Abstract
Microvascular obstruction (MVO) is a strong independent predictor of left ventricular remodelling and mortality following ST-segment elevation myocardial infarction (STEMI). Microvascular obstruction can be identified at angiography or with gadolinium-enhanced cardiac MRI (CMR). First-pass perfusion CMR also allows a novel quantitative evaluation of myocardial blood flow (MBF) that might provide superior predictive data in the assessment of MVO. We sought to compare angiographic and CMR derived methodologies in the assessment of MVO to determine the optimal methodology that best predicts the surrogate outcome marker of left ventricular function post STEMI. Following primary-PCI angiographic assessment of ‘no-reflow’ with TIMI myocardial perfusion grade (TMPG) and myocardial blush grade (MBG) were documented. Assessment of CMR derived MVO was assessed on day 3, with MVO on first-pass perfusion imaging termed ‘early MVO’ and on late gadolinium enhancement, ‘late MVO’. Furthermore on the same day 3 CMR scan, myocardial blood flow in the infarct region was quantified at adenosine stress and rest utilizing standard perfusion imaging sequences. Assessment of remodelling, structure and function was undertaken via standard CMR imaging assessment on day 90 post-STEMI and was used as the surrogate marker for long term clinical outcome. Forty patients (age 59 ± 12 years, 84% males) were appraised. Late MVO had the strongest correlation with LVEF at 90 days compared to the CMR parameters of early MVO, stress infarct region MBF and rest infarct region MBF (r = −0.754, r = −0.588, r = 0.595 and r = 0.345 respectively). Of the angiographic parameters used to assess MVO, TMPG had the strongest relationship with MVO when assessed via CMR. Myocardial blush grade however showed no relationship to CMR derived assessment of MVO. On multivariate analysis, of all angiographic and CMR variables, late MVO was the strongest predictor of LVEF at 90 days (p = 0.004). Cardiac magnetic resonance imaging derived assessment of microvascular obstruction on late gadolinium enhancement strongly predicts left ventricular function following STEMI at 90 days.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In acute ST-segment elevation myocardial infarction (STEMI), reperfusion therapy has contributed to the reduction in mortality by limiting the extent of myocardial necrosis. However despite successful recanalisation of the infarct-related artery, as many as 30–75% of patients have impaired myocardial perfusion due to microvascular obstruction (MVO) [1]. This phenomenon is angiographically referred to as the ‘no-reflow’ phenomenon [2]. Patients with microvascular obstruction are known to exhibit a higher prevalence of early post infarct complications, adverse left ventricular remodeling, recurrent hospitalisations for heart failure and mortality [3–5]. The diagnosis of microvascular obstruction can be made using angiography [6, 7], electrocardiography [8], nuclear scintigraphy [9], myocardial contrast echocardiography [10] or cardiovascular magnetic resonance imaging (CMR) [3].
The advantage of utilizing CMR in the measurement of MVO, is other valuable information such as myocardial viability, infarct size, ischaemia, left ventricular remodelling and systolic function can also be acquired. Microvascular obstruction assessed on CMR can be assessed by two methods utilising gadolinium contrast: first pass perfusion (early MVO) [3] and late gadolinium enhancement (late MVO) [11]. Many studies have assessed the impact of the presence and extent of early and late MVO on global and regional function [12, 13]. One recent study showed that in contrast to early MVO, the presence and extent of late MVO was a strong and independent predictor of the long-term occurrence of death, myocardial re-infarction and congestive heart failure, even when compared with traditional post-infarction risk stratification markers and scores after STEMI [14]. Quantification of regional resting of myocardial blood flow (MBF) on first pass perfusion has been described [15] but has never been correlated with MVO. Furthermore CMR offers a unique opportunity to evaluate not only fixed, but dynamic aspects to microvascular obstruction and subsequent MBF when imaging is undertaken in response to a microvascular dilator such as adenosine. To our knowledge, no studies have compared the predictive value of CMR indices (including resting and stress infarct region MBF) with angiographic derived assessment of MVO in the prediction of left ventricular function at 90 days post STEMI. This was the focus of this analysis.
