Abstract
We sought to determine the relationship between white blood cell count (WBCc) and infarct size assessed by cardiovascular magnetic resonance imaging (CMR) in patients undergoing primary percutaneous coronary intervention (PCI) for ST-elevation myocardial infarction (STEMI). In 198 patients undergoing primary PCI for STEMI, WBCc was measured upon arrival and CMR was performed a median of 7 days after the index event. Infarct size was measured on delayed enhancement imaging and the area at risk (AAR) was quantified on T2-weighted images. Baseline characteristics were not significantly different between the high WBCc group (>11,000/mm3, n = 91) and low WBCc group (≤11,000/mm3, n = 107). The median infarct size was larger in the high WBCc group than in the low WBCc group [22.0 % (16.7–33.9) vs. 14.7 % (8.5–24.7), p < 0.01]. Compared with the low WBCc group, the high WBCc group had a greater extent of AAR and a smaller myocardial salvage index [MSI = (AAR−infarct size)/AAR × 100]. The major adverse cardiovascular events (MACE) including cardiac death, nonfatal reinfarction, and rehospitalization for congestive heart failure at 12-month occurred more frequently in the high WBCc group (12.1 vs. 0.9 %, p < 0.01). In multivariate analysis, high WBCc significantly increased the risk of a large infarct (OR 3.04 95 % CI 1.65–5.61, p < 0.01), a low MSI (OR 2.08, 95 % CI 1.13–3.86, p = 0.02), and 1-year MACE (OR 16.0, 95 % CI 1.89–134.5, p = 0.01). In patients undergoing primary PCI for STEMI, an elevated baseline WBCc is associated with less salvaged myocardium, larger infarct size and poorer clinical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with an acute myocardial infarction (AMI), elevated white blood cell count (WBCc) at presentation is associated with a worse angiographic appearance of the culprit lesions and an increased risk of adverse clinical outcomes [1]. A recent observational study showed that an elevated baseline WBCc is an independent predictor of mortality, major bleeding, and infarct size as assessed by cardiac enzyme levels [2]. Although previous studies found a strong relationship between high WBCc, infarct size, and subsequent adverse clinical outcomes [1, 3, 4], the causality and pathological mechanisms underlying these associations are still unknown.
In the setting of AMI, cardiovascular magnetic resonance imaging (CMR) is useful in the accurate assessment of myocardial edema, infarcted tissue, microvascular obstruction (MVO), and myocardial hemorrhage [5]. In addition, CMR allows for quantification of the extent of salvaged myocardium, which is defined as the myocardium at risk for irreversible injury as indicated by the presence of acute myocardial edema by T2-weighted image (T2W) but negative for delayed enhancement image, after primary percutaneous coronary intervention (PCI) [6]. Although 1 previous CMR study reported that neutrophil count independently predicted large infarctions, other valuable information such as myocardial salvage or MVO was not assessed [7].
In the present study, we aimed to assess the relationship between WBCc at presentation, myocardial salvage, and infarct size as determined by CMR in patients undergoing primary PCI for ST-elevation myocardial infarction (STEMI). Furthermore, we evaluated the impact of higher WBCc on clinical outcomes.
Methods
Study population
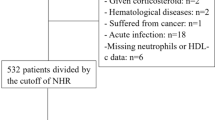
From January 2006 to November 2009, a total of 349 STEMI patients presented to Samsung Medical Center, Seoul, Korea. Patients were eligible if they had (1) chest pain for less than 12 h after symptom onset; (2) ST-segment elevation of more than 1 mm in two or more contiguous leads or a presumably new-onset left bundle branch block on electrocardiogram; and (3) had undergone successful primary PCI and CMR. All patients met the following criteria: no contraindication to CMR, no condition related to leukocytosis apart from the index MI, and no prior history of MI. In total, 198 patients were enrolled in this study and followed prospectively (Fig. 1). Baseline clinical data including past medical history, the presence of risk factors, medications, angiographic/procedural data, and clinical outcomes were recorded prospectively by the research coordinators of the dedicated registry. This study was approved by the local institutional review board. Informed consent was obtained from all subjects.
Blood samples
Blood samples were taken for routine WBC measurement on admission, before primary PCI (Sysmex XE-2100; TOA Medical Electronics Co., Kobe, Japan).
