Abstract
Although myocardial contrast echocardiography (MCE) can evaluate microvascular perfusion abnormalities, its prognostic value is uncertain in acute anterior wall ST-Segment elevation myocardial infarction (STEMI) with successful epicardial recanalization. Therefore, the study aims to investigate the prognostic role of qualitative and quantitative MCE in acute anterior wall STEMI with successful epicardial recanalization. 153 STEMI patients were assessed by MCE within 7 days after successful epicardial recanalization. Qualitative perfusion parameters (microvascular perfusion score index, MPSI) and quantitative perfusion parameters (A, β, and Aβ) were acquired using a 17-segment model. And corrected A and Aβ were calculated. Patients were all followed for major adverse cardiovascular events (MACEs). During median follow-up of 27 (4) months, 39 (25.49%) patients experienced MACEs, while 114 (74.51%) were free from MACEs. Patients with MACEs had higher MPSI (1.65 ± 0.13 vs. No-MACEs 1.35 ± 0.20, P < 0.001), lower β (1.09 ± 0.19 s−1 vs. No-MACEs 1.34 ± 0.30 s−1, P < 0.001), corrected A (0.17 ± 0.03 dB vs. No-MACEs 0.19 ± 0.04 dB, P = 0.039) and lower corrected Aβ (0.19 ± 0.06 dB/s vs. No-MACEs 0.25 ± 0.08 dB/s, P < 0.001). MPSI of 1.44 provided an area under the curve (AUC) of 0.872, while β of 1.18 s−1 and corrected Aβ of 0.22 dB/s provided AUCs of 0.759 and 0.724, respectively. The combination of MPSI, β and corrected Aβ provided an increased AUC of 0.964 (all P < 0.05). Time-dependent ROC analysis showed that the AUCs of the MPSI, β, corrected Aβ and the combination at 1, 1.5 and 2 years indicated a strong predictive power for MACEs (AUC = 0.900/0.894/0.881 for MPSI, 0.648/0.704/0.732 for β, 0.674/0.686/0.722 for corrected Aβ, and 0.947/0.962/0.967 for the combination, respectively). Patients with MPSI < 1.44, β > 1.18 s−1, or corrected Aβ > 0.22 dB/s had lower event rate (all Log Rank P ≤ 0.001). MPSI, β, corrected Aβ, GLS and WBC were independent predictors of MACEs with adjusted hazard ratio of 34.41 (8.18–144.87), P < 0.001 for MPSI; 39.29 (27.46–65.44), P < 0.001 for β; 8.93 (1.46–54.55), P = 0.018 for corrected Aβ; 10.88 (2.83–41.86), P = 0.001 for GLS; and 1.43 (1.16–1.75), P = 0.001 for WBC. Qualitative and quantitative MCE can accurately predict MACEs in acute anterior wall STEMI with successful epicardial recanalization, and their combined predictive value is higher.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Emergency percutaneous coronary intervention (ePCI) can effectively restore the epicardial coronary artery patency of acute ST-segment elevation myocardial infarction (STEMI) patients as early as possible, improve microvascular reperfusion and reduce complications [1, 2]. Although ePCI is currently accepted as the gold standard treatment of STEMI, the incidence of major adverse cardiovascular events (MACEs) such as recurrent myocardial infarction, stroke and death in the 1-year follow-up after ePCI is still up to 10.9%, with a mortality rate of 6.2% [3]. Previous studies [4, 5] have shown that the patency of epicardial culprit vessels and the restoration of microvascular perfusion (MVP) at the tissue level are independent predictors of MACEs after reperfusion. Therefore, for patients who achieve thrombolysis in myocardial infarction (TIMI) grade 3 epicardial flow after ePCI, it’s crucial to assess postoperative MVP for predicting the prognosis.
Myocardial contrast echocardiography (MCE), which indirectly visualizes the state of MVP by showing the refilling of the microbubbles after a high mechanical index flash, has been used to evaluate the function of myocardial microcirculation in patients with acute coronary syndrome [6, 7]. MCE parameters include qualitative perfusion parameters (microvascular perfusion score index, MPSI) and quantitative perfusion parameters (A, β, and Aβ). Qualitative parameters of MCE are more commonly used in recent studies to identity microvascular flow abnormalities which lead to worse function and prognosis in STEMI patients, especially in patients with anterior wall STEMI whose incidence of microvascular obstruction is up to 73% [8,9,10]. Nonetheless, the prognostic value of quantitative parameters of MCE for these patients is unclear. The purpose of this study was to investigate the prognostic value of the qualitative and quantitative measurements with MCE during the follow-up in a cohort of acute anterior wall STEMI patients with TIMI flow grade 3 after ePCI.
