Abstract
In this work, four ZSM-5 and HZSM-5 zeolites were synthesized hydrothermally with SiO2/Al2O3 ratio of 40 and 80 and the performance of these samples was studied in desulfurization of heavy naphtha. Samples were analyzed by X-ray diffraction, the Brunauer–Emmett–Teller, field emission scanning electron microscope (FE-SEM), and temperature-programmed desorption of ammonia (NH3–TPD). All samples were tested at 450 °C, 15 bar, H2/Oil = 150 Nml/ml, and weight hourly space velocity (WHSV) = 1 h−1 in a fixed-bed reactor (a Catatest reactor system). The standard of ASTM-D5453 was used for measuring the total sulfur of the feed and products. The result shows that the HZSM-5 zeolites have higher acidity compared to ZSM-5 zeolite samples; as a result, they are more capable of eliminating sulfuric compounds. The sample with SiO2/Al2O3 of 40 shows 87% desulfurization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As sulfur compounds in the engine fuel are finally changed to harmful SOX compounds, which are known as air pollutants, the environmental pollution due to exhaust of gasoline engines is considered as a global concern. In order to follow the new standard quality of gasoline, the number of sulfur components must be reduced. According to the vehicle fuel regulation, sulfur content has to be decreased to 10 mg/g and soon be set at zero percent (Charter and Association 2006; Wang et al. 2013).
The heavy straight-run naphtha from atmospheric distillation towers is one of the main gasoline resources in refinery units. The hydrodesulfurization (HDS) process is applied to remove the sulfur component from the gasoline before passing the gasoline pool unit (Gary et al. 2007). The HDS catalytic process is operated under high temperature and pressure, which changes organic sulfuric compounds to sulfuric hydrogen (H2S). Generally, the most useful catalysts for the HDS processes are Co–Mo/Al2O3 and Ni–Mo/Al2O3. Catalysts’ performance, activity, and selectivity are influenced by the properties of the catalysts (such as the concentration of active sites, supports properties, and syntheses paths), and the reaction conditions (including temperature and hydrogen partial pressure). Moreover, desulfurization efficiency in HDS varies by structure, entity, the concentration of sulfuric compounds, reactor, and process designing. However, the HDS process has been considered as one of the most expensive processes due to high operating and capital costs, H2 consuming, high pressure, and temperature (Shafi and Hutchings 2000; Babich and Moulijn 2003; Song 2003).
Materials with mesoporous and microporous pore size were used in many researches as pollutant removal candidate for the large pore volumes, high specific surface areas, hydrophobic properties, and tunable pore sizes (Sahu et al. 2017, 2018, 2019a, b, c). ZSM-5 with the medium porosity is one of the most practical acidic catalysts in the petrochemical and refinery industries due to morphology, crystalline structures, uniform pores, thermal stabilities, and high selectivity. As mentioned in the previous studies, this zeolite has a potential for gasoline desulfurization (Sharifi et al. 2018a, b; 2019). Furthermore, there are some other documents which are reported the eye-catching results of the ZSM-5 application for gasoline desulfurization (Yin and Liu 2004; Jaimes et al. 2009). According to the mentioned disadvantages of HDS process, extending a new deep desulfurization process using ZSM-5 zeolites can be considered a cheap and favorable process in industrial fields.
In this study, HZSM-5 and NaZSM-5 zeolites were synthesized with SiO2/Al2O3 ratios of 40 and 80 using the hydrothermal method. A fixed-bed reactor was applied to compare the performance of these catalysts in naphtha desulfurization, and also, the effect of cation exchange between NA and H on improving this process was investigated.
Materials and methods
Materials
Aluminum nitrate (ANN; Al(NO3)3.9H2O, 98.5 wt%), tetraethyl orthosilicate (TEOS; Si(OC2H5)4, 98 wt%), and tetrapropyl ammonium hydroxide solution (TPAOH; C12H29NO, 40% aqueous solution) were supplied from Merck company. The feed to be considered is Tehran refinery heavy straight-run naphtha.
