Abstract
Objective The aim of this study was to compare the diagnostic accuracy of myocardial perfusion imaging (MPI) by positron emission tomography (PET) with the diagnostic accuracy of MPI by single photon emission computed tomography (SPECT) in two comparable patient cohorts, using coronary angiography (CA) as the standard of reference. Methods A “SPECT-group” of 80 patients (15 female, 65 male; mean age 60 ± 9 years) and a “PET-group” of 70 patients (14 female, 56 male; mean age 57 ± 10 years) underwent a one day stress/rest examination either with attenuation-corrected 13N-ammonia PET or attenuation-corrected 201TlCl SPECT or 99mTc-hexakis-methoxy-isobutyl-isonitril (MIBI) SPECT. PET and SPECT results were semiquantitatively graded using a 6-segment heart model. All patients underwent CA, and stenoses were graded as a diameter reduction ≥50%. Results Coronary findings between both groups did not significantly differ at CA. For the SPECT-group overall sensitivity and specificity for localisation of stenoses was 77% and 84%. Respective values for the PET-group were 97% and 84%. The specificity of MPI by SPECT in the detection of ischemia was 74% and 91% for MPI by PET. The diagnostic accuracy of MPI improves when the individual coronary dominance and previous coronary revascularisations are taken into account. Conclusion MPI by 13N-ammonia PET is more sensitive in the detection and localisation of coronary stenoses, and more specific in the detection of ischemia than MPI by 201TlCl/99mMIBI SPECT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Almost 2 decades ago, first reports on the diagnostic accuracy of myocardial perfusion imaging (MPI) by positron emission tomography (PET) have described its superiority over single photon emission computed tomography (SPECT) in the diagnosis of coronary artery disease (CAD) [1]. Compared to SPECT, PET offers several potential advantages, such as: a higher spatial resolution, lower effective dose due to greater counting efficiency, and higher temporal resolution, allowing fast dynamic imaging of tracer kinetics and thus absolute quantification of myocardial perfusion [2–4]. These advantage have presumably influenced the results of consecutive studies, confirming the higher sensitivity and specificity of PET [1, 5–8].
However, such studies have remained sparse and comparability is hampered by a large variability of protocols used. Therefore, more data are required to define the added value of PET over SPECT justifying the use of a more complex and expensive technique. This would also help to increase the use of PET MPI, which has remained very limited in daily clinical routine, although the availability of PET cameras has dramatically increased, mainly because of its clinical role in oncology [9].
Therefore, the aim of this study was to compare the diagnostic accuracy of MPI by PET with the diagnostic accuracy of MPI by SPECT in two comparable patient cohorts, using coronary angiography (CA) as the standard of reference.
Methods
Patients
A “SPECT-group” of 80 patients (15 female, 65 male; mean age 60 ± 9 years; age range 36–76 years) underwent MPI by SPECT for further evaluation of known CAD (n = 55) or because of suspected CAD (n = 25). Similarly, a “PET-group” of 70 patients (14 female, 56 male; mean age 57 ± 10 years; age range 36–73 years) underwent MPI by PET for further evaluation of known CAD (n = 49) or because of suspected CAD (n = 21). The use of SPECT or PET for MPI was determined by the referring physician. All patients (n = 150) underwent coronary angiography.
MPI SPECT acquisition
Patients were instructed to fast and discontinue antiischemic cardiac medication 24 h before the examination. All patients underwent a 1 day stress/rest protocol. Rest data was acquired after approximately 75–85 MBq 201Thallium-Chlorid (TlCl) were injected into a right antecubital vein. For stress data patients underwent a treadmill exercise protocol: during the last 60–90 s of peak exercise (age-predicted maximal heart rate, estimated by 220—age) the patient received 600–800 MBq 99mTc-hexakis-methoxy-isobutyl-isonitril (MIBI) intravenously; heart rate and blood pressure were measured every 2 min, and ECG was monitored continuously.