Methods
Study population
This was a prospective study, and 163 consecutive patients with acute anterior wall STEMI underwent ePCI were enrolled in Renmin Hospital of Wuhan University. The inclusion criterion was continuous onset of chest pain without remission accompanied with ST-segment elevation at lowest 0.1 mV of at least 2 leads on the electrocardiogram (acute anterior wall myocardial infarction) [11]. All patients underwent coronary angiography and confirmed that the left anterior descending (LAD) was the culprit artery. Then all patients underwent ePCI and the TIMI flow of LAD were restored to grade 3. Patients with hypersensitivity to ultrasound enhancing agents (UEAs), structural heart disease, prior myocardial infarction and previous PCI were excluded. All patients provided written informed consent. Approval was obtained from the Ethics Committee of Renmin Hospital of Wuhan University.
Echocardiographic images acquisition
All patients underwent echocardiography using the Philips Epic 7C ultrasound diagnostic system (Philips Medical Systems Inc., Andover, MA, USA) equipped with a 2.0–3.5 MHz probe within 7 days after ePCI. We collected the unenhanced images of parasternal long-axis views, the parasternal short-axis views (at the levels of mitral valve, papillary muscle and apex), the apical views (at 4-chamber, 3-chamber and 2-chamber) for consecutive five cardiac cycles with a frame rate > 50 FPS.
Then, resting real-time MCE was performed. Power-modulation images were set at a very low mechanical index (0.16–0.20) with a frame rate of 25 FPS. Acquisition settings were optimized by adjusting the depth, focus, gain and time-gain compensation controls. The UEAs was SonoVue (Bracco, Milan, Italy). 2.5 ml reconstituted SonoVue was further diluted with 12.5 ml saline, which was administered intravenously at the rate of about 4 ml/min. Continuous dynamic images were collected in the apical 4-chamber, 3-chamber, 2-chamber and short-axis views, in which a brief high-mechanical index (1.1–1.3) ‘‘flash’’ impulse (duration 5–10 frames) was manually triggered, followed by very low mechanical index imaging for up to 15 cardiac cycles. The patient was observed for anaphylaxis after contrast infusion about 30 min. And all patients had no adverse reactions.
Echocardiographic data analysis
The left ventricular end-diastolic volume (LVEDV), end-systolic volume (LVESV), and ejection fraction (LVEF) were calculated with biplane Simpson’s method using contrast-enhanced images. The wall motion score index (WMSI) was determined by dividing left ventricular (LV) into 17 segments using the following scoring system: 1-normal, 2-hypokinetic, 3-akinetic or 4-dyskinetic. The speckle tracking analysis was performed off-line with stored dynamic unenhanced images using Qlab (version 10.7, Philips Medical Systems, Bothell, WA, USA). The longitudinal strain was determined using an automatic analysis process triggered by placing 3 points (2 at the basal segments along the mitral valve annulus and 1 at the apex) inside the endocardium at the apical views. Then the global longitudinal strain (GLS) was calculated.
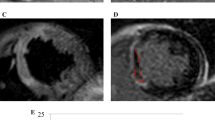
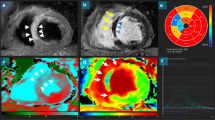
The MPSI was depended on LV 17-segment model and evaluated according to the following scoring system: 1-homogeneous contrast perfusion; 2-partial or reduced contrast perfusion; and 3-absent contrast perfusion [12]. The quantitative analysis of MCE was performed off-line with stored dynamic contrast-enhanced images using Qlab. Regions of interest were manually positioned, and realigned frame-by-frame to maintain the myocardium contained rather than the LV cavity and pericardium during the entire replenishment sequence. The automatic motion compensation mode was used when necessary. Segments with artifacts or attenuation were excluded. Qlab software automatically constructed a replenishment curve and fitted to an exponential function: y = A(1–e−βt) + C, in which A is the peak plateau contrast intensity while β represents the rate of UEAs replenishment, as shown in Fig. 1. The A, β and product of Aβ were expressed as the average values of all segments. “A” was subsequently corrected for the blood pool video intensity to acquire “corrected A” and “corrected Aβ” [13].