Catalysts preparation
Four ZSM-5 and HZSM-5 zeolites with SiO2/Al2O3 = 40,80 were synthesized hydrothermally. First, the aluminum source and distilled water were added to 40 wt% tetrapropyl ammonium hydroxide solution; then, the required amount of silica source was added dropwise into the solution. NaOH was used for setting the prepared gel pH. Afterward, the mix was placed on stirrer with 300 RPM at room temperature for 3 h to perform hydrolysis. The final solution was kept into a stainless steel autoclave to perform a hydrothermal reaction at 170 °C for 72 h. The product was washed three times to set its PH at seven. The product was then dried at 100 °C for 12 h in an oven. The obtained product was then converted to a powdery phase. The powder was calcined at the rate of 5 °C/min at 650 °C and for 3 h. After calcination, the ion exchange process was performed (for preparing the catalyst) by washing with one molar NH4NO3 solution four times (each time 4 h). The washing, filtrating, and drying processes were carried out to remove excess materials, and finally, the product was heated at 550 °C for 3 h to convert the catalyst into the HZSM-5.
Catalytic reaction
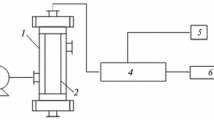
Figure 1 shows a Catatest reactor system which was used to determine the performance of the synthesized catalysts at different conditions. This system includes a fixed-bed reactor with three separated zones (to measure and set the temperature of the media) and an entrance which is located at the top of the reactor to supply the feed where the N2 and H2 components could be injected. The products were also discharged from the bottom, and this downstream could be cooled in two separate steps. To study the performance of the synthesized catalyst in HDS—according to the aim of this study—the naphtha was entered to the reactor under the following condition: 15 bar, 450 °C, H2/Oil = 150 ml/Nml, WHSV = 1 h−1.
Total sulfur concentration in samples was detected using a total nitrogen and sulfur analyzer by ultraviolet fluorescence (Analytik Jena, Multi EA®5000 nitrogen/sulfur analyzer, Germany) following the direct injection method (Standard method ASTM D-5453).
Catalyst characterization
-
1.
X-ray diffraction (XRD) was used to obtain the crystal structure using a D5000 Siemens instrument (scan speed 0.04 s−1, range 2, between 5° and 50° with Cu Ka radiation, and 0.154056 nm wavelengths in 30 kV and 40 mA). The quality of each product was analyzed through a comparison between XRD and the reference diagrams.
-
2.
Temperature-programmed desorption (TPD) was conducted using ammonia gas by an American Micrometrics TPD/TPR 2900 to calculate the total acidic strength and weak and strong acidic sites.
-
3.
Porosity and surface area were measured by BET method. To detect the Brunauer–Emmett–Teller surface area, the nitrogen adsorption/desorption isotherms with a Micromeritics ASAP 2010 analyzer at 77 K were applied to the system.
-
4.
Field emission scanning electron microscope (FE-SEM) was applied to determine morphology and particle size using FE-SEM, MIRA Tescan microscope.
Results and discussion
Catalytic characterization
X-ray diffraction
The XRD patterns of the zeolites with the SiO2/Al2O3 ratios of 40 are shown in Fig. 2. The XRD patterns for synthesized zeolites were within 2θ (2theta) radiation angle range and 5°–50° angular change with the rate of 1.5 min−1. All the XRD patterns of the catalysts match ICDD ref. code 00-044-0003 observed in all the samples and verify the crystal formation of samples.
NH3–TPD
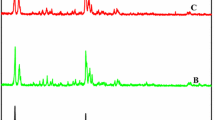
The acid strength of samples was measured using NH3–TPD. According to this method, the total and relative area of TPD peaks at both lower and higher temperatures can be represented by the strength distribution as well as the total acidity. The NH3–TPD diagrams of the synthesized ZSM-5 and HZSM-5 zeolites are shown in Fig. 3. All samples have shown two desorption peaks, one of them is on the low-temperature peak (LTP) at 200–300 °C, while the other is on the high-temperature peak (HTP) at 400–500 °C. All results are in accordance with the published results (Rodríguez-González et al. 2007). It should be noted that both the high- and low-temperature peak intensities decline with the reduction of the catalyst acidity. Table 1 represents the total acidity intensity of all samples obtained from TPD analysis. It can be seen that the amount of catalyst acidity significantly decreases with the increasing SiO2/Al2O3 ratio. On the other hand, HZSM-5 samples that involved ion exchange of Na cation show higher acidity in comparison with the ZSM-5 samples which is totally in accordance with the other references (Shirazi et al. 2008).
