ZSM-5 zeolite membranes were prepared by hydrothermal synthesis. Structural features of the synthesized zeolites were studied by x-ray diffraction (XRD) and scanning electron microscopy (SEM). Adsorptive separation of an n-hexane:thiophene mixture over the obtained zeolites was studied. The effect of the Na2O content in the feedstock for synthesizing the zeolite on the degree of desulfurization was investigated. The maximum amount of thiophene was extracted for zeolite prepared with an Al2O3:Na2O:SiO2:tetrapropylammonium-hydroxide:H2O ratio of 1:30:100:25:10,000. Zeolite modified by NaOH solution of the optimum concentration contained mesopores that could retain sulfur compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
New methods for reducing the sulfur content of diesel fuel to <0.1 ppm are currently being extensively developed [1, 2]. A standard according to which the sulfur content in gasoline and diesel fuel should be <10 ppm became effective last year in China. Chen et al. [3, 4] showed that pervaporation could effectively remove sulfides from fuel. ZSM-5 zeolite membrane modified by metal ions provided a sufficiently high degree of desulfurization during adsorptive purification [5, 6].
ZSM-5 zeolite membrane typically has high thermal stability, even microporosity, and exclusive size selectivity for molecules [7]. These membranes are produced mainly by hydrothermal synthesis [8–10].
Hydrothermal synthesis was used in the present work to prepare ZSM-5 membranes with different pore sizes that were modified by sodium ions. The synthesized membranes were found to be effective for adsorptive desulfurization of a model mixture.
Tetrapropylammonium hydroxide (TPAOH), sodium aluminate, thiophene (T), 2-methylthiophene (2-MT, 99%), 2,5-dimethylthiophene (2,5-DMT, 98.5%), and benzothiophene (BT, 97%) were purchased (Acros Organics). The substrate for the zeolite was б-Al2O3 (hollow porous cylinders of inner diameter 8 mm, outer diameter 12 mm, and pore diameter 3-5 мm). The substrate was polished with emery paper, placed into an ultrasonic bath for 30 min, rinsed with an excess of deionized H2O, and dried in a drying cabinet at 120°C for 24 h.
Preparation of suspension: Al2O3, Na2O, SiO2, TPAOH, and H2O in a ratio of 1:30:120:10:10,000 were stirred on a magnetic stirrer for 2 h and stored in the dark for 24 h. A previously conditioned substrate was leached by the prepared suspension, drawn several times by the Czochralski method, dried at room temperature for 4 h, and finally dried in a drying cabinet at 120°C for 10 h.
Hydrothermal synthesis of ZSM-5 membrane. Several solutions were prepared with Al2O3:Na2O:SiO2:TPAOH:H2O ratios of 1:x:100:25:10,000, where
The prepared substrate was placed into a stainless-steel container with a Teflon liner. The prepared solution was poured into the container, which was sealed with a fluoroplastic cap and placed into a furnace at 175°C for 36 h. The resulting zeolite membrane was cooled, rinsed with deionized H2O at room temperature, and dried in a drying cabinet at 120°C for 10 h. The zeolite membranes were placed into a round electric furnace and stored at 150°C for 1 h before conducting the experiments. Then, the membranes were dried at 300°C for 1 h, calcined at 500°C for 3 h, and cooled to 50°C.
The zeolites were analyzed by x-ray diffraction on a Rigaku D/Max-2500 diffractometer (Rigaku, Germany) using Cu Kб-radiation with a Ni monochromator, 40 kV, and 20 mA. Samples were studied by continuous scanning from 5° to 45° (2θ) at scan rate 0.1°/s and slit width 2 mm. The morphology and microstructure of the zeolites were investigated using a TM3000 table-top scanning electron microscope (Hitachi, Japan).
Model gasoline was prepared by dissolving T, 2-MT, 2,5-DMT, or BT (1 mg/g) in n-hexane. The sulfur content in the mixture after adsorption was determined using a GC-450 gas chromatograph (GC) (Bruker Co., Germany) and an SE-4 capillary column (30 m × 0.32 mm × 0.25 μm).