Clinical parameters and angiographic parameters
Baseline history, clinical characteristics, symptoms to reperfusion time and results of laboratory tests were recorded at the beginning of the enrollment period. Also, the indexes related to coronary angiography including the pre- and post-PCI TIMI flow grades, TIMI thrombus burden, lesion locations and multivessel lesions were recorded. The TIMI thrombus burden was recorded, in which scoring 4 points or above (thrombus length greater than 2 times vessel diameter or completely occlusive vessel) was considered to be high thrombus load.
Follow-up
Follow-up was started at the time of MCE and collected by periodic phone interviews and hospitalization records, with major adverse cardiovascular events (MACEs) as the end point. MACEs were defined as the composite of all-cause mortality, admission for congestive heart failure, recurrent MI, and need for implantable cardioverter-defibrillator placement [14]. According to the occurrence of MACEs during the follow-up, we divided the patients into No-MACEs and MACEs groups.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (version 21.0, SPSS, Inc., Chicago, U.S.) and R program (version 4.0.3). The Kolmogorov–Smirnov test was used to confirm the normal distribution of the data. Normally distributed continuous variables were expressed as the mean ± standard deviation and assessed by the independent-samples t-test. Skew-distributed continuous variables were reported as the median (interquartile range) and compared using a Mann–Whitney U test. Categorical variables were presented as the frequency (percentage) and analyzed using a χ2 test. The receiver operator characteristic (ROC) analyses were performed for ROC curve and area under curve (AUC) with sensitivity, specificity and predictable cutoff values of parameters. Time-dependent ROC curves were performed to further understand the prediction capabilities. The AUCs of parameters were compared by Wilcoxon rank sum test for predictive values. Kaplan–Meier survival analysis was performed with Log Rank test according to Mantel–Haenszel, where cutoff values were used from preceding ROC analysis. Univariate and multivariate Cox regression models were used to identify predictors of MACEs, and to adjust for known confounders and for variables that were significant on the univariate model. The reproducibility analysis used the intra-class correlation coefficient (ICC) of qualitative and quantitative parameters of MCE for intra- and inter-observer measurements. A 2-tailed P value less than 0.05 was considered statistically significant.
Results
We excluded 5 patients because of poor acoustic windows, and 5 patients who were lost to follow up. As a result, 153 patients (120 men, 33 women; aged 55.63 ± 10.87 years) were included in the final study, as shown in Table 1. The median follow-up time for all patients was 27 months, and the interquartile range was 4 months. During the follow-up, 114 (74.51%) patients were free from MACEs (No-MACEs group); 39 (25.49%) patients experienced MACEs (MACEs group): 17 patients were hospitalized due to decompensated congestive heart failure, and 8 patients had recurrent myocardial infarction, 9 patients used ICD and 5 patients were dead.
Baseline clinical characteristics
The baseline clinical characteristics are summarized in Table 1. The proportion of patients with peak troponin I (TNI) ≥ 50 ng/ml in the MACEs group was significantly higher than that in the No-MACEs group (n = 33, 84.62% vs. No-MACEs n = 72, 65.79%, P = 0.013). The prevalence of hyperlipidemia was also higher in the MACEs group (n = 21, 53.85% vs. No-MACEs n = 39, 34.21%, P = 0.030). Patients with MACEs had a higher WBC count (12.77 ± 2.69 vs. No-MACEs 11.11 ± 3.28, P = 0.005). And there were no differences in residual clinical characteristics between the MACEs group and No-MACEs group (all P > 0.05).
Angiographic parameters
Comparisons of angiographic parameters between patients with and without MACEs are presented in Table 2. Patients with MACEs exhibited higher proportions of LAD proximal lesions (n = 24, 61.54% vs. No-MACEs n = 48, 42.11%, P = 0.036) and lower proportions of middle lesions (n = 14, 35.90% vs. No-MACEs n = 65, 57.02%, P = 0.023) than patients without MACEs. Other parameters such as symptoms to reperfusion time, pre-PCI TIMI flow grade, TIMI thrombus burden and the multivessel lesions also didn’t achieve statistical significance between the two groups (all P > 0.05).