Methods
Study population
All subjects provided written informed consent in accordance with local human research and ethics committee approval. Patients with acute STEMI who underwent primary PCI between April 2008 and April 2009 were prospectively enrolled. STEMI was defined as chest pain for at least 30 min and an ECG demonstrating ST-segment elevation >0.1 mV in ≥2 contiguous leads. Patients aged <18 years, previous myocardial infarction in the same territory, atrioventricular block of grade II or higher, severe asthma of chronic obstructive airways disease, contraindications to CMR (e. g, pacemaker implantation or claustrophobia) contraindication to gadopentetate dimeglumine.(e. g, known hypersensitivity to gadopentetate dimeglumine or creatinine clearance ≤60 ml/min/1.73 m2) or pregnancy were excluded from the study. All patients abstained from caffeine for 24 h prior to CMR.
Coronary angiography
All angiographic findings were analyzed by 2 interventional cardiologists, by consensus agreement, each of whom were blinded to the CMR and ECG findings. The myocardial blush grade (MBG) and TIMI myocardial perfusion grade (TMPG) analyses were performed at the end of procedure utilizing standard methodology [6, 7]. In brief, we ensured the duration of cine filming exceeded 3 cardiac cycles in the washout phase to assess clearance of the myocardial blush. Blush was assessed distal to the culprit lesion and angiographic runs were made in identical views according to the infarct-related artery thereby assuring assessment in equivalent conditions.
Myocardial blush grade was identified according to a dye density score (0 = no myocardial blush or persistent blush; 1 = minimal blush; 2 = moderate blush, but less than obtained during angiography of the contralateral or ipsilateral noninfarct-related artery; 3 = normal myocardial blush) [6]. Impaired MBG was defined as MBG <2 [12].
TIMI myocardial perfusion grade was identified according to perfusion of dye score (0 = minimal or no myocardial blush; 1 = dye stains the myocardium, and this stain persists on the next injection; 2 = dye enters the myocardium but washes out slowly so that dye is strongly persistent at the end of the injection; 3 = there is normal entrance and exit of dye in the myocardium) [7]. Impaired TMPG was defined as TMPG < 2 [16].
Adenosine infusion protocol
Adenosine (Adenoscan®, Sanofi-Synthelabo) was infused at 140 μg/kg/min through an antecubital vein using an accurate syringe pump (Graseby® 3500) by a supervising cardiologist. The target time of the infusion was 3 min, however if patients developed persistent or symptomatic 3rd AV block, severe hypotension (systolic blood pressure <90 mmHg) or bronchospasm, infusion was discontinued. On standby was aminophylline for adenosine receptor antagonism, nitroglycerine for persistent chest pain, atropine for persistent AV block and a fully equipped crash trolley with defibrillator if required.
CMR protocol
Cardiac MRI was performed using a 1.5 Tesla MRI scanner (Magnetom Avanto, Siemens, Germany) equipped with a dedicated cardiac software package and cardiac phased array surface coil. Cine images were acquired in sequential short-axis slices covering the entire ventricular myocardium during breath-hold with retrospectively ECG-gated TrueFISP (Fast Imaging with Steady-State Precision) sequences. Section thickness was 6 mm with intersection gaps of 4 mm. Acquisition time was 90% of the RR-interval, image matrix 256 × 150, field of view (FOV) 380 mm, repetition time 52.05 ms, echo time (TE) 1.74 ms, flip angle 70°, and 25 heart phases were acquired per repetition time interval.
Following at least 3 min of adenosine infusion, a gadolinium-based contrast agent (Dimeglumine gadopentetate, Magnevist; Schering, Germany) was administered intravenously at 0.1 mmol/kg body weight (injection rate, 7 ml/s), followed by at least 30 ml saline flush at the same rate [17, 18]. Perfusion imaging (echo time 1.08 ms, repetition time 2.2 ms, saturation recovery time 100 ms, shot time 100 ms, voxel size 2.5 × 1.9 × 10 mm; flip angle 10°) was performed every cardiac cycle during the first pass, using a T1-weighted fast low-angle single shot gradient-echo sequence (GRE). Parallel acquisition method using generalised autocalibrating partially parallel acquisition (GRAPPA) was utilised [19]. Three short axis slices, positioned from base to the apex of the left ventricle, were obtained. The same imaging sequence with a second dose of Magnevist 0.1 mmol/kg body weight (total dose 0.2 mmol/kg) was repeated 10 min later without adenosine to obtain perfusion images at rest. Delayed enhancement imaging was obtained by acquiring an inversion-recovery segmented gradient echo T1 weighted sequence (TR = 2 heart beats, TE = 3.32 ms, flip angle = 25°, FOV = 38 cm, matrix size = 176 × 256) 10–15 min after the second contrast administration.