Percutaneous coronary intervention
All patients were given a loading dose of aspirin (300 mg) and clopidogrel (300 or 600 mg) before PCI. Coronary angiography and stent implantation were performed using standard interventional techniques [8]. The decision to use glycoprotein IIb/IIIa receptor inhibitors was made by individual operators. All baseline and procedural cine coronary angiograms were reviewed and analyzed quantitatively at the angiographic laboratory of our institution. Myocardial blush grade (MBG) was evaluated using the final angiogram, as described previously [9]. After PCI, aspirin (100–200 mg daily) was continued indefinitely and clopidogrel (75 mg daily) for at least 1 year was recommended.
CMR protocol
CMR was performed using a 1.5-T scanner (Achieva, Philips Medical Systems, Best, Netherlands) with a SENSE cardiac coil according to our laboratory protocol [10]. Images were acquired using electrocardiographic gating and expiratory breath holds. The CMR protocol consisted of cine, T2W, first-pass perfusion, and late-gadolinium enhancement (LGE) imaging. Cine imaging was carried out based on balanced steady-state free precession sequences along the long and short axes from the apex to the base of the left ventricle (LV). Next, T2Ws were acquired in the cardiac short-axis direction using a dark-blood T2W inversion-recovery fast-spin echo sequence. First-pass perfusion imaging was obtained with the T1-weighted dynamic sequence (turbo field echo with SENSE, repetition time/echo time, 2.6/1.3 ms) after intravenous infusion of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA, Magnevist, Bayer Schering Pharma, Berlin, Germany; 0.15 mmol/kg body weight in total amount at 3 mL/sec). The slice thickness was 6 mm with a field-of-view of 40 cm × 40 cm and an image matrix of 128 × 128. Images of 4 locations for every 2 heart beats were acquired for 40 phases. LGE and the extent of MVO were assessed 5, 10, and 15 min after Gd-DTPA administration in contiguous 10–12 slices of 6 mm thickness with a 4-mm interslice gap by use of a multi-shot turbo field echo breath-hold sequence with a non-selective inversion (typical repetition time/time to echo, 4.6/1.4 ms). The field-of-view and image matrix were 35 cm × 35 cm and 256 × 256, respectively. The inversion delay time was varied in a range of 200–300 ms. A Look-Locker sequence was used to determine optimal inversion time.
CMR analysis
The CMR images were analyzed using validated software (ARGUS, Siemens Medical System, Erlangen, Germany) at our MRI core laboratory by two experienced radiologists who were blinded to the clinical information of the patient. After acquiring the short-axis images at the end of diastole and the end of systole, endocardial borders were traced manually. LV end-diastolic volume, end-systolic volume, and ejection fraction were calculated using the Simpson rule. The infarct volume was quantified from the sum of the area with LGE within each segment of the short-axis images multiplied by the slice thickness to cover the entire LV. The infarct area was traced by the visual border detection using manual drawing method using commercialized analysis software. Interobserver and intraobserver variability of infarct size in our laboratory (intraclass correlation coefficient) is 0.82 and 0.87 as previously reported [11]. The extent of MVO was calculated in the same manner. The endocardial and epicardial borders were planimetered to calculate myocardial area and summed to calculate LV myocardial volume. The percent infarct volume was expressed as percentage of LV myocardial volume. T2Ws were used to determine the presence of myocardial hemorrhage [12]. The area at risk (AAR) was quantified on T2Ws by using a similar algorithm as above and expressed as percentage of LV myocardial volume. Myocardial salvage index (MSI) was computed as follows: MSI = (AAR−infarct size)/AAR × 100 [6]. The infarct transmurality of each segment was calculated by dividing the LGE area by the total area of the affected myocardium in each segment. The transmural extent of infarction was expressed as the sum of segments with >75 % of infarct transmurality.
End points
The primary objective was to compare myocardial infarct size assessed by CMR according to baseline WBCc (WBCc > 11,000 vs. ≤11,000 per 1 mm3). The cutoff value of WBCc was determined in reference to previous studies [2, 13]. The secondary objectives included (1) AAR, MSI, extent of MVO, number of segments with >75 % of infarct transmurality, and the presence of myocardial hemorrhage as assessed by CMR, (2) the incidence of stent thrombosis and the composite of major adverse cardiovascular events (MACE), including cardiac death, nonfatal reinfarction, and rehospitalization for congestive heart failure at the 12-month follow-up. Stent thrombosis was assessed based on the definitions of the Academic Research Consortium [14]. All deaths were considered cardiac unless a definite non-cardiac cause could be established. Reinfarction was defined as elevated cardiac enzymes (troponin or MB fraction of creatine kinase, CK-MB) greater than the upper limit of the normal value with ischemic symptoms or electrocardiography findings indicative of ischemia that were not related to the index procedure. Rehospitalization for congestive heart failure was defined as hospitalization due to exacerbation of congestive heart failure occurring after discharge.