Echocardiographic characteristics
As shown in Table 3, patients with MACEs had significantly larger WMSI (1.64 ± 0.23 vs. No-MACEs 1.48 ± 0.27, P = 0.001), MPSI (1.65 ± 0.13 vs. No-MACEs 1.35 ± 0.20, P < 0.001) and a smaller LVEF (44.13 ± 7.36 vs. No-MACEs 48.00 ± 9.75, P = 0.011), GLS (− 11.67 ± 1.81 vs. No-MACEs − 13.51 ± 3.71, P < 0.001) than patients without MACEs. Moreover, no significant differences in LVEDV and LVESV were identified between the two groups (all P > 0.05).
Quantitative MCE analysis was feasible in 2327 of 2601 segments (89.47%), unsuccessful in 274 segments (10.53%) due to not obtaining a satisfactory curve. Compared with patients without MACEs, β (1.09 ± 0.19 s−1 vs. No-MACEs 1.34 ± 0.30 s−1, P < 0.001), corrected A (0.17 ± 0.03 dB vs. No-MACEs 0.19 ± 0.04 dB, P = 0.039) and corrected Aβ (0.19 ± 0.06 dB/s vs. No-MACEs 0.25 ± 0.08 dB/s, P < 0.001) were significantly lower in patients with MACEs; however, no statistically significant differences were found in A (7.33 ± 1.27 dB vs. No-MACEs 7.54 ± 1.39 dB, P = 0.419), blood pool video intensity (41.11 dB, IQR 1.19 dB vs. No-MACEs 41.37 dB, IQR 2.93 dB, P = 0.598) (Table 3).
Prediction for MACEs after ePCI
The predicted values of above variables with statistical differences for MACEs after ePCI are listed in Table 4 and Fig. 2a. The results indicated that MPSI, β, corrected Aβ, WMSI, GLS, WBC count and peak ultra-TNI ≥ 50 ng/ml achieved an area under the curve (AUC) 0.872, 0.759, 0.724, 0.683, 0.644, 0.666 and 0.607 in turn for the ability to predict MACEs. A cut-off value for MPSI was 1.44 with sensitivity of 92.30% and specificity of 68.40%, for β was 1.18 s−1 with sensitivity of 76.90% and specificity of 71.10%, and for corrected Aβ was 0.22 dB/s with sensitivity of 69.20% and specificity of 65.80%. The ROC curve of the combined prediction for MACEs by the qualitative and quantitative MCE indicators MPSI, β and corrected Aβ was drawn, and the AUC was increased to 0.964 (P < 0.05) while the sensitivity and specificity were increased to 92.30% and 92.10%, respectively. In addition, time-dependent ROC analysis was performed on MPSI, β, corrected Aβ and the combination to further understand the prediction capabilities for 1-, 1.5- and 2- year MACEs. The AUCs of the MPSI, β, corrected Aβ and the combination at 1, 1.5 and 2 years, as shown in Fig. 2b-e, indicated a strong predictive power for MACEs (AUC = 0.900/0.894/0.881 for MPSI, 0.648/0.704/0.732 for β, 0.674/0.686/ 0.722 for corrected Aβ, and 0.947/0.962/ 0.967 for the combination, respectively).
Receiver operator characteristic curves for the prediction of MACEs in acute anterior wall STEMI patients with TIMI flow grade 3 after ePCI. aThe AUCs of MPSI, β and corrected Aβ were 0.872, 0.759 and 0.724. The combination of MPSI, β and corrected Aβ provided an increased AUC of 0.964 (sensitivity of 92.30% and specificity of 92.10%). b–e Time-dependent ROC analysis showed the AUCs at 1, 1.5 and 2 years were 0.900/0.894/0.881 for MPSI, 0.648/0.704/0.732 for β, 0.674/0.686/0.722 for corrected Aβ, and 0.947/0.962/0.967 for the combination, respectively. Abbreviations: MACEs major adverse cardiovascular events, STEMI ST-segment elevation myocardial infarction, TIMI thrombolysis in myocardial infarction, ePCI emergency percutaneous coronary intervention, AUC area under the curve, GLS global longitudinal strain, A peak plateau myocardial contrast intensity, β the replenishment curve slope, MPSI microvascular perfusion score index
After dichotomization of patients using MPSI < 1.44 or β > 1.18 s−1 or corrected Aβ > 0.22 dB/s as a cutoff value, Kaplan–Meier curves confirmed significant differences in the probability of free from MACEs (all Log Rank P < 0.001), as presented in Fig. 3.