CMR data analysis and definitions
Left ventricular ejection fraction (LVEF), volume and mass were derived from cine images using commercially available software (MASS, Medis, The Netherlands). Papillary muscles and pericardial fat were excluded from calculations. In brief, the end-diastolic and end-systolic cine frames were identified for each slice and the endocardial and epicardial borders were manually traced. The end-diastolic and end-systolic volumes were then calculated using Simpson’s true disk summation technique (i.e. sum of cavity sizes across all continuous slices), as previously described [20]. The change in end diastolic volume from baseline scan was used as the marker of left ventricular remodeling [21]. Ejection fraction at day 90 was used as the primary end-point for assessment of left ventricular function. Regional left ventricular function was evaluated by wall motion score index (WMSI). As recommended by the American Society for Echocardiography a 16-segment model was used for left ventricular segmentation [22]. This model consists of six segments at both the basal and mid-ventricular levels and four segments at the apex. Each segment’s function was scored as: normal or hyperkinesis = 1, hypokinesis = 2, akinesis = 3 and dyskinesis (or aneurysmatic) = 4. Wall motion score index was derived as the sum of all scores divided by the number of segments visualized [23].
First-pass perfusion was evaluated qualitatively. Microvascular obstruction was considered to be present if a region of hypoperfusion persisted for >1 min after contrast bolus arrival in the left ventricle and was located in the subendocardial layer of the infarct core in at least 1 of the slices [24]. The frame demonstrating peak signal intensity in the remote normal myocardium was chosen for quantification of the myocardial extent of the perfusion defect. To verify that a true perfusion deficit persisted after passage of the contrast agent, all acquired phases were evaluated. The perfusion defect was defined visually as hypoenhanced region and then planimetered manually [24] (Fig. 1). Microvascular obstruction on first-pass perfusion imaging was termed early MVO. The presence and extent of early MVO was determined.
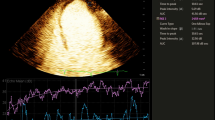
Comparison of myocardial blood flow (MBF) in the infarct and non infarct region. a Late gadolinium enhancement image showing scar and microvascular obstruction area (highlighted in yellow) in the inferior and inferolateral region. b First pass perfusion image showing segmentation of a short axis slice into 6 segments. c Myocardial (green and white) and blood pool (red) signal intensity versus time curves at stress. Stress infarct region MBF (white) is lower than the stress non infarct region MBF. d Signal intensity versus time curves at rest. The MBF at rest is lower than at stress. Resting infarct region MBF (white) is lower than the resting non infarct region MBF (green)
For perfusion analysis to quantify resting and stress infarct region MBF, we used the methodology as previously described [15]. In brief, the endocardial and epicardial contours of three short axis sections (base, mid and apex) were traced (MASS version 7.2, Medical Imaging Solutions, Leiden, the Netherlands) and corrected manually for displacements (e.g. breathing) by an examiner blinded to coronary angiography findings. In each short axis slice, MBF was determined for 6 myocardial segments in ml min−1 g−1 by deconvolution of signal intensity curves with an arterial input function measured in the left ventricular blood pool, with explicit accounting for any delay in the arrival of the tracer (Fig. 1). As basal MBF is closely related to the rate-pressure product, an index of left ventricular oxygen consumption, values for basal flow in each patient were also corrected for the respective rate-pressure product (i.e, the absolute values of flow were divided by rate-pressure product/10,000) [15]. Segments were assigned to vascular regions according to the segmental model of the American Society of Echocardiography, with modifications to correct for variable coronary dominances as previously described [25]. Segments 6, 1 and 2 were assigned to the left anterior descending artery; segments 2, 3 and 4 were assigned to the circumflex artery; and segments 4 and 5 were assigned to the right coronary artery (Fig. 2). The MBF in the infarct region at stress and at rest was determined, termed ‘stress infarct region MBF’ and ‘resting infarct region MBF’. The difference between stress infarct region MBF and resting infarct region MBF was calculated and termed ‘infarct region MBF difference’.
