Abstract
To assess the impact of adaptive statistical iterative reconstruction (ASIR) on coronary plaque volume and composition analysis as well as on stenosis quantification in high definition coronary computed tomography angiography (CCTA). We included 50 plaques in 29 consecutive patients who were referred for the assessment of known or suspected coronary artery disease (CAD) with contrast-enhanced CCTA on a 64-slice high definition CT scanner (Discovery HD 750, GE Healthcare). CCTA scans were reconstructed with standard filtered back projection (FBP) with no ASIR (0 %) or with increasing contributions of ASIR, i.e. 20, 40, 60, 80 and 100 % (no FBP). Plaque analysis (volume, components and stenosis degree) was performed using a previously validated automated software. Mean values for minimal diameter and minimal area as well as degree of stenosis did not change significantly using different ASIR reconstructions. There was virtually no impact of reconstruction algorithms on mean plaque volume or plaque composition (e.g. soft, intermediate and calcified component). However, with increasing ASIR contribution, the percentage of plaque volume component between 401 and 500 HU decreased significantly (p < 0.05). Modern image reconstruction algorithms such as ASIR, which has been developed for noise reduction in latest high resolution CCTA scans, can be used reliably without interfering with the plaque analysis and stenosis severity assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary computed tomography angiography (CCTA) has become an important non-invasive tool for the evaluation of coronary artery disease (CAD) with high accuracy compared to invasive coronary angiography in a large variety of patients [1–3]. In addition to depicting luminal narrowing CCTA allows early detection of coronary atherosclerotic lesions [4, 5]. Several attempts have been made to characterize the plaque nature by differentiating non-calcified from calcified and mixed plaques with CCTA [6, 7].
However, despite encouraging results this was limited to the most proximal coronary segments and hampered by a poor reproducibility as documented in studies using intravascular ultrasound (IVUS) as gold standard [8]. Clinically, accurate and reliable assessment of plaque compositions would be crucial as low attenuation plaques and positive vessel remodelling have been identified as key features of lesions with high risk for rupture causing an acute coronary syndrome [9]. Currently, a new generation of high definition CT scanners (Discovery HD 750, GE Healthcare), are being introduced with substantially improved spatial resolution (0.23 × 0.23 mm in-plane resolution) complemented by a new adaptive statistical iterative reconstruction (ASIR, GE Healthcare) algorithm to compensate for the increased noise due to the higher resolution [10, 11].
This may contribute to overcome issues of limited contrast resolution and blooming artefacts from coronary calcifications or stents as ASIR was developed to help reducing noise associated with standard convolution reconstruction algorithms [12, 13]. The impact of these technical innovations on calcium detection, plaque boundary identification, coronary plaque component characterization and luminal narrowing quantification is unknown. Therefore, the aim of the present study was to assess the impact of high definition (HD) CCTA combined with ASIR reconstruction on plaque volume and stenosis detection with a previously validated computed software for automated CCTA plaque analysis [14].
Materials and methods
Study population
This retrospective study included twenty-nine consecutive patients who were referred for the assessment of known or suspected CAD with contrast-enhanced CCTA on a 64-slice high definition CT scanner (Discovery HD 750, GE Healthcare). We included patients with at least one coronary artery segment with an atherosclerotic plaque. Exclusion criteria were motion artefacts of the plaque or diffuse coronary atherosclerosis.
The need for informed consent was waived by the institutional review board (local ethics committee) due to the purely retrospective nature of this study.
CT acquisition
All patients underwent contrast-enhanced CCTA during inspiration breath hold with prospective ECG-triggering as previously reported [15]. Metoprolol (up to 25 mg Beloc, AtraZeneca, London, UK) was administered intravenously prior to the examination if beats per minute were higher than 65 in order to obtain optimal image quality for CCTA. The patients received 2.5 mg isosorbiddinitrate (Isoket, Schwarz Pharma, Monheim, Germany) sublingually 2 min prior to the CCTA scan.
Iodixanol (Visipaque 320, 320 mg/ml, GE Healthcare, Buckinghamshire, UK) was injected into an antecubital vein followed by 50 ml saline solution via an 18-cauge catheter. Volume (40–105 ml) and flow rate (3.5–5 ml/s) were adapted to body surface area (BSA) [16]. For CCTA acquisition we used a collimation of 64 × 0.625 mm and gantry rotation time of 0.35 s, with a field of view of 25 cm. Tube voltage (100–120 kV) and tube current (450–700 mA) were adapted to body mass index (BMI) [17]. All scans were acquired in high resolution mode with an in-plane spatial resolution of 0.23 × 0.23 mm.