Kaplan–Meier survival curves comparing the probability of being free from MACEs: a patients with MPSI < 1.44 versus MPSI ≥ 1.44, b patients with β > 1.18 s−1 versus β ≤ 1.18 s−1, and c patients with corrected Aβ > 0.22 dB/s versus corrected Aβ ≤ 0.22 dB/s. MACEs major adverse cardiovascular events, A peak plateau myocardial contrast intensity, β the replenishment curve slope, MPSI microvascular perfusion score index
Table 5 showed univariate and multivariate Cox regression analyses of parameters associated with MACEs. Hyperlipemia, peak TNI ≥ 50 ng/ml, WBC, LVEF < 50%, WMSI > 1.44 were significant predictors of MACEs in univariate proportional hazard analyses, in addition to MPSI, β and corrected Aβ. Using multiple variable proportional hazard models (adjusting for significant variables in the univariate model), MPSI, β, corrected Aβ, GLS and WBC were independent predictors of MACEs, with adjusted hazard ratio of 34.41 (8.18–144.87), P < 0.001 for MPSI; 39.29 (27.46–65.44), P < 0.001 for β; 8.93 (1.46–54.55), P = 0.018 for corrected Aβ; 10.88 (2.83–41.86), P = 0.001 for GLS; and 1.43 (1.16–1.75), P = 0.001 for WBC, respectively.
Reproducibility
We randomly selected 15 STEMI patients for the reproducibility analysis. The intra-observer analysis indicated the results of MPSI, β, A, blood pool video intensity and corrected Aβ were 0.94, 0.81, 0.82, 0.87 and 0.81 respectively. The inter-observer analysis revealed the results of MPSI, β, A, blood pool video intensity and corrected Aβ were 0.93, 0.79, 0.78, 0.85 and 0.79 respectively.
Discussion
In this study, we investigated the prognostic impact of early qualitative and quantitative MCE evaluations on further risk stratification in 153 successfully reperfused acute anterior wall STEMI patients with TIMI flow grade 3. The major findings of our study were as follows: (1) patients experienced MACEs exhibited the poor qualitative perfusion parameter of MPSI, accompanied by lower quantitative perfusion parameters of β, corrected A and corrected Aβ; (2) early assessments of MPSI, β and corrected Aβ after ePCI could provide predictive value of MACEs, and the combination of the three had the best predictive power; (3) patients with MPSI < 1.44 or β > 1.18 s−1 or corrected Aβ > 0.22 dB/s might be less prone to MACEs.
MCE for evaluation of MVP
MCE indirectly reflects MVP by relying on the velocity and peak intensity of UEAs filling capillaries which including qualitative and quantitative analysis methods. The capillaries account for 90% of the myocardial blood volume [15]. When the UEAs within the capillaries achieve a steady state, A represents the myocardial blood volume. After a high mechanical index flash, β reflects the capillary blood velocity. The myocardial blood flow is expressed as Aβ. The quantitative values of A, β and Aβ are favorable supplements for qualitative visual evaluation of MVP. MCE has been used to detect myocardial viability, evaluate coronary artery flow reserve and diagnose coronary artery disease [16,17,18]. A meta-analysis [19] indicated the diagnostic efficiency of A, β and Aβ in determining coronary artery disease, and the AUCs were 0.637, 0.851 and 0.859, respectively. Additionally, animal as well as human studies have demonstrated that MCE is safe and effective in evaluating MVP in STEMI patients [20,21,22,23].