If patients were unable to undergo treadmill exercise, an intravenous infusion of dipyridamole (140 μg/kg/min) over 4 min [10] and a bolus of 600–800 MBq 99mTc-MIBI were injected 8 min after the beginning of the dipyridamole infusion.
SPECT data at rest were acquired directly after injection of the tracer while SPECT data for stress were acquired with one hour delay, both with a triple-detector camera (PRISM 3000, Picker, Bedford Heights, OH, USA) with the following parameters: a low-energy, high-resolution collimator; a 20% symmetric window at 140 keV; a 64 × 64 matrix; an elliptic orbit with step-and-shoot acquisition at 3° intervals over 180°; and a 20-s dwell time per stop.
For attenuation correction simultaneous transmission measurements with either a 99mTc line source for examinations with 201ThCl, or a 153Gadolinium line source for examinations with 99mTc-MIBI were acquired.
MPI PET acquisition
Patients were instructed to fast and discontinue antiischemic cardiac medication 24 h before the PET examination. Studies were performed with a GE Advance PET scanner (GE Medical Systems, Milwaukee, Wisconsin; axial field of view 35 × 4.25 mm). Rest data was acquired directly after an injection of 700–900 MBq of 13N-ammonia into a peripheral vein by the bolus technique. Stress data was acquired approximately 5 halftimes (1 hour) after the acquisition of the rest data, starting with a 4-min dipyridamole infusion (140 μg/kg/min), which was followed by a second bolus of 700–900 MBq of 13N-ammonia, 8 min after the beginning of the dipyridamole infusion. Stress data acquisition was started directly after the second bolus of 13N-ammonia. Serial transaxial tomographic images of the heart (12 × 10-s, 4 × 30-s, 1 × 60-s and 2 × 300-s frames) were acquired for all rest and stress data. After 15-min acquisition, a 20-min transmission scan for photon attenuation correction was performed using an external 68Germanium source as previously reported [11].
Analysis of MPI data
PET and SPECT images were transferred to external workstations. On reformatted short-axis, vertical long-axis, and horizontal long-axis slices, encompassing the entire left ventricle, the left ventricle was subdivided into six segments (anterior, apical, inferior, posterior, lateral, septal), as previously published [12]. Two independent observers, blinded to the results of coronary angiography, semiquantitatively graded tracer uptake for each segment in two planes in consensus: normal uptake (grade 0), mildly abnormal (grade 1), moderately abnormal (grade 2) or severely abnormal (grade 3) of uptake. After calculating the mean grade of both planes, a grade ≥1.5 in at least one segment was defined as pathologic.
Coronary angiography
CA was performed according to standard techniques and multiple views were stored on a CD-ROM. The angiograms were evaluated by an experienced observer who was blinded to the results from SPECT and PET. The coronary arteries were segmented according to the guidelines of the American Heart Association [13] and analysis was performed in all vessels with a luminal diameter of at least 1.5 mm, excluding those vessels distal to complete occlusions. Each vessel segment was scored as being stenosed, defined as a diameter reduction of ≥50%, which was considered the standard of reference for the assessment of coronary artery disease, as it is generally assumed that lesions above this cut off may induce detectable perfusion defects.
Left ventricular segments (as defined above) were assigned to vascular territories as previously published, with and without taking into account the individual coronary dominance [14, 15] (Table 1). Furthermore, previous coronary interventions were taken into account for the determination of the diagnostic accuracy of SPECT and PET.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation and categorical variables as frequencies, median (25th, 75th percentiles), or percentages.
All statistical analysis was performed using SPSS software (SPSS 12.0.1, Chicago, IL, USA). The clustered nature of the data [i.e., the fact that there were not 480 independent segments with SPECT (or 420 with PET) but instead clusters of segments in 80 (70 with PET) patients] was taken into account.