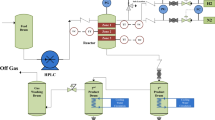
Figure 1 shows a diagram of the laboratory apparatus for membrane purification from the sulfur compounds. The zeolite membranes were loaded into the membrane modules through which the model mixture was pumped (flow rate 8 and 20 mL/h) at 25°C. Residual sulfur was determined periodically by a GC method. The degree of desulfurization (%) was determined using the formula:
where β is the sulfur content (μg/g) in the model mixture at the outlet from the membrane module.
Variation of Na2O content in hydrothermal synthesis feedstock
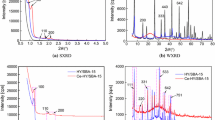
Figure 2 shows diffraction patterns of samples A-F.
Peaks at 7.834°, 8.756°, 9.671°, 23.034°, 23.813°, and 24.309° appeared in the diffraction patterns of samples B-E as compared with that of the substrate and confirmed the zeolite structure [11–13]. The diffraction pattern of sample A showed only one peak at 5-10°, in contrast with B-E. This indicated that the zeolite had a low degree of crystallinity. Sample F, for which the maximum amount of Na2O was used in the hydrothermal synthesis feedstock, had a characteristic peak at 15.602° that was missing in the diffraction patterns of the other zeolites. This indicated that sample F was mordenite [14]. The particle diameters (μm) were A, 2.4; B, 1.92; C, 2.64; D, 4.32; E, 4.8; and F, 5.28. Small particles were formed if the Na2O content in the feedstock for synthesizing the zeolite was low.
Figure 3 shows SEM images of samples A-F. The average particle diameter of sample A, which was prepared from feedstock with Na2O (10 mole fraction), was 2.4 μm. The particles were spherical and distributed unevenly. Sample F, which was prepared from feedstock with Na2O (35 mole fraction), had cubic particles that were very compacted. The mordenite structure did not provide effective retention of sulfur compounds. Sample E which was prepared from feedstock with Na2O (30 mole fraction), had particles with equally sized dimensions. It was shown before [15] that the zeolite particle size increased with increasing alkali-metal content in the preparation mixture.
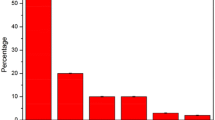
Figure 4 shows the degree of desulfurization of the model mixture containing T as a function of the module operating lifetime for membranes filled with samples A-F. The mixture flow rate was 8 mL/h. Sample A, which had the greatest distances between particles (Fig. 3), provided a low degree of desulfurization. The cubic particles of sample F retained a large amount of T. Sample E had the highest degree of desulfurization (40%). A mixture containing Na2O (30 mole fraction) was used for the hydrothermal synthesis of it.
Zeolite membranes modified by NaOH solutions
Sample E was immersed in NaOH solutions [0.1 (X), 0.18 (Y), 0.25 (Z) M] for 10 h, rinsed with deionized H2O, dried at 120°C for 10 h, and calcined at 300°C for 1 h and at 500°C for 3 h.
Figure 5 shows diffraction patterns of zeolites X-Z.
New peaks appeared at 7.834°, 8.756°, 9.671°, 23.034°, 23.813°, and 24.309°. The starting ZSM-5 structure persisted after treatment with NaOH solutions of concentrations 0.1 and 0.18 M. Storing the zeolite in NaOH solution of higher concentration (0.25 M) caused the peak to decrease slightly, which indicated that the zeolite structure may have changed. The average particle diameters of samples X, Y, and Z were 4.32, 3.36, and 2.88 μm, respectively.
A part of the facets of the cubic particles disappeared if the zeolite was modified by NaOH solution of concentration 0.1 M. The average particle diameter decreased from 4.8 to 4.32 μm. Treatment of the zeolite with NaOH solution of concentration 0.25 M disintegrated large particles into smaller ones and clearly destroyed the zeolite structure, i.e., the structure became irregular.