Potential mechanisms associations between poor MVP and MACEs
Our study showed that patients with MACEs exhibited increased MPSI as well as decreased β, corrected A and corrected Aβ. To date, it’s still uncertain on the mechanisms of adverse prognosis due to the MVP abnormality. The potential mechanisms are as follows. The self-regulation of myocardial microcirculation is a vital mechanism to ensure the flow supply of coronary arteries. And the injured myocardial microcirculation results in poor MVP, which accounts for angina and recurrent myocardial infarction in the condition of increased oxygen demand [24]. Baks et al. [25] demonstrated that LV segments with abnormal MVP didn’t gain improvements in terms of the wall thickening, even became thinner during 5 months follow-up. The thinning wall can cause increased tension of the involved and adjacent myocardium, promote myocardial extension and expansion, and further contribute to myocardial remodeling. The resulting electroanatomical substrate is characterized by increased nonuniform anisotropy and macroscopic slowing of conduction, which is associated with a higher risk of reentry phenomenon, arrhythmia and sudden death [26]. A report from Nijveldt et al. [27] showed that most STEMI patients with MVP defect had enlarged LVEDV and unimproved LVEF in the 4-month follow-up, which supports the hypothesis that MVP abnormalities are closely related to LV remodeling, chronic heart failure, even cardiac death.
Prognostic value of qualitative and quantitative MCE in STEMI patients
Several studies have found that qualitative and quantitative parameters of MCE can reflect the recovery of myocardial structure and function in reperfused STEMI patients, and serve as independent predictors for short-term or long-term prognosis [28, 29]. According to Galiuto et al. [12], only TIMI flow grade less than 3 and contrast defect size more than 25% were independently related with LV remodeling; among patients with TIMI flow grade 3, contrast defect size was the only independent variable in connection with LV remodeling. A study conducted by Dwivedi et al. [30] investigated that MPSI was a strong predictor for reinfarction and death in patients with STEMI. When the cutoff point of MPSI was 1.67, the sensitivity and specificity were 80% and 76%, respectively. A more recent study from the Mayo clinic [31] revealed that MPSI, β, corrected A and corrected Aβ provided AUCs of 0.873, 0.858, 0.747 and 0.842 in the matter of predicting MACEs in STEMI patients after PCI. And MPSI > 1.58 and corrected Aβ < 0.32 were most valuable predictors of MACEs with an adjusted hazard ratio of 3.41 and 4.19. Our data extended these clinical observations through enlarging the sample size and focusing on particular acute anterior wall STEMI patients with TIMI flow grade 3 after ePCI. As in the previous studies, our findings also confirmed that poorer MPSI and lower myocardial blood flow were predictive of a poor outcome.
Unlike other studies, our results proposed that the combination of qualitative and quantitative parameters of MCE had higher clinical significance for the first time, with the AUC of predicting MACEs rising to 0.964. We also discovered quantitative parameter A was less prognostic than β and Aβ. Furthermore, MPSI, β and Aβ had more effect on predicting outcomes than other noninvasive parameters such as GLS and WMSI, especially with high specificity. GLS has the same sensitivity with MPSI, while the specificity was much lower, which may be explained by the early postoperative myocardial perfusion was restored but the wall motion was not in some patients without MACEs.
Clinical implications
All these findings suggest that the changes in MVP are related to the new-onset of MACEs after ePCI. Therefore, early determination of post-ePCI MVP may help identify the risk of MACEs. If the MVP is critically poor, optimal or additional strategies may be required. MCE, radionuclide perfusion imaging and cardiac magnetic resonance are noninvasive imaging modalities widely used to assess MVP. Unlike radionuclide perfusion imaging, MCE is portable, nonradiative, inexpensive, and can obtain myocardial blood flow. Cardiac magnetic resonance has excellent spatial resolution, however its application was limited in patients with renal insufficiency, cardiac pacemaker, claustrophobia and critically ill in intensive care unit. Also, MCE, which can be performed at the bedside, acquire data rapidly and have no side effect on kidney, may be the technique of prior choice for early evaluation of MVP in STEMI patients after ePCI.
Study limitations
This study has several limitations. The main limitation is the evaluation of qualitative and quantitative parameters of MCE all highlight global MVP at the cost of more detailed information between the non-ischemic and ischemic segments. In addition, the patient sample was single center. Furthermore, we should collect more ultrasound data during follow-up to assess the effects of MVP on recovery of LV structure and function.
Conclusions
Qualitative and quantitative parameters of MCE can accurately predict MACEs in acute anterior wall STEMI patients with TIMI flow grade 3 following ePCI, and their combined predictive value is higher, which is expected to provide a practical method for early clinical risk stratification of acute anterior wall STEMI patients after ePCI.