Sensitivity and specificity were calculated from chi-squared-tests of contingency. Because of the interdependencies between different segments, the statistics were calculated on a per-segment and on a per-patient basis (presence of at least one positive finding or absence of positive findings in each patient). CA was considered the standard of reference.
To determine differences between the two groups (SPECT and PET), Student’s t-tests for independent samples were performed for the variables: age and BMI; chi-squared-tests were performed for all other variables. A P-value of <0.05 was considered to indicate statistical significance.
Results
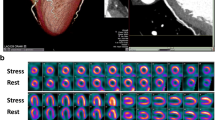
CA and MPI (SPECT or PET) were successfully performed in all patients (n = 150) (Figs. 1 and 2). CA revealed 122 patients (81%) to have CAD (defined as at least one coronary lesion with luminal narrowing ≥50%). Patients undergoing SPECT or PET did not differ among each other with regard to age, gender, BMI, cardiovascular risk, history of myocardial infarction, and particularly not with regard to coronary findings (Tables 2 and 3).
Stress and rest SPECT images reveal an irreversible inferior basal perfusion defect (open arrow heads), representing a myocardial scar. At stress an additional minor lateral perfusion defect (solid arrow heads) is indicative of minor ischemia. VLA: vertical long axis; HLA: horizontal long axis; SA: short axis
Seventeen patients (21%) in the SPECT-group had previous coronary revascularisation [10 × coronary artery bypass graft surgery (CABG), 7 × percutanous coronary intervention (PCI)], and 13 patients (19%) in the SPECT-group had previous coronary revascularisation (7 × CABG, 4 × PCI).
The diagnostic accuracy of MPI was calculated for both groups with and without taking into account successful revascularization and considering coronary dominance (Table 3).
MPI SPECT analysis
The overall sensitivity of SPECT to determine CAD in a patient was 85%. Sensitivity was unchanged when coronary arteries with previous successful revascularisation (PCI or bypass) were rated as “not stenosed”. Specificities were not calculated, due to the small number of patients without coronary stenoses [16].
With respect to the localisation of the SPECT findings (in 480 segments) and the localisation of CA findings in the three coronary arteries, overall sensitivity and specificity was 77% and 84% (values for each coronary artery are demonstrated in Fig. 3). In general, sensitivities and specificities increased when previous revascularisation procedures and/or coronary dominances were taken into account (Table 4, Fig. 3).
Regional sensitivity and specificity for the localisation of SPECT and PET findings in the territory subtended by the left anterior descending artery (LAD) (a), the circumflex artery (CX) (b), and the right coronary artery (RCA) (c). Results are demonstrated overall, considering previous revascularisation, considering coronary dominance, and considering coronary revasc. (revascularisation) and coronary dominance
Thirty patients (38%) had reversible perfusion defects (ischemia); specificity was 74%; sensitivity was not calculated because MPI is the accepted reference standard for the detection of ischemia.
MPI PET analysis
Overall sensitivity for the determination of CAD with PET was 96%. Sensitivity improved when coronary arteries with previous revascularisation (PCI or bypass) were rated as “not stenosed” (Table 4).
Overall sensitivity and specificity for the localisation of the PET findings (in 420 segments) with respect to the localisation of CA findings, was 97% and 84% (values for each coronary artery are demonstrated in Fig. 3). Similar to SPECT, sensitivities and specificities increased when previous revascularisation procedures and/or coronary dominances were taken into account (Table 4, Fig. 3).
Ischemia was detected by PET in 16 patients (23%); specificity was 91% and 95%; sensitivity was not calculated.
Discussion
Despite the suggested superiority of MPI by PET over MPI by SPECT, SPECT is primarily used in clinical routine today. Our study adds to the previous knowledge on MPI using SPECT and PET: (a) in a direct comparison of 2 patient population with comparable prevalence and extent of CAD, MPI by 13N-ammonia PET is more sensitive and more specific in the detection and localisation of coronary stenoses than MPI by 201TlCl/99mMIBI SPECT; (b) the specificity in the overall detection of ischemia is higher with MPI by 13N-ammonia PET; (c) individual coronary dominance and previous coronary revascularisations need to be taken into account when determining the diagnostic accuracy of MPI.