N2 adsorption
Figure 4 represents the nitrogen adsorption–desorption isotherm diagrams. Regarding the BET class, all shapes of the adsorption isotherms are classified as Type I, which is the microporous solids type (Brunauer et al. 1940). It can be observed that with increasing SiO2/Al2O3 ratio, nitrogen adsorption in the samples increases. The amount of nitrogen adsorption for HZSM-5 samples was lower than ZSM-5 samples. The properties of synthesized catalysts such as the pore volume, surface area, and median pore diameter are summarized in Table 2.
N2 adsorption
The results of FE-SEM analyzed are shown in Fig. 5. The crystalline structure was observed for all samples.
Catalytic runs
Heavy straight-run naphtha of Tehran refinery under the mentioned operating conditions was used as a feed to evaluate the catalyst’s capabilities on the desulfurization process.
Reactions mechanism
HSRG feed was used in this study containing sulfur compounds, such as mercaptans (RSH), sulfides (R2S), disulfides (RSSR), thiophene, and alkylated derivatives with the boiling point lower than 225 °C. Thus, it is free from heavier sulfur compounds. Figure 6 shows the overall reaction for the desulfurization of the mentioned cut (Babich and Moulijn 2003; Song 2003). All the reactions associated with H2 consumption and their overall reaction can generally be defined as follows:
Reaction pathway for mercaptans, sulfides, disulfides, and thiophene (Babich and Moulijn 2003)
Process operation conditions
In order to achieve the best condition which leads to an effective process, temperature and operating pressure should be chosen carefully. In the sulfur removal process, the desulfurization reaction rate increases with temperature. However, it is possible to reproduce the mercaptans due to the combination of H2S and olefinic compounds at such a high-temperature condition. Therefore, for HDS processes with the high amount of olefinic compounds in the feed (such as the naphtha of the FCC process), the temperature of the reactor cannot be dramatically increased, and temperature should be limited to 315–340 °C. Unlike the olefinic compounds in the HDS process, HSRG with low olefinic compounds has shown a good performance at high-temperature condition with no cracking (Babich and Moulijn 2003; Song 2003). Furthermore, high-pressure conditions during the HDS process prevent coke formation on the catalyst surface, especially in the case of using zeolite catalysts. In regard to this situation, 15 bar and 450 °C were suggested for the reaction for HSRG feed.
Desulfurization of naphtha
The analytical results of sulfur compounds in the feeds and products are represented in Table 3. As it could be seen, the catalyst is highly effective for sulfur compounds reduction. HZSM-5 sample with SiO2/Al2O3 ratio of 40 indicated an 87% reduction. The result has shown that HZSM-5 zeolites have better performance for the HDS process of heavy naphtha in comparison with ZSM-5 zeolites. This can be attributed to the higher acidity of HZSM-5 zeolites, which leads to better performance of the catalyst in breaking the C-S bond. Nevertheless, desulfurization was performed properly for both catalysts at the lower ratio of SiO2/Al2O with the higher acidity.
Experimental design
Since the HZSM-5 sample has the best performance in the desulfurization process, the experimental design was performed for this sample using the Taguchi method. HSRG with 160 ppmwt was applied as the feed. For this purpose, four variables in three levels were investigated as shown in Table 4. The obtained results from the Taguchi method and analysis of variance (ANOVA) are summarized in Tables 5 and 6. Figure 7 shows experimental data and predicted values comparison using the suggested model. Then, model optimization was used to find optimum conditions and reach the lowest sulfuric compounds. The experimental conditions obtained from optimization were applied to the reactor test. The experiment was performed using HZSM-5 with SiO2/Al2O3 ratio 20, at 450 °C, 15 bar, and 1 h−1 WHSV. The final product analysis indicated that the sulfur amount decreased to 19 ppm, which is comparable with the predicted value (i.e., 14) ppm using the model. It can be said that predicted values agree with experimental data.
Conclusion
Four ZSM-5 zeolites were synthesized, and ion exchange between Na and H cation was conducted for two samples. Physical and chemical properties for all catalyst samples were investigated using various analyses, and the performance of the catalysts was evaluated in rector tests. Results showed that HZSM-5 with SiO2/Al2O ratio of 40 has the best performance for sulfuric compounds reduction of HSRG feed. Furthermore, zeolite catalysts with high acidity were the most suitable for sulfuric compounds removal, even without metals presence. Finally, regarding the need for production of high-quality fuel besides the decrease in operating and capital costs, the development of cheaper processes using zeolites as alternatives to conventional HDS processes can be recommended as a matter of interest for researchers.
References
Babich I, Moulijn J (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review☆. Fuel 82(6):607–631
Brunauer S, