Abstract
Dilated inferior vena cava (IVC) is prevalent among patients with heart failure (HF), but whether its presence predicts worsening renal function (WRF) or adverse outcomes is unclear. This cohort study analyzed patients with left ventricular ejection fraction <40 % and repeated hospitalizations (≥2 times) for HF between August 2009 and August 2011. The study endpoints were death and HF re-hospitalization. Among baseline parameters, IVC diameter was the most powerful predictor for the development of WRF (area under the curve = 0.795, cut-off value = 20.5 mm). During the 2-year follow-up, 36 patients (49 %) were re-hospitalized for HF and 14 patients (19 %) died. The event rates were significantly greater in the WRF group than in the non-WRF group (71 vs. 30 %, P < 0.001 for HF re-hospitalization; 29 vs. 10 %, P = 0.03 for death). In Cox regression model, the risk of combined end-points was increased in patients with aging, elevated blood urine nitrogen, IVC >21 mm, and WRF. When adjusted for confounding factors, IVC >21 mm [hazard ratio (HR) 3.73, 95 % confidence interval (CI) 1.66–8.34] and WRF (HR 2.68, 95 % CI 1.07–6.75) were significant predictors for adverse outcomes. In patients with advanced decompensated HF, dilated IVC (>21 mm) predicted the development of WRF and could be a predictor for adverse outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired renal function is highly prevalent among patients with heart failure (HF). Moreover, the coexistence of renal and cardiac dysfunction in the same patient, known as cardiorenal syndrome, has an extremely poor prognosis [1, 2]. For patients with chronic abnormalities in cardiac function (e.g., chronic HF), hypoperfusion alone cannot explain renal function decline in this setting. The presence of systemic venous congestion has been considered to be one of the mechanisms for the cardiorenal syndrome [3]. Systemic venous congestion may worsen renal function from the implication of experimental animal data [4, 5] and the evaluation study of congestive HF and pulmonary catheterization effectiveness (ESCAPE) trial [6]. Systemic venous congestion is highly prevalent among patients with advanced HF, but whether its presence predicts worsening renal function (WRF) or adverse outcomes is unclear. Echocardiography allows non-invasive evaluation of systemic venous congestion by measuring the size and collapsibility of the inferior vena cava (IVC) [7]. The aims of this study were to evaluate the relation between dilated IVC and WRF, and the prognostic significance of dilated IVC and WRF in patients with advanced decompensated HF.
Methods
Patient population
This cohort study analyzed consecutive patients aged 18 years or older with repeated hospitalizations (≥2 times) for decompensated HF, who visited our emergency room between August 2009 and August 2011. Patients were included if they had left ventricular (LV) ejection fraction (EF) <40 % and had echocardiography within 24 h after going to the emergency room to decrease the effects of intravenous diuretics that may interfere the measurements of the IVC size and collapsibility. Exclusion criteria included mechanical ventilation, end-stage renal disease under renal replacement therapy, intravenous inotropic support, congenital heart disease, prior valvular cardiac surgery, or poor echocardiographic image quality. The ischemic etiology of HF was defined by one of the following criteria: (1) significant epicardial coronary artery stenosis (≥50 %); or (2) history of myocardial infarction or coronary revascularization. The study conforms with the principles outlined in the Declaration of Helsinki. The research protocol was approved by the local Institutional Review Board.
Two-dimensional echocardiography
Conventional two-dimensional echocardiography was performed using commercially available equipment (Vivid 7, General Electric Vingmed Ultrasound, Horten, Norway) with a 2.5-MHz transducer. LV EF was determined by the biplane Simpson’s method. According to the guidelines for the echocardiographic assessment of the right heart in adults, (1) dilated IVC is defined if diameter >2.1 cm; (2) the estimated right atrial (RA) pressure is 3 mmHg if an IVC diameter is ≤2.1 cm and collapse is >50 %; RA pressure is 15 mmHg if an IVC diameter is >2.1 cm and collapse is <50 %; RA pressure is 8 mmHg if the IVC diameter and collapsibility do not fit the above paradigm, respectively [7]. Peak velocity of tricuspid regurgitation (TR) was recorded by continuous wave Doppler and TR was graded qualitatively using the Framingham Heart Study criteria: mild if regurgitant jet area/RA area was <20 %, moderate if 20–40 %, or severe if >40 % [8]. The pressure gradient (PG) between right ventricle and right atrium was calculated by using the simplified Bernoulli equation [9, 10].
WRF
Serum creatinine in the emergency room was recorded. Glomerular filtration rate (GFR) was estimated using the four-variable modification of diet in renal disease study equation [11]. The development of WRF was defined as a rise in serum creatinine of >0.3 mg/dl, similar to prior studies [12–14]. The subjects were divided to whom developed WRF during hospitalization versus those who did not.
Study endpoints
The intermediate end-point was defined as the occurrence of WRF during hospitalization. The final end-point was the combined death and re-hospitalization during the 2-year follow-up. Re-hospitalization was defined as an unplanned overnight stay in our hospital because of progression of HF or as a direct result of HF. Patients had to have typical symptoms and signs of HF, using standard criteria. All events were evaluated and adjudicated by two independent observers.
Statistical analysis
Data are presented as mean ± standard deviation or as a count (percentage). Statistical analysis was performed using the Statistical Package for Social Sciences statistical software, version 19 for Windows. Chi square test for categorical variables and two-sample t test for continuous variables were used for comparisons between patients who developed WRF and those who did not. Receiver operating characteristic (ROC) curves were generated to determine the predictive ability of clinical and echocardiographic parameters for WRF. Stepwise logistic regression analysis was used to evaluate the independent effect of our aimed variables for WRF. Cox proportional model was used to evaluate the time-to-event associations with death and re-hospitalization for HF during the 2-year follow-up. Kaplan–Meier curves were plotted, and the log-rank test used to compare between groups. For all analysis, a P value <0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics for the study patients are shown in Table 1. The mean age was 63.6 ± 16.2 years and approximately three fourths of the patients were male. No significant differences were found in age, sex, systolic blood pressure, heart rate, ischemic etiology, risk factors of coronary artery disease, atrial fibrillation, biochemistry data (serum creatinine, estimated glomerular filtration rate, hemoglobin, serum sodium), and medications for HF between patients with WRF and patients without WRF. The values of blood urine nitrogen (BUN) and brain natriuretic peptide (BNP) were significantly higher in patients with WRF than in those without WRF. Thirty-six patients (49 %) were re-hospitalized for decompensated HF and 14 patients (19 %) died during the follow-up period. The clinical event rate over 2 years was significantly greater in the WRF group than in the non-WRF group (71 vs. 30 %, P < 0.001 for re-hospitalization for worsening HF; 29 vs. 10 %, P = 0.03 for all-cause mortality).
Echocardiographic parameters
There were significant differences in LV EF, LV end-systolic volume (ESV), RA pressure, TR PG, IVC diameter, IVC >21 mm, and TR severity ≥moderate degree between patients with and without WRF (Table 2). In the logistic-regression model, dilated IVC was associated with WRF [odds ratio (OR) 1.37, 95 % confidence interval (CI) 1.18–1.59, P < 0.01], and IVC diameter remained significant after adjustment for the BUN, LV EF and RA pressure, or adjustment for the BNP, LV ESV and TR PG (Table 3). In the ROC curve analysis for the prediction of WRF, several indices including LV EF, LV ESV, BUN, BNP, RA pressure, TR PG, and IVC diameter were analysed. Among these factors, IVC diameter was the most powerful predictor for the development of WRF (area under the ROC curve = 0.795, cut-off value = 20.5 mm, 79 % sensitivity, 82 % specificity) (Fig. 1).
Receiver operating characteristic curve analysis for the prediction of WRF including: a IVC diameter, right atrial pressure (RAP), and tricuspid regurgitant pressure gradient (TR PG) and b BUN, BNP, LVESV, and EF. A, area under the ROC curve. *All parameters had P values <0.05, except for EF (P = 0.05)
Clinical events and event-free survival
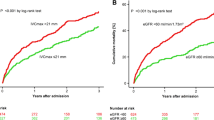
In Cox regression model, the risk of combined end-points including death and re-hospitalization for HF was increased in patients with aging, elevated BUN, IVC >21 mm, and WRF. When adjusted for age, BUN, and BNP, IVC >21 mm (HR 3.73, 95 % CI 1.66–8.34) and WRF (HR 2.68, 95 % CI 1.07–6.75) were significant predictors for adverse events (Table 4). Figure 2 shows the Kaplan–Meier event-free survival curves for patients with or without IVC dilatation and WRF.