Data of direct comparison of MPI using SPECT and PET is sparse and inhomogeneous [1, 5–8]. Tamaki and colleagues [1] have compared 13N-ammonia PET with 201TlCl SPECT, not using attenuation correction, and more importantly, in groups of patients with a prevalence of CAD of over 90%, leading to very high and not significantly different sensitivities for SPECT and PET.
Comparing 82Rubidium PET with 201TlCl SPECT Stewart and colleagues [5] have demonstrated a higher specificity of PET in localising coronary stenoses, but this observation might have been influenced by a referral bias, associated with their study design, where patients with more advanced disease were preferentially referred to PET scanning [17]. On the other hand, their results were confirmed by Go and colleagues [6], using the same tracers, but with a different study design, less prone to referral bias.
Furthermore, Marwick and colleagues [7] concluded in their study, that PET can identify smaller and less ischemic areas subtended by milder coronary stenoses, but in their comparison of 82Rubidium PET and 201TlCl SPECT, attenuation correction was only used for PET. Recently, Bateman and colleagues [8] described a higher diagnostic accuracy of 82Rubidium PET compared to 99mTc sestamibi SPECT, but again attenuation correction was only used for PET, although it has been shown to substantially improve accuracy in SPECT [18–20].
As far as comparisons of studies with different study designs are reasonable, the results of our study are largely in line with previous work [1, 5–8], placing PET above SPECT with regard to diagnostic accuracy of coronary stenosis detection and localisation. However, the fact that we have used attenuation correction for SPECT, may explain why the difference between PET and SPECT was slightly smaller in our study compared to the previous reports.
In addition, we could demonstrate for the first time, that PET is more specific in the detection of ischemia than SPECT. As MPI is the reference standard for the detection of ischemia, the determination of the sensitivity of PET and SPECT is not possible. Nonetheless, hypothesising that normal myocardial perfusion must be associated with coronary arteries being free of stenoses, we calculated specificities of both methods, and could demonstrate the superiority of MPI by PET.
Finally, when using CA as the standard of reference, the knowledge of the individual coronary dominance is crucial for an adequate (but blinded) assignment of significant coronary artery stenoses to myocardial segments with reduced perfusion in MPI with SPECT and PET. Similarly, previous coronary revascularisations may interfere with the results of a study when a myocardial scar in MPI is attributed to an unstenosed vessel in CA (after reperfusion), which would lead to an incorrect false positive rating. Our study confirms a relevant impact of coronary dominance and of previous coronary revascularisations on the accuracy of segmental MPI, which should be kept in mind when planning upcoming studies on diagnostic accuracy.
Limitations of the study
The clinical assignment of patients to either the SPECT-or the PET-group might have been biased by their referring physicians. However, we could demonstrate no significant differences in age, gender, BMI, CCS classification, or prevalence or extent of CAD between both groups, indicating an excellent match of the two groups.
Conclusion
13N-ammonia PET is more sensitive and specific in the detection and localisation of coronary stenoses, and more specific in the detection of ischemia than 201TlCl/99mMIBI SPECT, even when attenuation correction is used for MPI and SPECT. The diagnostic accuracy of MPI can be improved when the individual coronary dominance and previous coronary revascularisations are taken into account.