CT image reconstruction and analysis
All scans were reconstructed using the ASIR-assisted high-definition kernel with a display field of view of 25 cm, using FBP (0 % ASIR) and increasing ASIR blending factors, i.e. 20, 40, 60, 80 and 100 % (no FBP). ASIR is an iterative reconstruction algorithm, which has been described in details previously [11]. ASIR is a modified iterative reconstruction algorithm which models the photon statistics in x-ray attenuation, resulting in significant noise reduction, which potentially improves image quality and allows reduction in radiation dose. By particular correction for the fluctuations in projection measurement due to limited photon statistics, ASIR allows reduction of pixel variance that is statistically unlikely to represent anatomic structures without trade-off in spatial resolution. In clinical practice variably blended images created with FBP and ASIR to produce different levels of ASIR can be obtained. However, high levels of ASIR blending factors may result in a smooth appearance of image texture and noise characteristic unfamiliar to the observer. In brief, ASIR reconstructs pictures by comparing measured projection with a synthesized projection using both statistical fluctuation calculations and system optics.
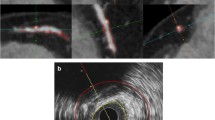
Coronary arteries were automatically tracked on a dedicated workstation (Advantage Workstation 4.6, GE Healthcare) using CardIQ Xpress software package (GE Healthcare) and curved-multiplanar-reconstructions were obtained. Stenosis degree was calculated by using the mean proximal and distal diameter as reference and plaque boundaries were automatically detected to asses plaque volume as previously validated versus IVUS [14]. The software identified three plaque components: i.e. soft, intermediate and calcified using a lower threshold (fixed at 30 HU) and a higher threshold at an average of 540 ± 75 HU automatically adapted by the software to each individual contrast bolus density (Fig. 1).
Upper row shows cross-sectional CT image of a coronary artery plaque reconstructed by FBP (0 % ASIR) a 20 % b 40 % c 60 % d 80 % e and 100 % ASIR f contribution. The plaque is automatically detected and components are colour illustrated: blue corresponds to soft, pink to intermediate and yellow to calcified component (lower row)
In order to asses which components of the coronary plaque are most susceptible to ASIR we have systematically analyzed the impact of ASIR on the plaque volume components in different HU strata, i.e. <30 HU, 30–130 HU, 131–200 HU, 201–300 HU, 301–400 HU, 401–500 HU, 501–600 HU and >600 HU.
Radiation dose estimation
Effective radiation dose from CCTA was calculated as the product of dose-length product (DLP) times a conversion coefficient for chest (k = 0.014 mSv/(mGy/cm)) [18].
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation (SD) if not stated otherwise and categorical variables as frequencies or percentages. The statistical software package SPSS 19.0 (SPSS, Chicago, IL) was used for analysis. Comparisons of continuous variables with non-normal distributions between groups were performed with the Kruskual-Wallis test. The data were tested for normal distribution by Shapiro–Wilk test. P values of less than 0.05 were considered statistically significant.
Results
Study population
The study population consisted of 29 patients with a mean age of 58 ± 9 years (Table 1) in whom 50 plaques were analyzed in the left anterior descending (n = 26), the circumflex (n = 10) and the right coronary artery (n = 14). In 7 plaques the edge-detection algorithm of the software tool failed to correctly identify the plaque boundaries. Therefore, the final analysis included 43 plaques in 27 patients. Effective radiation dose was 1.68 ± 0.52 mSv.
Lesion quantification
Mean minimal luminal diameters were 2.1 ± 0.7 mm, 2.1 ± 0.7 mm, 2.2 ± 0.7 mm, 2.2 ± 0.7 mm, 2.2 ± 0.7 mm, and 2.3 ± 0.7 mm with 0, 20, 40 60, 80 and 100 % ASIR without significant difference between the reconstruction algorithms (p = 0.7; Fig. 2a). The respective values for mean minimal luminal area were 4.7 ± 2.6 mm2, 4.8 ± 2.4 mm2, 5.1 ± 2.8 mm2, 5.1 ± 2.7 mm2, 5.1 ± 2.7 mm2, and 5.2 ± 2.8 mm2 (p = 0.9; Fig. 2b). The respective mean percent luminal narrowing values (and ranges) from the different algorithms were 34 ± 18 % (0–70 %), 33 ± 15 % (0 –67 %), 32 ± 16 % (0–68 %), 29 ± 17 % (0–68 %), 29 ± 16 % (0–61 %), and 30 ± 16 % (4–57 %) (p = 0.6; Fig. 2c).