Discussion
Our study shows that in patients with advanced decompensated HF, WRF during hospitalization is common and is associated with adverse outcomes. Dilated IVC identifies patients who may develop WRF during hospitalization, and is associated with increased risk of adverse events during 2-year follow up.
The mechanisms responsible for WRF in patients with HF are complex and not well-defined. Hemodynamic abnormalities, such as hypotension or low cardiac output, might be expected to play an important role. In addition, there is increasing evidence to support the role of systemic venous congestion in the development of WRF in patients with advanced decompensated HF [3]. In patients with advanced HF, LV systolic dysfunction causes increased left atrial pressure. The pressure is transmitted back through the pulmonary circulation to cause pulmonary arterial hypertension. The pulmonary artery hypertension can worsen pre-existing right ventricular dysfunction and exacerbate TR, leading to systemic venous congestion [15]. If venous congestion and elevated central venous pressure are the hallmarks of HF, then distention of the IVC by echocardiography may be a good prognostic marker in patients with decompensated HF.
Increased BUN and natriuretic peptide have been associated with WRF and poor outcome in patients hospitalized for HF [16–20]. Increased urea reabsorption by proximal tubules or collecting ducts as a result of angiotensin II or vasopressin increase has been showed in HF patients with worsening symptoms and increased central venous pressure [4, 21–23]. Plasma BNP rises in various pathologic states, particular where there is increased cardiac wall stretch, an expanded fluid volume [24, 25] or reduced clearance [26]. Compatible with previous studies, the present study demonstrated that HF patients with WRF had higher BUN and BNP levels at hospital admission than those without WRF. The association between dilated IVC and WRF remains significant after adjustment for BUN, BNP or TR PG. These results implicate that HF patients with WRF had marked venous congestion. Actually, venous congestion had been demonstrated to be correlated with impaired renal function [6, 27–30]. Our findings confirm and support the venous congestion might be an important determinant of WRF in patients with HF. Moreover, our findings also contribute to the growing evidence that dilated IVC could be a marker of adverse outcomes in patients with decompensated HF [31]. The present study also indicated WRF was a significant prognostic factor for adverse events compatible with the results of a large body of literature [32].
Noninvasive measurement of the diameter of the IVC and the change in diameter with respiration by echocardiography has demonstrated fair to excellent correlation with RAP [33–38]. Instead of invasive nature of catheterization and complications such as pneumothorax, air embolism, or injury of great vessels [39, 40], when echocardiography is available, IVC diameter might provide similar information in HF patients. In addition, a rapid assessment of IVC physiology could be performed at the bedside by a non-cardiologist in the emergency department [41, 42].
Apart from the intrinsic limitations associated with a retrospective study and thereby possibly subject to selection bias, the other limitations were this work being a single-centre study and a small sample size. However, we applied rigorous inclusion criteria to ensure that our model is as valid as possible. Although we utilized IVC diameter >21 mm to define IVC dilatation according to the literature [7], the cut-off value of IVC diameter derived from the ROC analysis for predicting WRF was close to the guideline criteria for a dilated IVC. We also had attempt to avoid the influence of the use of medications influencing renal function; therefore, to limit the timing of echocardiography within 24 h, but we did not know how far the influence we could really avoid. In this study, we did not exclude patients with atrial fibrillation, prior cardiac surgery or nitrate use that may potentially affect the IVC diameter. However, they are frequently observed in patients with HF and the inclusion of these patients yields a realistic view of what is observed in daily clinical practice. Additional studies with the exclusion of these confounding factors could provide more specific information in this regard. Although we assessed EF and systolic blood pressure in our study, blood flow to the kidneys could not be assessed directly, and thus we cannot exclude reduced renal blood flow as an additional major contributor to impaired renal function.
Conclusions
In this cohort study, WRF was commonly observed and was associated with adverse outcomes. Dilated IVC predicted the development of WRF and provides similar prognostic information as WRF.