Late gadolinium enhancement (LGE) images were assessed both for infarct mass and MVO area (Fig. 3). The endocardial and epicardial borders on each slice were traced manually. Infarcted tissue was defined as areas with late gadolinium enhancement. These regions were identified and then quantified using a semi-automated algorithm. Areas of enhancement were defined by full width at half maximum technique which has been shown to be accurate for assessing infarct size [26]. The mass of infarcted myocardium was then automatically calculated. Transmural extent of LGE was evaluated using a two point scale: 0 = less than 50%, 1 = greater than 50% of wall thickness. A 16-segment model was used for left ventricular segmentation and a maximal LGE score was derived [22]. We defined MVO on LGE imaging as a region of subendocardial hypo-enhancement within a hyper-enhanced region as previously described [24, 27]. The area of MVO was manually planimetered (Fig. 1). Microvascular obstruction was included in the calculation of the total infarct size. Microvascular obstruction on late gadolinium enhancement imaging was termed late MVO. The presence and extent of late MVO was determined.
a Late gadolinium enhancement image on cardiac MRI showing regions of myocardial necrosis (white) and microvascular obstruction area ‘late MVO’ (black blood lakes within myocardial necrosis, highlighted in orange and purple). b First pass perfusion imaging on cardiac MRI showing microvascular obstruction area ‘early MVO’, highlighted in orange
Statistical analysis
Values are expressed as mean ± standard deviation or median (interquartile ranges) as appropriate. The primary endpoint was to evaluate the relationship between CMR indices of MVO and LVEF at 90 days. Association between variables were assessed by Chi square analysis, Mann–Whitney or Spearmans correlation when appropriate. Variables were normalised for multiple linear regression, with p < 0.2 on simple linear regression defining variables used in this analysis. Likelihood ratio test was performed to compare two linear regression models (i) Late MVO, (ii) Late MVO and stress infarct region MBF) for prediction of LVEF at 90 days. Statistical analysis was performed with SPSS 17.0 (SPSS Inc, Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
Baseline patient characteristics and medication are listed in Table 1. Fifty patients were prospectively recruited, however six patients did not undertake the follow up study and a further four patients did not undergo adenosine stress protocol due to contraindications. The remaining 40 patients form the basis of this report.
All 40 patients completed the adenosine stress CMR study, which was undertaken at a mean of 3 ± 2 days post primary PCI. During adenosine stress CMR perfusion, most patients reported at least one mild symptoms of flushing, breathlessness or chest discomfort. No patients developed significant AV block or hypotension.
Angiographic assessment of microvascular obstruction
Thirty-five patients (88%) achieved TIMI grade 3 flow at the end of the primary-PCI procedure. There were 26 patients (65%) with impaired TMPG while 5 patients (12%) had impaired MBG (Table 1). Impaired TMPG correlated both with the extent of late MVO (704.5 vs. 298, p = 0.034) and early MVO (354.8 vs. 133.4, p = 0.036). In addition, impaired TMPG correlated with stress infarct region MBF (19.3 vs. 26.5, p = 0.039) but not resting infarct region MBF (p = 0.116). MBG did not correlate with the extent of late MVO (p = 0.159), extent of early MVO (p = 0.102), resting (p = 0.098) or stress infarct region MBF (p = 0.595). There was no statistical association between impaired TMPG and MBG with pain-to-balloon time.
Cardiac MRI assessment of MVO
The CMR examinations were performed 3 ± 2 days and 90 ± 8 days after primary PCI. The LVEF at baseline was 57.5 ± 9.4%, which significantly improved to 62.0 ± 9.6% at 90 days (p < 0.001). The total infarct mass at baseline was 33.5 g (21.8; 41.9) which decreased to 23.1 g (14.6; 30.2) at 90 days. The analysis of CMR derived MVO assessment were performed on the first CMR scan performed at 3 ± 2 days. The stress infarct region MBF was higher than the resting infarct region MBF [24.3 ± 10.1 vs 14.8 ± 5.1 ml min−1 g−1 (mmHg bpm/104)−1, p < 0.001]. Stress infarct region MBF correlated with the extent of early MVO (r = −0.748) and late MVO (r = −0.682). The resting infarct region MBF also correlated with the extent of early MVO (r = −0.524) and late MVO (r = −0.46). Infarct region MBF difference correlated with the extent of late MVO (r = −0.474) and early MVO (r = −0.528). Late MVO was present in 31 patients (78%) while early MVO was present in 29 patients (73%). There were 2 patients (5%) who had late MVO without early MVO. The presence of late (60 vs. 70%, p = 0.005) and early MVO (59 vs. 69%, p = 0.001) predicted LVEF at 90 days respectively. The presence of late (p = 0.015) and early MVO (p = 0.04) correlated with impaired TMPG. However MBG did not correlate with the presence of late (p = 0.886) or early (p = 0.688) MVO.