Plaque characterization
Mean plaque volume tended to be higher with increasing (0, 20, 40, 60, 80, 100 %) ASIR, i.e. 60.4 ± 14 mm3, 63.5 ± 15 mm3, 63.7 ± 15 mm3, 67.8 ± 16 mm3, 66.4 ± 17 mm3, 66.8 ± 16 mm3 (mean ± SEM) although this fell short of statistical significance (p = 1.0). Similarly, this holds true for the different plaque components, i.e. soft (2.1 ± 0.5 mm3, 2.5 ± 0.7 mm3, 2.8 ± 0.8 mm3, 3.3 ± 0.8 mm3, 2.9 ± 0.8 mm3, 3.0 ± 0.8 mm3; mean ± SEM, p = 1.0) and intermediate (39.6 ± 7.9 mm3, 42.0 ± 8.5 mm3, 41.6 ± 8.7 mm3, 45.3 ± 9.6 mm3, 44.3 ± 10.4 mm3, 45.5 ± 10.3 mm3, mean ± SEM; p = 0.6). Finally, there was no impact of the reconstruction algorithms on the volume of the calcified component, i.e. 18.8 ± 6.1 mm3, 18.9 ± 6.0 mm3, 19.4 ± 6.4 mm3, 19.2 ± 6.3 mm3, 19.1 ± 6.3 mm3, and 18.3 ± 5.7 mm3 (mean ± SEM; p = 1.0, Fig. 3). The automatically determined threshold was 544 ± 66 HU, 550 ± 71 HU, 552 ± 74 HU, 555 ± 77 HU, 534 ± 100 HU, and 549 ± 67 at 0, 20, 40, 60, 80 and 100 % ASIR. With increasing ASIR the mean percentage of the plaque volume component at 401-500HU significantly decreased from 9.4 ± 0.5 % to 8.8 ± 0.5 %, 8.8 ± 0.5 %, 8.1 ± 0.5 %, 7.9 ± 0.5 %, and 7.4 ± 0.5 % (mean ± SEM; p = 0.03) while there was no significant difference in all other tissue components throughout all reconstruction algorithms (Fig. 4).
Graph shows mean fraction of total plaque depending on HU. With increasing ASIR contributions, there was a significant decrease in plaque volume components of 401–500 HU, while there was no significant difference measured in all other tissue components throughout all reconstruction algorithms. Values are given as mean ± SEM. *p < 0.05
Discussion
Latest generation CT scanners offer increased spatial resolution but require specific post processing methods with modern reconstruction algorithms (such as ASIR) to compensate for increased noise. Our results are the first to document that high resolution CCTA scans can be safely reconstructed with ASIR without affecting reliability of coronary plaque analysis.
Non-invasive coronary plaque analysis including plaque component characterization and luminal narrowing quantification have emerged as important measurements from CCTA. Several studies have compared CCTA with IVUS [8, 14, 19] and plaque characterization by CCTA has been shown to predict outcome in patients with acute coronary syndrome [9].
The latest generation of HD CT scanner offers an improved in-plane spatial resolution (0.23 × 0.23 mm) compared to standard CT (0.64 × 0.64 mm). As this is paralleled by an increase in noise, this technical refinement has been complemented by new reconstruction algorithms such as ASIR to compensate for the noise increase. ASIR has been shown to improve image quality and to reduce noise in chest and abdominal CT as well as in CCTA [10]. Proof of reliability in the assessment of stenosis severity and quantitative plaque component analysis is essential before introducing ASIR into the daily clinical routine of CCTA scanning.
It is not yet known which grade of ASIR is the best to reveal good results. Our results document that the use of ASIR at any blending factor does not significantly affect measurements of minimal luminal diameter and area or lesion severity. So far no standard blending factor for ASIR has been defined, although 30–40 % has been recommended by the vendor and confirmed in clinical experience [10, 20].
Consequently total plaque volume measurements did not differ significantly with increased percentage of ASIR algorithm. Although this was associated with a tendency of increased soft and intermediate components of the plaque, this fell short of statistical significance. Interestingly, the calcified component of the plaque remained unaffected, which is in line with a study recently demonstrating that ASIR is not more effective than FBP in suppression of blooming artefacts [21].