Statistical analysis
Continuous variables are expressed as the mean ± SD or the median and interquartile range and were compared using the independent t test or Wilcoxon rank sum test. Categorical variables were compared with Pearson’s Chi square or Fisher’s exact tests. Multivariate logistic regression analysis was performed with a stepwise, backward selection process to determine the independent predictors of a large infarct (percent infarct volume > median infarct size in the present study), a low MSI (MSI < median MSI in the present study), and MACE. Age, sex, diabetes, high baseline WBCc, anterior myocardial infarction, aspiration thrombectomy during PCI, angiographic no-reflow, and a loading dose of 600 mg of clopidogrel were included in the multivariate logistic regression model. The criteria for the entry and removal of variables were set at 0.05 and 0.10, respectively. A value of p < 0.05 in the two-tailed test was considered significant. Statistical analysis was performed with the SPSS 17.0 statistical package (SPSS Inc., Chicago, Illinois).
Results
Patient characteristics
The clinical characteristics of the patients stratified by baseline WBCc are shown in Table 1. Compared to patients with WBCc ≤ 11,000 per 1 mm3 (low WBCc group), those with WBCc > 11,000 per 1 mm3 (high WBCc group) were more likely to be smokers, have a lower body mass index, a higher glucose level at admission, and a higher peak CK-MB level. The incidence of an LV ejection fraction of less than 40 % was significantly higher in the high WBCc group than in the low WBCc group. Other baseline clinical characteristics were not different according to WBCc.
Angiographic and procedural data
Angiographic and procedural variables stratified by baseline WBCc are shown in Table 2. There were no statistically significant differences between the two groups except for stent length. The most frequent culprit vessel was the left anterior descending artery in both groups. The baseline TIMI flow grade was 0 or 1 in most patients.
CMR analysis
The results of CMR are presented in Table 3. Figure 2 shows a representative CMR of a reperfused anterior STEMI with high WBCc versus low WBCc. CMR was performed at a median of 7 days after the index event [interquartile range (IQR), 4–15 days]. There was no difference in the interval from procedure to CMR between the groups [7 days (IQR, 4–15) in the high WBCc group versus 7 days (IQR, 3–16) in the low WBCc group, p = 0.98]. LV ejection fraction was significantly lower in the high WBCc group than in the low WBCc group. Patients with high WBCc had a significantly larger AAR than those with low WBCc. The median infarct size was significantly larger in the high WBCc group compared with the low WBCc group. Moreover, MSI was significantly lower in the high WBCc group than the low WBCc group. The extent of MVO and the number of segments with a >75 % infarct transmurality were significantly greater in the high WBCc group compared with the low WBCc group. Myocardial hemorrhage was detected more frequently in the high WBCc group than in the low WBCc group. In addition, WBCc was related to infarct size by univariate linear regression analysis. (β ± SE 0.841 ± 0.269, p ≤ 0.01).
A representative CMR of a reperfused anterior ST-elevation myocardial infarction with high WBCc (a, b) versus low WBCc (c, d); short-axis slices of a T2-weighted image (a, c) and the corresponding late-gadolinium enhancement image (b, d). In these cases, the extent of area at risk and of infarct size were 46.5 versus 28.2 % and 34.1 versus 15.0 %, respectively, yielding myocardial salvage index of 26.7 versus 46.8
Multivariate analysis showed that the independent predictors of a large infarct (>18.6 % of median infarct size) were an elevated baseline WBCc, aspiration thrombectomy during PCI, and angiographic no-reflow [odds ratio (OR) 3.04, 95 % confidence interval (CI) 1.65–5.61, p < 0.01; OR 0.20, 95 % CI 0.23–0.89, p = 0.02; OR 3.54, 95 % CI 1.17–10.7, p = 0.03, respectively]. Moreover, high baseline WBCc, anterior myocardial infarction, angiographic no-reflow, and a loading dose of 600 mg of clopidogrel were the independent predictors of a low MSI (<42.2 of median MSI) in multivariate analysis (OR 2.08, 95 % CI 1.13–3.86, p = 0.02; OR 2.54, 95 % CI 1.32–4.89, p < 0.01; OR 3.03, 95 % CI 1.04–8.83, p = 0.04; OR 0.40, 95 % CI 0.21–0.75, p = 0.04, respectively).