Predictors of end diastolic volume change
Resting infarct region MBF correlated best with EDV change compared to the extent of late MVO and early MVO (r = −0.618, −0.5 and −0.515 respectively). Stress infarct region MBF (p = 0.169) and infarct region MBF difference (p = 0.685) did not correlate with EDV change. Maximal LGE correlated with EDV change (r = −0.32, p = 0.044). When evaluating angiographic parameters of MVO both TMPG (p = 0.431) and MBG (p = 0.984) did not correlate with EDV change.
Predictors of wall motion score index at 90 days follow up
The extent of late MVO correlated best with WMSI at 90 days compared to the extent of early MVO, stress infarct region MBF, resting infarct region MBF and infarct region MBF difference (r = −0.822, r = 0.641, r = −0.651, r = −0.474 and r = −0.445 respectively). Maximal LGE correlated with WMSI at 90 days (r = 0.549, p < 0.001). When evaluating angiographic parameters of MVO, TMPG (p = 0.048) but not MBG (p = 0.313) correlated with WMSI at 90 days.
Predictors of LVEF at 90 days follow up
The extent of late MVO correlated best with LVEF at 90 days compared to the extent of early MVO, stress infarct region MBF, infarct region MBF difference and resting infarct region MBF (r = −0.754, r = −0.595, r = 0.595, r = 0.431 and r = 0.345 respectively). Infarct mass at baseline also correlated with LVEF at 90 days (r = −0.384). Maximal LGE score correlated with ejection fraction at day 90 (r = −0.431, p = 0.006) When evaluating angiographic parameters of MVO, TMPG (p = 0.002) but not MBG (p = 0.115) correlated with LVEF at 90 days. On univariate linear regression, extent of late MVO (β = −0.68) and stress infarct region MBF (β = 0.492) were better predictors of LVEF at 90 days compared to TMPG (β = −0.486). On multiple linear regression, extent of late MVO was the strongest predictor of LVEF at 90 days (p = 0.016), independent of infarct mass and other assessments of microvascular injury (Table 2). On post hoc analysis utilising likelihood ratio test, the addition of ‘Stress infarct region MBF’ to late MVO significantly improved the prediction of LVEF at 90 days (Chi-square (1) = 6.20, p = 0.013).
Discussion
This study is the first to directly compare both CMR (including stress and resting infarct region MBF) and angiographic derived indices of MVO to assess which is best to predict the surrogate marker of LVEF 90 days post STEMI. The main outcome was that CMR derived late MVO was the strongest predictor of LVEF at 90 days.
Angiographic assessment of microvascular obstruction
Despite improvement in reperfusion strategies, patients who experience microvascular dysfunction following STEMI continue to have a poor clinical outcome. The “time dependent open artery and open microvascular hypothesis” represents a paradigm shift in the treatment of STEMI, which is now targeted to restoring and maintaining myocardial tissue perfusion [28]. Restoration of myocardial perfusion can be assessed angiographically by two main techniques; TIMI myocardial perfusion grade (TMPG) and myocardial blush grade (MBG). Gibson et al. [7] showed that independent of flow in the epicardial artery and other covariates such as age, blood pressure, and heart rate, TMPG was a strong predictor of mortality in acute STEMI at 2 years. Meanwhile, Appelbaum et al. [29] showed that post-PCI TMPG correlated with CMR measures of MVO and infarct size . This is consistent with the finding of our study showing correlation between TMPG and all CMR measures of MVO including stress infarct region MBF. Furthermore our study found no relationship between MBG and the CMR derived assessments of MVO studied, including stress and rest infarct region MBF. This finding is consistent with several other recent studies which have called into question MBG’s correlation with microvascular obstruction in context of myocardial infarction [12].