In an in-depth analysis of the plaque, using several HU strata, our study revealed significant decrease of plaque volume components with 401–500 HU. Although these HU strata seem to be most susceptible for ASIR reconstructions, further studies will be needed to clearly assess the effect of ASIR on assessment of plaque composition, i.e. for example a comparative characterization of atheroma by CT versus microscopy of plaque specimens either by simple light microscopy or by more complex methods such as immuno-fluorescence studies targeting different plaque structures [22]. This should also help to clarify whether the component between 401 and 500 HU is indeed negligible for evaluation of plaque vulnerability. This is important as it appears that the improved HD image resolution combined with the ASIR technique has a particular impact on the discrimination of structures with attenuation values representing the edge of non-calcified versus calcified structures.
As ASIR has been shown to reduce noise in CCTA as well as in non-cardiac CT scans, it could emerge as the preferred reconstruction algorithm in patients with low image quality and therefore higher noise, due to further reduced tube voltage and current [23] for dose reduction or due to obesity. This may pave the way for a broad application over a high scale of different ASIR blending factors. It is, therefore, crucial that our data document the reliability for reconstructions throughout the entire spectrum of 0 to 100 % ASIR.
Several limitations of this study have to be considered. The patient number was limited, although this seems justified due to the pilot nature of the study. Second, the plaque measurement techniques were based on automatically detection of plaque boundaries. Therefore, plaques associated with motion artefacts were not measureable as the tool could not accurately detect plaque boundaries. However, as there is no operator interaction after defining the coronary segment of interest, this excludes virtually any intra- and inter-observer variability which further strengthens our results. Finally, we did not evaluate the performance of ASIR compared to FBP in correlation to other imaging modalities such as for example intravascular ultrasound or optical coherence tomography. Therefore, our data do not allow commenting with final certainty on this, although it appears reasonable to assume that results would be comparable.
In conclusion this study confirms that ASIR has no significant influence on measured minimal luminal diameter or area or plaque composition.
References
Schroeder S, Achenbach S, Bengel F, Burgstahler C, Cademartiri F, de Feyter P, George R, Kaufmann P, Kopp AF, Knuuti J, Ropers D, Schuijf J, Tops LF, Bax JJ (2008) Cardiac computed tomography: indications, applications, limitations, and training requirements: report of a writing group deployed by the working group nuclear cardiology and cardiac CT of the European society of cardiology and the european council of nuclear cardiology. Eur Heart J 29(4):531–556
Pelliccia F, Pasceri V, Evangelista A, Pergolini A, Barilla F, Viceconte N, Tanzilli G, Schiariti M, Greco C, Gaudio C (2012) Diagnostic accuracy of 320-row computed tomography as compared with invasive coronary angiography in unselected, consecutive patients with suspected coronary artery disease. Int J Cardiovasc Imaging. doi:10.1007/s10554-012-0095-4
Lee JH, Chun EJ, Choi SI, Vembar M, Lim C, Park KH, Choi DJ (2011) Prospective versus retrospective ECG-gated 64-detector coronary CT angiography for evaluation of coronary artery bypass graft patency: comparison of image quality, radiation dose and diagnostic accuracy. Int J Cardiovasc Imaging 27(5):657–667
Lim S, Choi HJ, Shin H, Khang AR, Kang SM, Yoon JW, Choi SH, Jeong IK, Cho SI, Park KS, Jang HC (2012) Subclinical atherosclerosis in a community-based elderly cohort: the Korean longitudinal study on health and aging. Int J Cardiol 155(1):126–133
Ueda H, Harimoto K, Tomoyama S, Tamaru H, Miyawaki M, Mitsusada N, Yasuga Y, Hiraoka H (2011) Association between cardiovascular risk factors and the presence of coronary plaque in a zero or low coronary artery calcium score. Int J Cardiol 147(3):475–477
Kristanto W, van Ooijen PM, Groen JM, Vliegenthart R, Oudkerk M (2012) Small calcified coronary atherosclerotic plaque simulation model: minimal size and attenuation detectable by 64-MDCT and MicroCT. Int J Cardiovasc Imaging 28(4):843–853
Ovrehus KA, Marwan M, Botker HE, Achenbach S, Norgaard BL (2012) Reproducibility of coronary plaque detection and characterization using low radiation dose coronary computed tomographic angiography in patients with intermediate likelihood of coronary artery disease (ReSCAN study). Int J Cardiovasc Imaging 28(4):889–899
Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, Ohnesorge B, Fayad ZA, Becker CR, Reiser M, Steinbeck G, Boekstegers P (2006) Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol 47(3):672–677
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54(1):49–57
Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, Taylor C, Dunning A, Earls JP (2010) Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195(3):649–654
Gebhard C, Fiechter M, Fuchs TA, Ghadri JR, Herzog BA, Kuhn F, Stehli J, Muller E, Kazakauskaite E, Gaemperli O, Kaufmann PA (2012) Coronary artery calcium scoring: Influence of adaptive statistical iterative reconstruction using 64-MDCT. Int J Cardiol. doi:10.1016/j.ijcard.2012.08.003
Prakash P, Kalra MK, Ackman JB, Digumarthy SR, Hsieh J, Do S, Shepard JA, Gilman MD (2010) Diffuse lung disease: CT of the chest with adaptive statistical iterative reconstruction technique. Radiology 256(1):261–269
Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, Samei E (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm–initial clinical experience. Radiology 254(1):145–153
Pedrazzini GB, D’Angeli I, Vassalli G, Faletra FF, Klersy C, Pasotti E, Corbacelli C, Moccetti T, Auricchio A (2011) Assessment of coronary stenosis, plaque burden and remodeling by multidetector computed tomography in patients referred for suspected coronary artery disease. J Cardiovasc Med (Hagerstown) 12(2):122–130
Husmann L, Schepis T, Scheffel H, Gaemperli O, Leschka S, Valenta I, Koepfli P, Desbiolles L, Stolzmann P, Marincek B, Alkadhi H, Kaufmann PA (2008) Comparison of diagnostic accuracy of 64-slice computed tomography coronary angiography in patients with low, intermediate, and high cardiovascular risk. Acad Radiol 15(4):452–461
Pazhenkottil AP, Husmann L, Buechel RR, Herzog BA, Nkoulou R, Burger IA, Vetterli A, Valenta I, Ghadri JR, von Schulthess P, Kaufmann PA (2010) Validation of a new contrast material protocol adapted to body surface area for optimized low-dose CT coronary angiography with prospective ECG-triggering. Int J Cardiovasc Imaging 26(5):591–597
Tatsugami F, Husmann L, Herzog BA, Burkhard N, Valenta I, Gaemperli O, Kaufmann PA (2009) Evaluation of a body mass index-adapted protocol for low-dose 64-MDCT coronary angiography with prospective ECG triggering. AJR Am J Roentgenol 192(3):635–638
Ghadri JR, Kuest SM, Goetti R, Fiechter M, Pazhenkottil AP, Nkoulou RN, Kuhn FP, Pietsch C, von Schulthess P, Gaemperli O, Templin C, Kaufmann PA (2012) Image quality and radiation dose comparison of prospectively triggered low-dose CCTA: 128-slice dual-source high-pitch spiral versus 64-slice single-source sequential acquisition. Int J Cardiovasc Imaging 28:1217–1225
Akutagawa O, Kijima Y, Kume K, Sakai T, Okura A, Ide K, Iwasaki S, Hata T (2011) Feasibility and limitation of coronary plaque volumetry by contrast-enhanced 64-row multi-detector computed tomography. Int J Cardiol 150(1):118–120
Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, Taylor C, Dunning A, Earls JP (2010) Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am J Roentgenol 195(3):655–660
Scheffel H, Stolzmann P, Schlett CL, Engel LC, Major GP, Karolyi M, Do S, Maurovich-Horvat P, Hoffmann U (2012) Coronary artery plaques: Cardiac CT with model-based and adaptive-statistical iterative reconstruction technique. Eur J Radiol 81(3):e363–e369
Fiechter M, Frey K, Fugmann T, Kaufmann PA, Neri D (2011) Comparative in vivo analysis of the atherosclerotic plaque targeting properties of eight human monoclonal antibodies. Atherosclerosis 214(2):325–330
Herzog BA, Husmann L, Buechel RR, Pazhenkottil AP, Burger IA, Valenta I, Altorfer U, Wolfrum M, Nkoulou RN, Ghadri JR, Wyss CA, Kaufmann PA (2011) Rapid cardiac hybrid imaging with minimized radiation dose for accurate non-invasive assessment of ischemic coronary artery disease. Int J Cardiol 153(1):10–13
Acknowledgments
The study was supported by grants from the Swiss National Science Foundation (SNSF) to PAK (Grant No. 320030-127604/1) and to MF (Grant No. 323630-128868/1). Furthermore, we thank our Cardiac Radiographer Ennio Mueller for his excellent technical support.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tobias A. Fuchs and Michael Fiechter have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fuchs, T.A., Fiechter, M., Gebhard, C. et al. CT coronary angiography: impact of adapted statistical iterative reconstruction (ASIR) on coronary stenosis and plaque composition analysis. Int J Cardiovasc Imaging 29, 719–724 (2013). https://doi.org/10.1007/s10554-012-0134-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0134-1