviance of each vertebral fracture term (fracture presence and worst fracture grade) and their associated p value [48].
Results
Study population and CT findings
During a median follow-up of 4.4 (interquartile range 3.6–5.0) years 493 (8.6 %) patients suffered a cardiovascular event. These events included 134 myocardial infarctions, 70 strokes and 289 other events. Cases were more likely to be male (69 vs 61 %) and older (66 vs 62 years old), compared to the subcohort. Cases had more vertebral fractures (39 vs 32 %; Table 1) and a higher burden of coronary, aortic and cardiac valve calcifications.
Association of vertebral fractures with future cardiovascular events
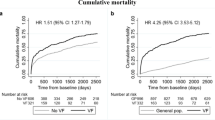
Having at least one vertebral fracture was predictive of future cardiovascular events (HR 1.37, CI 1.15–1.64). After correction for age and gender this effect weakened, but remained statistically significant (HR 1.28, 1.07–1.54). After further correction for vascular imaging findings the predictive effect of vertebral fractures was slightly reduced again (HR 1.20, 0.99–1.44; Table 2) so that it was borderline significant. Appropriately the Chi squared associated p value for fracture presence was marginally significant in the fully adjusted model (p = 0.078).
A similar pattern was seen for worst fracture grade. The HRs associated with the different grades of vertebral fracture diminished somewhat after correction for age and gender, from 1.94 (1.29–2.91) for severe fractures to 1.58 (1.05–2.39). The HR was slightly lower after additional correction for vascular calcifications, to 1.29 (0.85–1.97) (Table 2).
Incremental prognostic value of vertebral fractures for cardiovascular events
In terms of model discrimination, the C-statistic values associated with the progressively more elaborate models also repeated this pattern. When fracture presence was added to a model already containing age, gender and cardiovascular calcifications, the bootstrap corrected C-statistic was only marginally affected, increasing from 0.68 (0.66–0.71) to 0.68 (0.66–0.71, Table 3).
Discussion
We found that prevalent vertebral fractures on routine clinical CT are associated with future cardiovascular events with an adjusted HR of 1.20 (0.99–1.44). This borderline significant result suggests that vertebral fractures are moderately predictive of future cardiovascular events independently of cardiovascular calcifications on CT. However, in terms of model discrimination, the incremental prognostic value of adding vertebral fractures to a model already containing age, gender and cardiovascular calcifications was modest. This suggests that vertebral fractures would not be a useful predictor of cardiovascular events in settings where cardiovascular calcifications may also be assessed.
Epidemiological publications on the association between osteoporosis, fractures and cardiovascular calcifications have yielded conflicting results. While some studies found clear associations [29, 49], others did not [50–52]. Physiologically, nature of the mechanism linking arterial calcification to osseous fragility remains unclear, despite the existence of a large body of basic science in this field [9–17]. There are suggestions that hyperlipidemia may play a role in osteoporosis [19, 53, 54] and there are also indications that overlapping bone mineralization signaling pathways may be deregulated in both disease clusters [55]. Estrogen deficiency and possibly inflammation and homocysteine have also been suggested [56]. How these factors interplay to cause increased bone formation in arteries and decreased mineralization in the skeleton remains fundamentally unanswered but this study helps to shed some light on the possible prognostic implications of this link. Bone structures, particularly the spine, are visualized on every cardiac CT and on all routine chest CTs, along with cardiovascular calcifications. As such assessing the spine yields gratis extra information with potential prognostic information for both osteoporosis and cardiovascular disease.
Whilst this study provides further evidence of an association between increased arterial calcification both in the aorta, coronary arteries and heart valves and vertebral fractures that is independent of age and gender, it also indicates that the effect is likely to be modest, at least amongst a routine clinical population. Furthermore, our data suggest that vertebral fractures have at most a marginal incremental prognostic value on top of cardiovascular calcifications. As cardiovascular calcifications are also readily accessible on CT, there seems to be little prognostic gain in additionally assessing vertebral fractures when seeking to predict cardiovascular events. Further research on common pathophysiological mechanisms may in future identify new treatment targets and inform management strategies for both disease clusters.
Whilst the association we observed is statistically significant in this relatively large cohort, the results observed in this study are essentially negative in that they found at most a marginal added value of vertebral fractures in prognostic terms. The strength of this study is the large number of events providing us enough power to examine the independent prognostic value of vertebral fractures for CVD outcomes. By using a routine clinical population, rather than a more specialized and less generalizable study population, the results of this study are more likely to reflect the associations that may be expected in routine clinical care. The marginal gain in prognostic performance observed here thus indicates that systematic assessment of vertebral fractures is unlikely to contribute towards cardiovascular risk stratification in a general clinical setting.
Limitations
A limitation is that our study was unable to correlate the observed vertebral fractures to DXA-defined bone mineral density. DXA is currently still the preferred method for assessing osteoporosis. However, vertebral fractures and bone mineral density measured by DXA are strongly correlated [57]. This suggests that bone mineral density would probably show a similar association to cardiovascular events. Thirdly, as chest CT was used, there was no information on cervical and mid and lower lumbar fractures. This may have caused an underreporting of vertebral fractures in this cohort and the prognostic effect of vertebral fractures may be in fact be larger if the whole spine was included. However, in the clinical setting of dedicated cardiac CT or routine or screening chest CT the lumber spine will also not be visualized and our findings can be expected to apply.
In conclusion, prevalent vertebral fractures have moderate predictive power for future cardiovascular events, after adjustment for age, gender and cardiovascular calcifications also visible on thoracic CT, in a routine clinical population. The incremental improvement offered by vertebral fractures here to the predictive power of cardiovascular prediction models is slight.
References
Alwan A, World Health Organization (2011) Global status report on noncommunicable diseases 2010. World Health Organization, Geneva
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018. doi:10.1016/S0140-6736(06)68891-0
Gondrie MJA, van der Graaf Y, Jacobs PC et al (2011) The association of incidentally detected heart valve calcification with future cardiovascular events. Eur Radiol 21:963–973. doi:10.1007/s00330-010-1995-0
Gondrie MJA, Mali WPTM, Jacobs PC et al (2010) Cardiovascular disease: prediction with ancillary aortic findings on chest CT scans in routine practice. Radiology 257:549–559. doi:10.1148/radiol.10100054
Jacobs PC, Gondrie MJ, Mali WP et al (2011) Unrequested information from routine diagnostic chest CT predicts future cardiovascular events. Eur Radiol 21:1577–1585. doi:10.1007/s00330-011-2112-8
Persy V, D’Haese P (2009) Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 15:405–416. doi:10.1016/j.molmed.2009.07.001
Doherty TM, Fitzpatrick LA, Inoue D et al (2004) Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev 25:629–672. doi:10.1210/er.2003-0015
Lampropoulos CE, Papaioannou I, D’Cruz DP (2012) Osteoporosis–a risk factor for cardiovascular disease? Nat Rev Rheumatol 8:587–598. doi:10.1038/nrrheum.2012.120
Enneman AW, Swart KMA, Zillikens MC et al (2014) The association between plasma homocysteine levels and bone quality and bone mineral density parameters in older persons. Bone 63:141–146. doi:10.1016/j.bone.2014.03.002
Zhang H, Tao X, Wu J (2014) Association of homocysteine, vitamin B12, and folate with bone mineral density in postmenopausal women: a meta-analysis. Arch Gynecol Obstet 289:1003–1009. doi:10.1007/s00404-013-3075-6
McLean RR, Jacques PF, Selhub J et al (2008) Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J Clin Endocrinol Metab 93:2206–2212. doi:10.1210/jc.2007-2710
McLean RR, Jacques PF, Selhub J et al (2004) Homocysteine as a predictive factor for hip fracture in older persons. N Engl J Med 350:2042–2049. doi:10.1056/NEJMoa032739
Spence JD (2007) Homocysteine-lowering therapy: a role in stroke prevention? Lancet Neurol 6:830–838. doi:10.1016/S1474-4422(07)70219-3
Spence JD (2013) B vitamin therapy for homocysteine: renal function and vitamin B12 determine cardiovascular outcomes. Clin Chem Lab Med CCLM FESCC 51:633–637. doi:10.1515/cclm-2012-0465
Smith WS, Johnston SC, Skalabrin EJ et al (2003) Spinal manipulative therapy is an independent risk factor for vertebral artery dissection. Neurology 60:1424–1428
Beaudry M, Spence JD (2003) Motor vehicle accidents: the most common cause of traumatic vertebrobasilar ischemia. Can J Neurol Sci J Can Sci Neurol 30:320–325
Miley ML, Wellik KE, Wingerchuk DM, Demaerschalk BM (2008) Does cervical manipulative therapy cause vertebral artery dissection and stroke? Neurologist 14:66–73. doi:10.1097/NRL.0b013e318164e53d
Krumholz HM, Weintraub WS, Bradford WD et al (2002) Task force #2–the cost of prevention: can we afford it? Can we afford not to do it? 33rd Bethesda Conference. J Am Coll Cardiol 40:603–615
Uzzan B, Cohen R, Nicolas P et al (2007) Effects of statins on bone mineral density: a meta-analysis of clinical studies. Bone 40:1581–1587. doi:10.1016/j.bone.2007.02.019
Schoofs MWCJ, Sturkenboom MCJM, van der Klift M et al (2004) HMG-CoA reductase inhibitors and the risk of vertebral fracture. J Bone Miner Res 19:1525–1530. doi:10.1359/JBMR.040607
Santos LL, Cavalcanti TB, Bandeira FA (2012) Vascular effects of bisphosphonates-a systematic review. Clin Med Insights Endocrinol Diabetes 5:47–54. doi:10.4137/CMED.S10007
Smith-Bindman R, Miglioretti DL, Johnson E et al (2012) Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA 307:2400–2409. doi:10.1001/jama.2012.5960
Mets OM, de Jong PA, Prokop M (2012) Computed tomographic screening for lung cancer: an opportunity to evaluate other diseases. JAMA 308:1433–1434. doi:10.1001/jama.2012.12656
Bartalena T, Giannelli G, Rinaldi MF et al (2009) Prevalence of thoracolumbar vertebral fractures on multidetector CT: underreporting by radiologists. Eur J Radiol 69:555–559. doi:10.1016/j.ejrad.2007.11.036
Woo EK, Mansoubi H, Alyas F (2008) Incidental vertebral fractures on multidetector CT images of the chest: prevalence and recognition. Clin Radiol 63:160–164. doi:10.1016/j.crad.2007.01.031
Williams AL, Al-Busaidi A, Sparrow PJ et al (2009) Under-reporting of osteoporotic vertebral fractures on computed tomography. Eur J Radiol 69:179–183. doi:10.1016/j.ejrad.2007.08.028
Müller D, Bauer JS, Zeile M et al (2008) Significance of sagittal reformations in routine thoracic and abdominal multislice CT studies for detecting osteoporotic fractures and other spine abnormalities. Eur Radiol 18:1696–1702. doi:10.1007/s00330-008-0920-2
Hasserius R, Karlsson MK, Nilsson BE et al (2003) Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 14:61–68. doi:10.1007/s00198-002-1316-9
Bandeira E, Neves AP, Costa C, Bandeira F (2012) Association between vascular calcification and osteoporosis in men with type 2 diabetes. J Clin Densitom Off J Int Soc Clin Densitom 15:55–60. doi:10.1016/j.jocd.2011.07.002
Silva HC, Pinheiro MM, Genaro PS et al (2013) Higher prevalence of morphometric vertebral fractures in patients with recent coronary events independently of BMD measurements. Bone 52:562–567. doi:10.1016/j.bone.2012.11.004
Mazziotti G, Baracca M, Doga M et al (2012) Prevalence of thoracic vertebral fractures in hospitalized elderly patients with heart failure. Eur J Endocrinol Eur Fed Endocr Soc 167:865–872. doi:10.1530/EJE-12-0566
Lai S-W, Liao K-F, Lai H-C et al (2013) Risk of major osteoporotic fracture after cardiovascular disease: a population-based cohort study in Taiwan. J Epidemiol Jpn Epidemiol Assoc 23:109–114
Chung DJ, Choi HJ, Chung Y-S et al (2013) The prevalence and risk factors of vertebral fractures in Korean patients with type 2 diabetes. J Bone Miner Metab 31:161–168. doi:10.1007/s00774-012-0398-5
Den Uyl D, Nurmohamed MT, van Tuyl LH et al (2011) (Sub)clinical cardiovascular disease is associated with increased bone loss and fracture risk; a systematic review of the association between cardiovascular disease and osteoporosis. Arthritis Res Ther 13:R5. doi:10.1186/ar3224
Von der Recke P, Hansen MA, Hassager C (1999) The association between low bone mass at the menopause and cardiovascular mortality. Am J Med 106:273–278
Schulz E, Arfai K, Liu X et al (2004) Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89:4246–4253. doi:10.1210/jc.2003-030964
Szulc P, Kiel DP, Delmas PD (2008) Calcifications in the abdominal aorta predict fractures in men: MINOS study. J Bone Miner Res 23:95–102. doi:10.1359/jbmr.070903
Naves M, Rodríguez-García M, Díaz-López JB et al (2008) Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 19:1161–1166. doi:10.1007/s00198-007-0539-1
Gondrie MJA, Mali WPTM, Buckens CFM et al (2010) The PROgnostic Value of unrequested Information in Diagnostic Imaging (PROVIDI) Study: rationale and design. Eur J Epidemiol 25:751–758. doi:10.1007/s10654-010-9514-9
De Bruin A, Karduan J, Gast F et al (2004) Record linkage of hospital discharge register with population register: experiences at Statistics Netherlands. Stat J UN Econ Comm Eur 1:23–32
World Health Organization (2011) International statistical classification of diseases and related health problems. World Health Organization, Geneva
American Medical Association, Medicode (Firm) (1998) ICD-9-CM: international classification of diseases, 9th revision, clinical modification, volumes 1 and 2, color-coded, illustrated, 1999. American Medical Association, Dover
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. doi:10.1002/jbmr.5650080915
Buckens CF, de Jong PA, Mol C et al (2013) Intra and Interobserver Reliability and Agreement of Semiquantitative Vertebral Fracture Assessment on Chest Computed Tomography. PLoS ONE 8:e71204. doi:10.1371/journal.pone.0071204
Jacobs PCA, Isgum I, Gondrie MJA et al (2010) Coronary artery calcification scoring in low-dose ungated CT screening for lung cancer: interscan agreement. AJR Am J Roentgenol 194:1244–1249. doi:10.2214/AJR.09.3047
Onland-Moret NC, van der A DL, van der Schouw YT, et al. (2007) Analysis of case-cohort data: a comparison of different methods. J Clin Epidemiol 60:350–355. doi:10.1016/j.jclinepi.2006.06.022
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361:AID-SIM168>3.0.CO;2-4
Graubard BI, Korn EL (1993) Hypothesis Testing with Complex Survey Data: the Use of Classical Quadratic Test Statistics with Particular Reference to Regression Problems. J Am Stat Assoc 88:629–641. doi:10.1080/01621459.1993.10476316
Kammerer CM, Dualan AA, Samollow PB et al (2004) Bone mineral density, carotid artery intimal medial thickness, and the vitamin D receptor BsmI polymorphism in Mexican American women. Calcif Tissue Int 75:292–298. doi:10.1007/s00223-004-0215-9
Samelson EJ, Cupples LA, Broe KE et al (2007) Vascular calcification in middle age and long-term risk of hip fracture: the Framingham Study. J Bone Miner Res 22:1449–1454. doi:10.1359/jbmr.070519
Sinnott B, Syed I, Sevrukov A, Barengolts E (2006) Coronary calcification and osteoporosis in men and postmenopausal women are independent processes associated with aging. Calcif Tissue Int 78:195–202. doi:10.1007/s00223-005-0244-z
Mussolino ME, Madans JH, Gillum RF (2003) Bone mineral density and stroke. Stroke J Cereb Circ 34:e20–e22. doi:10.1161/01.STR.0000065826.23815.A5
Parhami F (2003) Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids 68:373–378
Danilevicius CF, Lopes JB, Pereira RMR (2007) Bone metabolism and vascular calcification. Braz J Med Biol Res Rev Bras Pesqui Médicas E Biológicas Soc Bras Biofísica Al 40:435–442
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292:490–495. doi:10.1001/jama.292.4.490
Koshiyama H, Ogawa Y, Tanaka K, Tanaka I (2006) The unified hypothesis of interactions among the bone, adipose and vascular systems: “osteo-lipo-vascular interactions”. Med Hypotheses 66:960–963. doi:10.1016/j.mehy.2005.11.024
Pickhardt PJ, Pooler BD, Lauder T et al (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588–595. doi:10.7326/0003-4819-158-8-201304160-00003
Acknowledgments
The PROVIDI Study Group consists of J. Laméris (Department of Radiology, Academic Medical Center, Amsterdam), C. van Kuijk (Department of Radiology, VU University Medical Center Amsterdam), W. ten Hove (Department of Radiology, Gelre Hospitals, Apeldoorn), M. Oudkerk (Department of Radiology, University Medical Center Groningen), Ay L. Oen (Department of Radiology, Elkerliek Hospital, Helmond), J. Wildberger (Department of Radiology, Academic Hospital Maastricht), J. van Heesewijk (Department of Radiology, St Antonius Hospital, Nieuwegein), W. Mali (Department of Radiology, University Medical Center Utrecht), and Y. van der Graaf (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht). This study was funded by a program Grant from the Netherlands Organization for Scientific Research-Medical Sciences (NWO-MW); Grant 40-00812-98-07-005. The funder had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Substudies from the PROVIDI cohort have been previously published, including substudies using cardiovascular endpoints. These patient groups partly overlap with the current manuscript. The current manuscript includes a different subset of patients from the previous studies and investigates a different set of covariates, most notably vertebral fractures.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Buckens, C.F., de Jong, P.A., Verkooijen, H.M. et al. Vertebral fractures on routine chest computed tomography: relation with arterial calcifications and future cardiovascular events. Int J Cardiovasc Imaging 31, 437–445 (2015). https://doi.org/10.1007/s10554-014-0567-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-014-0567-9