Infarct region MBF
To the best of our knowledge, this is the first study reporting the use of quantitative CMR perfusion imaging to evaluate stress and resting MBF within the infarct region and evaluate its predictive impact on LVEF at follow up. Using quantitative CMR perfusion imaging, this study shows that stress and resting infarct region MBF correlated with the extent of early MVO, late MVO and LVEF at 90 days. The utility of quantitative CMR perfusion imaging to assess resting regional MBF has previously been described [15]. Selvanayagam et al. utilising this methodology, demonstrated that resting MBF is abnormal within hibernating myocardial segments supplied by severely stenosed coronary arteries. The methodology from this study formed the template for our study. The findings from our MBF assessment showed that hyperaemia induced by adenosine resulted in higher infarct region MBF at stress compared to rest (24.3 ± 10.1 vs 14.8 ± 5.1 ml min−1 g−1). In addition, stress infarct region MBF had stronger correlation with the extent of early (r = −0.748 vs. r = −0.524) and late MVO (r = −0.682 vs. r = −0.46) than resting infarct region MBF. Furthermore, we found that the addition of stress infarct region MBF to late MVO has incremental benefit in predicting LVEF at 90 days. This would infer that dynamic microvascular integrity does play a role in the healing process of the left ventricle post myocardial infarction. While many studies to date have evaluated the effects of fixed microvascular obstruction (i.e. late MVO) on left ventricular function post MI [3, 14], we can find no evidence to date to support the microvascular dynamic function impacts on this measure. As this finding was not independently predictive of LVEF on multivariate analysis, it is possible that the ‘landscape’ of microvascular function in the infarct area is largely overwhelmed by fixed structural abnormality when evaluating the impact on myocardial function at 90 days. Interestingly while the adenosine responses in our infarcted area were severely reduced (i.e. only 80% increase, where previous studies report a three to fivefold increase in MBF in non-infarcted regions) [30], it is possible that this measure of dynamic function may play a more prominent role in predicting long term left ventricular systolic function in situations where less fixed structural microvascular damage (i.e. smaller myocardial damage) is seen. We find this novel observation extremely intriguing and worthy of further studies.
Microvascular obstruction
There has been active debate regarding the relative superiority of either MVO measure. Previous studies by Lund et al. and Nijveldt et al. have found differences in sensitivity between first pass and late gadolinium enhancement for quantification of MVO [12, 31]. It is however generally accepted that late MVO is less sensitive than early MVO because small ‘no-reflow’ zones become rapidly enhanced owing to diffusion of the extracellular contrast medium from surrounding regions without impaired microvasculature. Whereas late MVO might therefore be more specific as it reflects regions of ‘true’ no-reflow in contrast to early MVO, which represent areas with minimal residual blood flow. The interpretation that late MVO reflects areas of a more severely disturbed microcirculation is supported by the finding of a recent study by de Waha et al. [14] who found that the presence of late MVO was not only a better predictor than early MVO but was also the strongest independent predictor for the occurrence of death, non-fatal myocardial re-infarction and congestive heart failure after STEMI. Our study finding that the extent of late MVO was a superior predictor of LVEF at follow-up (than early MVO) is therefore in agreement.
Predictors of LVEF 90 days post-primary PCI
The present study directly compared the angiographic, gadolinium-enhanced CMR characteristics of MVO (early and late MVO) and the novel quantitative CMR perfusion imaging (stress and resting infarct region MBF) and their predictive value upon LVEF at 90 days. We showed that the extent of late MVO was a better predictor of LVEF at 90 days than the two angiographic assessments of MVO; TMPG and MBG. One possible explanation of why late MVO assessment on CMR is a better predictor of LVEF at 90 days compared to angiographic assessment is that MVO continues to evolve over the hours and days following STEMI meaning an assessment made angiographically immediately after reperfusion may lack accuracy [32]. Stress and resting infarct region MBF correlated with early and late MVO as well as predicted LVEF at 90 days on univariate analysis. However on multivariate analysis, late MVO was the best independent predictor of LVEF at 90 days.
Finally, the findings of this study suggest that late MVO is an early predictor of adverse left ventricular function after STEMI. This may become relevant in the clinical setting as late MVO could be a future early index used to assess the utility of novel therapies beyond current standard of care post acute myocardial infarction, in this high risk group.
Limitations
The number of patients in the present study was relatively small which may limit the interpretation of multivariate analysis. This study was also not powered to compare the predictive value of the angiographic and CMR parameters for evaluation of MVO with clinical end-points. We used LVEF at 90 days as a marker of left ventricular function rather than change from baseline as LVEFs on CMR prior to STEMI were unavailable. The predictive value of early MVO may have been hampered by the incomplete left ventricular coverage by first-pass perfusion. We utilised 3 short axis slices as previously described by Yan et al. [13]. Further studies are required to determine whether the new accelerated k–t SENSE first-pass perfusion described by Mather et al. [33] which allows full left ventricular coverage improves the predictive value of early MVO. MBF analysis was undertaken on a segment by segment basis with myocardial segments assigned based on coronary territory. Despite correction for dominant circulation, the distribution of myocardial segments based on coronary artery territories varies between individuals. Hence the infarct region MBF may have included the MBF values of non-infarcted segments, which might dilute its predictive accuracy.