Clinical outcomes
At the 12-month follow-up, there had been four cardiac deaths (4.4 %) in the high WBCc group and none in the low WBCc group (p = 0.08). Nonfatal reinfarction and rehospitalization for congestive heart failure occurred in a similar pattern between the 2 groups (5.5 vs. 0.9 %, p = 0.16, and 3.3 vs. 0 %, p = 0.15, respectively). The composite MACE rates at the 12-month follow-up were higher in the high WBCc group than in the low WBCc group (12.1 vs. 0.9 %, p < 0.01). Multivariate analysis showed that high WBCc was an independent predictor of a 1-year MACE (OR 16.0, 95 % CI 1.89–134.5, p = 0.01).
Myocardial blush grade and CMR findings
Since we evaluated MBG after PCI, we analyzed post PCI MBG (MBG 0/1 versus MBG 2/3) and the correlation with WBCc and CMR findings. WBCc was not different between MBG 0/1 group and MBG 2/3 group [10,970/m3 (8,485–13,575) vs. 10,380/m3 (8,640-12,530), p = 0.23). The rates of WBCc ≥ 11,000/m3 was numerically higher in MBG 0/1 group than in MBG 2/3 group, but it was not statistically significant (53.6 vs. 41.9 %, p = 0.11). The median infarct size [22.0 % (16.7–33.9) vs. 17.0 % [9.1–26.9], p = 0.01) and MVO area [1.3 % (0–4.2) vs. 0.8 % (0–2.4), p = 0.04] were larger in MBG 0/1 group than in MBG 2/3 group, respectively. Compared with MBG 2/3 group, the MBG 0/1 group showed tendencies of a greater extent of AAR [63.3 % (47.6–76.5) vs. 53.0 % (40.2–72.7), p = 0.06] and a smaller MSI [36.7 (23.5–52.4) vs. 47.0 (10.2–72.7), p = 0.06]. Number of segments with ≥75 % of infarct transmurality was greater in MBG 0/1 group than in MBG 2/3 group (5 [3–7] versus 4 [2–5], p = 0.03). However, the MACE rates did not differ according to the MBG (7.2 vs. 5.4 %, p = 0.76).
Discussion
The salient findings of this study are as follows: (1) an elevated baseline WBCc in patients with STEMI who undergo primary PCI is associated with a larger extent of myocardial edema (AAR), less myocardial salvage, and larger infarct size as assessed by CMR; (2) patients with an elevated baseline WBCc have poor midterm clinical outcomes compared to those without leukocytosis in the setting of STEMI.
WBC count, myocardial salvage, and final infarct size
The results of the present study correspond well with those of earlier studies that established an association between elevated WBCc and infarct size as assessed by cardiac enzymes[1, 15, 16], single photon emission computed tomography [17], and CMR [7]; however, the causality and pathological mechanisms underlying these associations have not been fully elucidated. In the setting of AMI, CMR can identify the pathological consequences of reperfusion strategies in vivo and provide more information such as infarct related myocardial edema (so-called AAR) by T2W, acute irreversible infarcted myocardium by LGE image, and the extent of salvaged myocardium [18–20]. In the present study, elevated WBCc at the time of presentation of STEMI was associated with a larger AAR assessed by T2W, which suggests that a greater extent of ischemia-induced inflammation promotes leukocytosis. On the other hand, salvaged myocardium was also significantly reduced in the high WBCc group. These findings support the contention that leukocytosis may play a significant role in infarct expansion. After an AMI, the release of chemoattractants draws neutrophils into the infarct zone during the first 6 h of myocardial reperfusion, and during the next 24 h they migrate into the myocardial tissue [21]. Neutrophil infiltration is regulated through a complex sequence of molecular steps involving the selectins and the integrins, which mediate leukocyte rolling and adhesion to the endothelium [22]. These neutrophils cause proteolytic and oxidative damage to the endothelial cells, plug the microvasculature, and induce hypercoagulability and may promote infarct expansion [21–23]. In patients with STEMIs undergoing primary PCI, a high neutrophil count at presentation is associated with more severe microvascular dysfunction after primary PCI [24]. Of note, our study showed that the extent of MVO and hemorrhagic infarction was significantly greater in patients with high WBCc. Therefore, our findings suggest that a higher WBCc is not only a consequence of wider myocardial damage, but also directly responsible for increased infarct size by inducing infarct expansion. However, it should be noted that these are hypothesis-generating findings. Experimental data have demonstrated that reductions in infarct size are observed after the depletion or pharmacologic inhibition of neutrophils, supporting the primary role of these cells in myocardial and microvascular injury [21, 22].
WBC count and clinical outcomes
Prior studies have reported conflicting findings regarding the association between WBCc and mortality in patients with STEMI undergoing primary PCI [16, 25–27]. A recent large-scale study showed that an elevated baseline WBCc is an independent predictor of infarct size, as assessed by peak CK-MB level, and of 1-year cardiac mortality, noncardiac mortality, and major bleeding in STEMI patients treated by primary PCI [2]. Another study using CMR to assess infarct size in STEMI patients reported that neutrophil count independently predicts large infarctions and MACE [7]. The findings of the present study are consistent with these recent studies. Collectively, wider myocardial damage and infarct expansion induced by elevated baseline WBCc might lead to poor clinical outcomes.
Myocardial blush grade and CMR findings
Successful microcirculatory reperfusion, defined as MBG 2 or 3, is associated with smaller enzymatic infarct size [28]. Recent studies comparing infarct size measured by CMR in AMI patients showed that MBG 2/3 was associated with reduction of infarct size and MVO [29] or infarct transmurality [30]. In the present study, MBG 2/3 was achieved in 65.2 % of patients and associated with smaller infarct size, less MVO and lower infarct transmurality. The results of our study correspond with prior studies. These data might provide the mechanistic link between MBG and mortality [31].
Study limitations
There are several limitations to this study. First, this was a nonrandomized, observational study, which may have significantly affected the results due to confounding factors. Second, information on the WBC subtypes was not available. Nevertheless, a prior study suggested that total WBCc is correlated better with long-term prognosis than WBC differential count [32]. Third, serial data on WBCc were not available. It has been reported that neutrophil count at 12 h after revascularization independently predicted large infarctions. Although serial measurements can increase the predictive power of WBCc, it is more practical and feasible to measure WBCc on admission. Fourth, despite that there was no difference in the interval from procedure to CMR between the groups, but infarct size may vary significantly if measured 3–45 days post PCI. Lastly, low event rate in our study population and the wide C.I suggest multivariate model over fitting and does not provide convincing evidence toward poorer outcomes.
Conclusions
The present study shows that an elevated baseline WBCc in patients with STEMI undergoing primary PCI is associated with less myocardial salvage and larger infarct size as assessed by CMR and higher 1-year MACE rates. WBCc on admission is a marker of infarct severity, and furthermore, can aggravate infarcts per se. The potential therapeutic implications of these conclusions deserve further investigation.
References
Barron HV, Cannon CP, Murphy SA et al (2000) Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation 102(19):2329–2334
Palmerini T, Mehran R, Dangas G et al (2011) Impact of leukocyte count on mortality and bleeding in patients with myocardial infarction undergoing primary percutaneous coronary interventions: analysis from the harmonizing outcome with revascularization and stent in acute myocardial infarction trial. Circulation 123(24):2829–2837 2827 p following 2837
Barron HV, Harr SD, Radford MJ et al (2001) The association between white blood cell count and acute myocardial infarction mortality in patients >or = 65 years of age: findings from the cooperative cardiovascular project. J Am Coll Cardiol 38(6):1654–1661
Cannon CP, McCabe CH, Wilcox RG et al (2001) Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. OPUS-TIMI 16 Investigators. Am J Cardiol 87(5):636–639 A610
Dall’Armellina E, Karamitsos TD, Neubauer S et al (2010) CMR for characterization of the myocardium in acute coronary syndromes. Nat Rev Cardiol 7(11):624–636
Eitel I, Desch S, Fuernau G et al (2010) Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol 55(22):2470–2479
Husser O, Bodi V, Sanchis J et al (2011) White blood cell subtypes after STEMI: temporal evolution, association with cardiovascular magnetic resonance–derived infarct size and impact on outcome. Inflammation 34(2):73–84
Jo HS, Park JS, Sohn JW et al (2011) Culprit-lesion-only versus multivessel revascularization using drug-eluting stents in patients with ST-segment elevation myocardial infarction: a korean acute myocardial infarction registry-based analysis. Korean Circ J 41(12):718–725
Van ‘t Hof AW, Liem A, Suryapranata H et al (1998) Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 97(23):2302–2306
Song YB, Hahn JY, Gwon HC et al (2012) A high loading dose of clopidogrel reduces myocardial infarct size in patients undergoing primary percutaneous coronary intervention: a magnetic resonance imaging study. Am Heart J 163(3):500–507
Choe YH, Choo KS, Jeon ES et al (2008) Comparison of MDCT and MRI in the detection and sizing of acute and chronic myocardial infarcts. Eur J Radiol 66(2):292–299
Ganame J, Messalli G, Dymarkowski S et al (2009) Impact of myocardial haemorrhage on left ventricular function and remodelling in patients with reperfused acute myocardial infarction. Eur Heart J 30(12):1440–1449
Kratz A, Ferraro M, Sluss PM et al (2004) Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Laboratory reference values. N Engl J Med 351(15):1548–1563
Cutlip DE, Windecker S, Mehran R et al (2007) Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17):2344–2351
Patel MR, Mahaffey KW, Armstrong PW et al (2005) Prognostic usefulness of white blood cell count and temperature in acute myocardial infarction (from the CARDINAL Trial). Am J Cardiol 95(5):614–618
Prasad A, Stone GW, Stuckey TD et al (2007) Relation between leucocyte count, myonecrosis, myocardial perfusion, and outcomes following primary angioplasty. Am J Cardiol 99(8):1067–1071
Dogan I, Karaman K, Sonmez B et al (2009) Relationship between serum neutrophil count and infarct size in patients with acute myocardial infarction. Nucl Med Commun 30(10):797–801
Abdel-Aty H, Zagrosek A, Schulz-Menger J et al (2004) Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation 109(20):2411–2416
Friedrich MG, Abdel-Aty H, Taylor A et al (2008) The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol 51(16):1581–1587
Abdel-Aty H, Cocker M, Meek C et al (2009) Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol 53(14):1194–1201
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357(11):1121–1135
Frangogiannis NG, Smith CW, Entman ML (2002) The inflammatory response in myocardial infarction. Cardiovasc Res 53(1):31–47
Madjid M, Awan I, Willerson JT et al (2004) Leukocyte count and coronary heart disease: implications for risk assessment. J Am Coll Cardiol 44(10):1945–1956
Lee HY, Kim JH, Kim BO et al (2011) Effect of aspiration thrombectomy on microvascular dysfunction in ST-segment elevation myocardial infarction with an elevated neutrophil count. Korean Circ J 41(2):68–75
Pellizzon GG, Dixon SR, Stone GW et al (2003) Relation of admission white blood cell count to long-term outcomes after primary coronary angioplasty for acute myocardial infarction (The Stent PAMI Trial). Am J Cardiol 91(6):729–731
Smit JJ, Ottervanger JP, Slingerland RJ et al (2008) Comparison of usefulness of C-reactive protein versus white blood cell count to predict outcome after primary percutaneous coronary intervention for ST elevation myocardial infarction. Am J Cardiol 101(4):446–451
Ndrepepa G, Braun S, Iijima R et al (2009) Total leucocyte count, but not C-reactive protein, predicts 1-year mortality in patients with acute coronary syndromes treated with percutaneous coronary intervention. Clin Sci Lond 116(8):651–658
Henriques JP, Zijlstra F, van ‘t Hof AW et al (2003) Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation 107(16):2115–2119
Brener SJ, Maehara A, Dizon JM et al (2013) Relationship between myocardial reperfusion, infarct size, and mortality: the INFUSE-AMI (intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction) trial. JACC Cardiovasc Interv 6(7):718–724
Riedle N, Dickhaus H, Erbacher M et al (2010) Early assessment of infarct size and prediction of functional recovery by quantitative myocardial blush grade in patients with acute coronary syndromes treated according to current guidelines. Catheter Cardiovasc Interv 76(4):502–510
Brener SJ, Cristea E, Mehran R et al (2011) Relationship between angiographic dynamic and densitometric assessment of myocardial reperfusion and survival in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: the harmonizing outcomes with revascularization and stents in AMI (HORIZONS-AMI) trial. Am Heart J 162(6):1044–1051
Gurm HS, Bhatt DL, Lincoff AM et al (2003) Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart 89(10):1200–1204
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seungmin Chung and Young Bin Song have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chung, S., Song, Y.B., Hahn, JY. et al. Impact of white blood cell count on myocardial salvage, infarct size, and clinical outcomes in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a magnetic resonance imaging study. Int J Cardiovasc Imaging 30, 129–136 (2014). https://doi.org/10.1007/s10554-013-0303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-013-0303-x