References
Levine GN, Bates ER, Blankenship JC et al (2011) 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the american college of cardiology foundation/american heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. J Am Coll Cardiol 58:e44-122. https://doi.org/10.1016/j.jacc.2011.08.007
Ogara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction: Executive summary: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol 61:485–510. https://doi.org/10.1016/j.jacc.2012.11.018
Savic L, Mrdovic I, Asanin M et al (2019) Using the RISK-PCI score in the long-term prediction of major adverse cardiovascular events and mortality after primary percutaneous coronary intervention. J Interv Cardiol 2019:2679791. https://doi.org/10.1155/2019/2679791
Bethke A, Halvorsen S, Bøhmer E et al (2015) Myocardial perfusion grade predicts final infarct size and left ventricular function in patients with ST-elevation myocardial infarction treated with a pharmaco-invasive strategy (thrombolysis and early angioplasty). EuroIntervention 11:518–524. https://doi.org/10.4244/eijy15m04_02
Fearon WF, Low AF, Yong AS et al (2013) Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation 127:2436–2441. https://doi.org/10.1161/circulationaha.112.000298
Wei K, Peters D, Belcik T et al (2010) A predictive instrument using contrast echocardiography in patients presenting to the emergency department with chest pain and without ST-segment elevation. J Am Soc Echocardiogr 23:636–642. https://doi.org/10.1016/j.echo.2010.03.013
Jiang L, Yao H, Liang ZG (2017) Postoperative assessment of myocardial function and microcirculation in patients with acute coronary syndrome by myocardial contrast echocardiography. Med Sci Monit 23:2324–2332. https://doi.org/10.12659/msm.901233
Xie F, Qian L, Goldsweig A et al (2020) Event-free survival following successful percutaneous intervention in acute myocardial infarction depends on microvascular perfusion. Circ Cardiovasc Imaging 13:e010091. https://doi.org/10.1161/CIRCIMAGING.119.010091
Aggarwal S, Xie F, High R et al (2018) Prevalence and predictive value of microvascular flow abnormalities after successful contemporary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction. J Am Soc Echocardiogr 31:674–682. https://doi.org/10.1016/j.echo.2018.01.009
Porter TR, Xie F (2010) Myocardial perfusion imaging with contrast ultrasound. JACC Cardiovasc Imaging 3:176–187. https://doi.org/10.1016/j.jcmg.2009.09.024
O’Gara PT, Kushner FG, Ascheim DD et al (2013) 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61:e78–e140. https://doi.org/10.1016/j.jacc.2012.11.019
Galiuto L, Garramone B, Scarà A et al (2008) The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: results of the multicenter AMICI study. J Am Coll Cardiol 51:552–559. https://doi.org/10.1016/j.jacc.2007.09.051
Min SY, Song JM, Shin Y et al (2017) Quantitative segmental analysis of myocardial perfusion to differentiate stress cardiomyopathy from acute myocardial infarction: A myocardial contrast echocardiography study. Clin Cardiol 40:679–685. https://doi.org/10.1002/clc.22714
Galea N, Dacquino GM, Ammendola RM et al (2019) Microvascular obstruction extent predicts major adverse cardiovascular events in patients with acute myocardial infarction and preserved ejection fraction. Eur Radiol 29:2369–2377. https://doi.org/10.1007/s00330-018-5895-z
Kaul S, Jayaweera AR (1997) Coronary and myocardial blood volumes: noninvasive tools to assess the coronary microcirculation? Circulation 96:719–724. https://doi.org/10.1161/01.cir.96.3.785
Porter TR, Adolphson M, High RR et al (2011) Rapid detection of coronary artery stenoses with real-time perfusion echocardiography during regadenoson stress. Circ Cardiovasc Imaging 4:628–635. https://doi.org/10.1161/circimaging.111.966341
Sun L, Wang Z, Xu T et al (2018) The value of real-time myocardial contrast echocardiography for detecting coronary microcirculation function in coronary artery disease patients. Anatol J Cardiol 19:27–33. https://doi.org/10.14744/AnatolJCardiol.2017.8041
Wu J, Barton D, Xie F et al (2016) Comparison of fractional flow reserve assessment with demand stress myocardial contrast echocardiography in angiographically intermediate coronary stenoses. Circ Cardiovasc Imaging 9:e004129. https://doi.org/10.1161/circimaging.116.004129