Abbreviations
- CA:
-
Coronary angiography
- CAD:
-
Coronary artery disease
- MPI:
-
Myocardial perfusion imaging
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
References
Tamaki N, Yonekura Y, Senda M et al (1988) Value and limitation of stress thallium-201 single photon emission computed tomography: comparison with nitrogen-13 ammonia positron tomography. J Nucl Med 29:1181–1188
Di Carli MF, Hachamovitch R (2007) New technology for noninvasive evaluation of coronary artery disease. Circulation 115:1464–1480
Klocke FJ, Baird MG, Lorell BH et al (2003) ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 42:1318–1333
Slart RH, Bax JJ, van Veldhuisen DJ, van der Wall EE, Dierckx RA, Jager PL (2006) Imaging techniques in nuclear cardiology for the assessment of myocardial viability. Int J Cardiovasc Imaging 22:63–80
Stewart RE, Schwaiger M, Molina E et al (1991) Comparison of rubidium-82 positron emission tomography and thallium-201 SPECT imaging for detection of coronary artery disease. Am J Cardiol 67:1303–1310
Go RT, Marwick TH, MacIntyre WJ et al (1990) A prospective comparison of rubidium-82 PET and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med 31:1899–1905
Marwick TH, Go RT, MacIntyre WJ, Saha GB, Underwood DA (1991) Myocardial perfusion imaging with positron emission tomography and single photon emission computed tomography: frequency and causes of disparate results. Eur Heart J 12:1064–1069
Bateman TM, Heller GV, McGhie AI et al (2006) Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99 m sestamibi SPECT. J Nucl Cardiol 13:24–33
Di Carli MF, Hachamovitch R (2006) Should PET replace SPECT for evaluating CAD? The end of the beginning. J Nucl Cardiol 13:2–7
Lee SJ, Lee KH, Park SM et al (2007) Myocardial perfusion defects and coronary risk factors in symptomatic and asymptomatic elderly women. Int J Cardiovasc Imaging
Koepfli P, Wyss CA, Namdar M et al (2004) Beta-adrenergic blockade and myocardial perfusion in coronary artery disease: differential effects in stenotic versus remote myocardial segments. J Nucl Med 45:1626–1631
Margonato A, Chierchia SL, Xuereb RG et al (1995) Specificity and sensitivity of exercise-induced ST segment elevation for detection of residual viability: comparison with fluorodeoxyglucose and positron emission tomography. J Am Coll Cardiol 25:1032–1038
Austen WG, Edwards JE, Frye RL et al (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51:5–40
Fagret D, Marie PY, Brunotte F et al (1995) Myocardial perfusion imaging with technetium-99 m-Tc NOET: comparison with thallium-201 and coronary angiography. J Nucl Med 36:936–943
Berman DS, Kiat H, Friedman JD et al (1993) Separate acquisition rest thallium-201/stress technetium-99 m sestamibi dual-isotope myocardial perfusion single-photon emission computed tomography: a clinical validation study. J Am Coll Cardiol 22:1455–1464
Hor G, Sebening H, Sauer E et al (1979) 201Tl-redistribution analysis in early and delayed myocardial scintigrams of patients with coronary heart disease (CHD). Eur J Nucl Med 4:343–350
Garcia EV, Eisner RL, Patterson RE (1993) What should we expect from cardiac PET? J Nucl Med 34:978–980
Heller GV, Bateman TM, Johnson LL et al (2004) Clinical value of attenuation correction in stress-only Tc-99 m sestamibi SPECT imaging. J Nucl Cardiol 11:273–281
Links JM, DePuey EG, Taillefer R, Becker LC (2002) Attenuation correction and gating synergistically improve the diagnostic accuracy of myocardial perfusion SPECT. J Nucl Cardiol 9:183–187
Masood Y, Liu YH, Depuey G et al (2005) Clinical validation of SPECT attenuation correction using X-ray computed tomography-derived attenuation maps: multicenter clinical trial with angiographic correlation. J Nucl Cardiol 12:676–686
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husmann, L., Wiegand, M., Valenta, I. et al. Diagnostic accuracy of myocardial perfusion imaging with single photon emission computed tomography and positron emission tomography: a comparison with coronary angiography. Int J Cardiovasc Imaging 24, 511–518 (2008). https://doi.org/10.1007/s10554-007-9288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-007-9288-7