Figure 6 shows data for the effectiveness of samples X-Z for adsorptive desulfurization of model mixtures containing T and 2-MT. The experiments were carried out at mixture flow rate 8 mL/h. Sample Y, which was modified by NaOH solution of concentration 0.18 M, gave the greatest desulfurization of both mixtures. Modification of the zeolite by NaOH solution of a certain concentration may have formed mesopores [16], which favored the retention of sulfur compounds. Only the outer zeolite structure changed whereas the inner structure remained unchanged if the NaOH solution concentration was too low (0.1 M).
Figure 7 shows the effectiveness of samples X-Z for adsorptive desulfurization of model mixtures containing 2,5-DMT and BT. The initial mixture flow rate was 8 mL/h. After the pores were partially clogged, the flow rate was increased to 20 mL/h in order to compensate for the throughput losses of the membrane module. The degree of desulfurization and; therefore, the degree of zeolite-pore clogging decreased in the order Y > X > Z whereas the pore size increased in this order.
Figures 6 and 7 show that the greatest degrees of T, 2-MT, 2,5-DMT, and BT extraction were 42, 45, 49, and 55%, respectively. This exceeded the degree of extraction of T on zeolite that was not modified by NaOH solution. The kinetic diameters of T, 2-MT, 2,5-DMT, and BT were 0.63, 0.671, 0.707, and 0.711 nm, respectively. The greater the size of the sulfur molecule was, the greater the degree of desulfurization was. Methyls or a benzene ring increased the molecular volume and facilitated its retention in the zeolite pores. Thus, the effectiveness of the zeolites for retaining the investigated sulfur compounds decreased in the order BT > 2,5-DMT > 2-MT > T.
It could be concluded that introducing an alkali metal into the zeolite composition increased the degree of desulfurization. Zeolite modified by NaOH solution of the optimum concentration formed mesopores that also facilitated extraction of the sulfur compounds.
References
B. Li, B. Sun, and X. F. Qian, J. Am. Chem. Soc., 135, 1181-1184 (2013).
T. Xu, S. Q. Lu, and W. Tan, Fluid Mechanics, 35, 10-14 (2007).
Y. Chen, J. S. Li, and L. J. Wang, Prog. Chem., 18, 221-229 (2006).
T. Miao, S. G. Ju, and F. Xue, J. Rare Earths, 30, 807-814 (2012).
Q. Liu and S. G. Ju, Chem. Ind. Eng. Prog. (Beijing, China), 30, 886-890 (2011).
N. Xue, Q. Liu, and S. G. Ju, J. Nanjing Univ. Technol. (Nat. Sci. Ed.), 33, 63-67 (2011).
L. G. Lin and Y. Z. Zhang, Acta Petrolei Sin. (Petroleum Process. Sect.), 26, 476-485 (2010).
J. Chen, G. W. Song, and J. X. Chen, Membr. Sci. Technol., 31, 68-73 (2011).
J. Lisette, B. Miguel, and L. Hugo, Fuel, 90, 2016-2025 (2011).
B. Li, W. P. Liu, and H. Wu, J. Membr. Sci., 415-416, 278-287 (2012).
J. S. Xu, J. Liang, and S. F. Xue, J. Guizhou Univ. (Nat. Sci. Ed.), 15, 260-263 (1998).
Y. Y. Gao, M. Chen, and T. Zhang, Mater. Lett., 65, 2789-2792 (2011).
K. Zhang, Y. Q. Liu, and S. Tian, Fuel, 104, 201-207 (2013).
X. L. Huang, R. R. Zhang, and Z. B. Wang, Chin. J. Catal., 33, 1290-1298 (2012).
Y. Chen, Bull. Chin. Ceram. Soc., 28, 511-515 (2009).
L. Zhao, S. Gao, and W. D. Bu, Chem. Eng. Equip., 4, 103-105 (2010).
Acknowledgments
We thank the National Natural Science Foundation of China (21176118) for sponsoring this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 11 – 14, July – August, 2015.
Rights and permissions
About this article
Cite this article
Dan, G., Tong, M., Yuli, C. et al. Effect of Alkali on Desulfurization Efficiency of ZSM-5 Zeolite Membrane. Chem Technol Fuels Oils 51, 345–352 (2015). https://doi.org/10.1007/s10553-015-0611-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-015-0611-z