Conclusion
Quantitative CMR perfusion assessment of resting and stress infarct region myocardial blood flow correlates strongly with left ventricular function 90 days post-primary PCI. TMPG (but not MBG) also correlated with the presence as well as extent of early and late MVO. However, of all the characteristics of microvascular injury studied, CMR-derived extent of late MVO was the strongest predictor of LVEF at 90 days following STEMI.
Abbreviations
- STEMI:
-
ST-segment elevation myocardial infarction
- MVO:
-
Microvascular obstruction
- CMR:
-
Cardiovascular magnetic resonance
- MBF:
-
Myocardial blood flow
- MBG:
-
Myocardial blush grade
- TMPG:
-
TIMI myocardial perfusion grade
- LVEF:
-
Left ventricular ejection fraction
- LGE:
-
Late gadolinium enhancement
References
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54(4):281–292. doi:10.1016/j.jacc.2009.03.054
Kloner RA, Ganote CE, Jennings RB (1974) The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest 54(6):1496–1508. doi:10.1172/JCI107898
Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA (1998) Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97(8):765–772
Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D (2004) Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109(9):1121–1126
Brosh D, Assali AR, Mager A, Porter A, Hasdai D, Teplitsky I, Rechavia E, Fuchs S, Battler A, Kornowski R (2007) Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol 99(4):442–445. doi:10.1016/j.amjcard.2006.08.054
van ‘t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra F (1998) Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle myocardial infarction study group. Circulation 97(23):2302–2306
Gibson CM, Cannon CP, Murphy SA, Marble SJ, Barron HV, Braunwald E (2002) Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 105(16):1909–1913
Schroder R, Dissmann R, Bruggemann T, Wegscheider K, Linderer T, Tebbe U, Neuhaus KL (1994) Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol 24(2):384–391
Kondo M, Nakano A, Saito D, Shimono Y (1998) Assessment of “microvascular no-reflow phenomenon” using technetium-99 m macroaggregated albumin scintigraphy in patients with acute myocardial infarction. J Am Coll Cardiol 32(4):898–903
Ito H, Okamura A, Iwakura K, Masuyama T, Hori M, Takiuchi S, Negoro S, Nakatsuchi Y, Taniyama Y, Higashino Y, Fujii K, Minamino T (1996) Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation 93(11):1993–1999
Nijveldt R, Beek AM, Hofman MB, Umans VA, Algra PR, Spreeuwenberg MD, Visser CA, van Rossum AC (2007) Late gadolinium-enhanced cardiovascular magnetic resonance evaluation of infarct size and microvascular obstruction in optimally treated patients after acute myocardial infarction. J Cardiovasc Magn Reson 9(5):765–770
Nijveldt R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Rossum AC (2008) Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52(3):181–189
Yan AT, Gibson CM, Larose E, Anavekar NS, Tsang S, Solomon SD, Reynolds G, Kwong RY (2006) Characterization of microvascular dysfunction after acute myocardial infarction by cardiovascular magnetic resonance first-pass perfusion and late gadolinium enhancement imaging. J Cardiovasc Magn Reson 8(6):831–837
de Waha S, Desch S, Eitel I, Fuernau G, Zachrau J, Leuschner A, Gutberlet M, Schuler G, Thiele H (2010) Impact of early vs. late microvascular obstruction assessed by magnetic resonance imaging on long-term outcome after ST-elevation myocardial infarction: a comparison with traditional prognostic markers. Eur Heart J 31(21):2660–2668. doi:10.1093/eurheartj/ehq247
Selvanayagam JB, Jerosch-Herold M, Porto I, Sheridan D, Cheng AS, Petersen SE, Searle N, Channon KM, Banning AP, Neubauer S (2005) Resting myocardial blood flow is impaired in hibernating myocardium: a magnetic resonance study of quantitative perfusion assessment. Circulation 112(21):3289–3296. doi:10.1161/CIRCULATIONAHA.105.549170
Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E (2000) Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 101(2):125–130
Lyne JC, Gatehouse PD, Assomull RG, Smith GC, Kellman P, Firmin DN, Pennell DJ (2007) Direct comparison of myocardial perfusion cardiovascular magnetic resonance sequences with parallel acquisition. J Magn Reson Imaging 26(6):1444–1451. doi:10.1002/jmri.21167
Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E (2008) Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson 10:35. doi:10.1186/1532-429X-10-35
Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47(6):1202–1210. doi:10.1002/mrm.10171
Teo KS, Carbone A, Piantadosi C, Chew DP, Hammett CJ, Brown MA, Worthley SG (2008) Cardiac MRI assessment of left and right ventricular parameters in healthy Australian normal volunteers. Heart Lung Circ 17(4):313–317
Bolognese L, Neskovic AN, Parodi G, Cerisano G, Buonamici P, Santoro GM, Antoniucci D (2002) Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation 106(18):2351–2357
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18(12):1440–1463. doi:10.1016/j.echo.2005.10.005
Klein P, Holman ER, Versteegh MI, Boersma E, Verwey HF, Bax JJ, Dion RA, Klautz RJ (2009) Wall motion score index predicts mortality and functional result after surgical ventricular restoration for advanced ischemic heart failure. Eur J Cardiothorac Surg 35(5):847–852; discussion 852-843. doi: 10.1016/j.ejcts.2008.12.046
Mather AN, Lockie T, Nagel E, Marber M, Perera D, Redwood S, Radjenovic A, Saha A, Greenwood JP, Plein S (2009) Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J Cardiovasc Magn Reson 11(1):33
Al-Saadi N, Nagel E, Gross M, Bornstedt A, Schnackenburg B, Klein C, Klimek W, Oswald H, Fleck E (2000) Noninvasive detection of myocardial ischemia from perfusion reserve based on cardiovascular magnetic resonance. Circulation 101(12):1379–1383
Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA (2004) Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 44(12):2383–2389
Hombach V, Grebe O, Merkle N, Waldenmaier S, Hoher M, Kochs M, Wohrle J, Kestler HA (2005) Sequelae of acute myocardial infarction regarding cardiac structure and function and their prognostic significance as assessed by magnetic resonance imaging. Eur Heart J 26(6):549–557
Gibson CM, Schomig A (2004) Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation 109(25):3096–3105
Appelbaum E, Abraham JM, Pride YB, Harrigan CJ, Peters DC, Biller LH, Manning WJ, Gibson CM (2009) Association of thrombolysis in myocardial infarction myocardial perfusion grade with cardiovascular magnetic resonance measures of infarct architecture after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am Heart J 158(1):84–91
Zun Z, Varadarajan P, Pai RG, Wong EC, Nayak KS (2011) Arterial spin Labeled CMR detects clinically relevant increase in myocardial blood flow with vasodilation. JACC Cardiovasc Imaging 4(12):1253–1261. doi:10.1016/j.jcmg.2011.06.023
Lund GK, Stork A, Saeed M, Bansmann MP, Gerken JH, Muller V, Mester J, Higgins CB, Adam G, Meinertz T (2004) Acute myocardial infarction: evaluation with first-pass enhancement and delayed enhancement MR imaging compared with 201Tl SPECT imaging. Radiology 232(1):49–57
Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, Becker LC, Melin JA (1998) Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation 98(10):1006–1014
Mather AN, Lockie T, Nagel E, Marber M, Perera D, Redwood S, Radjenovic A, Saha A, Greenwood JP, Plein S (2009) Appearance of microvascular obstruction on high resolution first-pass perfusion, early and late gadolinium enhancement CMR in patients with acute myocardial infarction. J Cardiovasc Magn Reson 11:33. doi:10.1186/1532-429X-11-33
Acknowledgments
Mr Thomas Sullivan; Statistician, Data Management & Analysis Centre. Discipline of Public Health, University of Adelaide for his assistance with statistical analysis. DW is supported by NHMRC and NHF Post Graduate Scholarship. MW is supported by SA Health Practitioner Fellowship.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, D.T.L., Leung, M.C.H., Richardson, J.D. et al. Cardiac magnetic resonance derived late microvascular obstruction assessment post ST-segment elevation myocardial infarction is the best predictor of left ventricular function: a comparison of angiographic and cardiac magnetic resonance derived measurements. Int J Cardiovasc Imaging 28, 1971–1981 (2012). https://doi.org/10.1007/s10554-012-0021-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-012-0021-9