References
Heywood JT (2004) The cardiorenal syndrome: lessons from the ADHERE database and treatment options. Heart Fail Rev 9(3):195–201
McAlister FA et al (2004) Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation 109(8):1004–1009
Ronco C et al (2008) Cardiorenal syndrome. J Am Coll Cardiol 52(19):1527–1539
Firth JD et al (1988) Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet 1(8593):1033–1035
Winton FR (1931) The influence of venous pressure on the isolated mammalian kidney. J Physiol 72(1):49–61
Nohria A et al (2008) Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol 51(13):1268–1274
Rudski LG et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713 (quiz 86–88)
Singh JP et al (1999) Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 83(6):897–902
Yock PG et al (1984) Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation 70(4):657–662
Currie PJ et al (1985) Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol 6(4):750–756
Levey AS et al (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4):247–254
Krumholz HM et al (2000) Correlates and impact on outcomes of worsening renal function in patients > or =65 years of age with heart failure. Am J Cardiol 85(9):1110–1113
Gottlieb SS et al (2002) The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail 8(3):136–141
Forman DE et al (2004) Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43(1):61–67
Simonneau G et al (2009) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54(1 Suppl):S43–S54
Filippatos G et al (2007) Prognostic value of blood urea nitrogen in patients hospitalized with worsening heart failure: insights from the Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Chronic Heart Failure (ACTIV in CHF) study. J Card Fail 13(5):360–364
Fonarow GC et al (2005) Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293(5):572–580
Aronson D et al (2004) Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am J Med 116(7):466–473
Blair JE et al (2011) Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J 32(20):2563–2572
Pfister R et al (2011) NT-pro-BNP predicts worsening renal function in patients with chronic systolic heart failure. Intern Med J 41(6):467–472
Schrier RW (2006) Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol 47(1):1–8
Schrier RW et al (1999) Hormones and hemodynamics in heart failure. N Engl J Med 341(8):577–585
Leithe ME et al (1984) Relationship between central hemodynamics and regional blood flow in normal subjects and in patients with congestive heart failure. Circulation 69(1):57–64
Yasue H et al (1994) Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 90(1):195–203
Mukoyama M et al (1990) Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med 323(11):757–758
Tsutamoto T et al (2006) Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol 47(3):582–586
Uthoff H et al (2011) Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail 13(4):432–439
Damman K et al (2010) Congestion in chronic systolic heart failure is related to renal dysfunction and increased mortality. Eur J Heart Fail 12:974–982
Damman K et al (2009) Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 53(7):582–588
Damman K et al (2007) Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail 9(9):872–878
Pellicori P et al (2013) IVC diameter in patients with chronic heart failure: relationships and prognostic significance. JACC Cardiovasc Imaging 6(1):16–28
Damman K et al (2007) Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail 13(8):599–608
Mintz GS et al (1981) Reat-time inferior vena caval ultrasonography: normal and abnormal findings and its use in assessing right-heart function. Circulation 64(5):1018–1025
Moreno FL et al (1984) Evaluation of size and dynamics of the inferior vena cava as an index of right-sided cardiac function. Am J Cardiol 53(4):579–585
Kircher BJ et al (1990) Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol 66(4):493–496
Jue J et al (1992) Does inferior vena cava size predict right atrial pressures in patients receiving mechanical ventilation? J Am Soc Echocardiogr 5(6):613–619
Bendjelid K et al (2002) Correlation between measured inferior vena cava diameter and right atrial pressure depends on the echocardiographic method used in patients who are mechanically ventilated. J Am Soc Echocardiogr 15(9):944–949
Brennan JM et al (2007) Reappraisal of the use of inferior vena cava for estimating right atrial pressure. J Am Soc Echocardiogr 20(7):857–861
Sznajder JI et al (1986) Central vein catheterization. Failure and complication rates by three percutaneous approaches. Arch Intern Med 146(2):259–261
Merrer J et al (2001) Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA 286(6):700–707
Kimura BJ et al (2007) Value of a cardiovascular limited ultrasound examination using a hand-carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol 100(2):321–325
Jang T et al (2004) Ultrasonography of the internal jugular vein in patients with dyspnea without jugular venous distention on physical examination. Ann Emerg Med 44(2):160–168
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HF., Hsu, LA., Chang, CJ. et al. Prognostic significance of dilated inferior vena cava in advanced decompensated heart failure. Int J Cardiovasc Imaging 30, 1289–1295 (2014). https://doi.org/10.1007/s10554-014-0468-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-014-0468-y