Abdelmoneim SS, Dhoble A, Bernier M et al (2009) Quantitative myocardial contrast echocardiography during pharmacological stress for diagnosis of coronary artery disease: a systematic review and meta-analysis of diagnostic accuracy studies. Eur J Echocardiogr 10:813–825. https://doi.org/10.1093/ejechocard/jep084
Janardhanan R, Moon JC, Pennell DJ, Senior R (2005) Myocardial contrast echocardiography accurately reflects transmurality of myocardial necrosis and predicts contractile reserve after acute myocardial infarction. Am Heart J 149:355–362. https://doi.org/10.1016/j.ahj.2004.06.018
Janardhanan R, Swinburn JM, Greaves K, Senior R (2003) Usefulness of myocardial contrast echocardiography using low-power continuous imaging early after acute myocardial infarction to predict late functional left ventricular recovery. Am J Cardiol 92:493–497. https://doi.org/10.1016/s0002-9149(03)00713-6
Coggins MP, Sklenar J, Le DE et al (2001) Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation 104:2471–2477. https://doi.org/10.1161/hc4501.098954
Mathias W, Tsutsui JM, Tavares BG et al (2016) Diagnostic ultrasound impulses improve microvascular flow in patients with STEMI receiving intravenous microbubbles. J Am Coll Cardiol 67:2506–2515. https://doi.org/10.1016/j.jacc.2016.03.542
Li X, He S, Zhang YS et al (2016) Resting myocardial contrast echocardiography for the evaluation of coronary microcirculation dysfunction in patients with early coronary artery disease. Clin Cardiol 39:453–458. https://doi.org/10.1002/clc.22548
Baks T, van Geuns RJ, Biagini E et al (2006) Effects of primary angioplasty for acute myocardial infarction on early and late infarct size and left ventricular wall characteristics. J Am Coll Cardiol 47:40–44. https://doi.org/10.1016/j.jacc.2005.09.008
Sattler SM, Skibsbye L, Linz D et al (2019) Ventricular arrhythmias in first acute myocardial infarction: epidemiology, mechanisms, and interventions in large animal models. Front Cardiovasc Med 6:158. https://doi.org/10.3389/fcvm.2019.00158
Nijveldt R, Beek AM, Hirsch A et al (2008) Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol 52:181–189. https://doi.org/10.1016/j.jacc.2008.04.006
Yang L, Xia C, Mu Y et al (2016) Prognostic value of real time myocardial contrast echocardiography after percutaneous coronary intervention. Echocardiography 33:421–430. https://doi.org/10.1111/echo.13061
Bolognese L, Carrabba N, Parodi G et al (2004) Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109:1121–1126. https://doi.org/10.1161/01.cir.0000118496.44135.a7
Dwivedi G, Janardhanan R, Hayat SA et al (2007) Prognostic value of myocardial viability detected by myocardial contrast echocardiography early after acute myocardial infarction. J Am Coll Cardiol 50:327–334. https://doi.org/10.1016/j.jacc.2007.03.036
Abdelmoneim SS, Martinez MW, Mankad SV et al (2015) Resting qualitative and quantitative myocardial contrast echocardiography to predict cardiac events in patients with acute myocardial infarction and percutaneous revascularization. Heart Vessels 30:45–55. https://doi.org/10.1007/s00380-013-0460-9
Funding
This study was supported by “the Fundamental Research Funds for the Central Universities” (2042021kf0121).
Author information
Authors and Affiliations
Contributions
YXZ and YGH carried out the studies, participated in data collection, established the framework of this study, performed the statistical analysis, and drafted the manuscript under the supervision of QZ and JLC. The MCE examination was performed by SC, JRL and YX. WYY helped to proofread the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Ethical approval
The study protocol was approved by the local medical ethics committee (WDRY2020- K189).
Informed consent
Informed consent was obtained from all patients participants included in the study.
Consent for publication
All patients signed informed consent regarding publishing their anonymized data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Yx., Hu, Yg., Cao, S. et al. Prognostic value of myocardial contrast echocardiography in acute anterior wall ST-segment elevation myocardial infarction with successful epicardial recanalization. Int J Cardiovasc Imaging 38, 1487–1497 (2022). https://doi.org/10.1007/s10554-022-02545-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02545-8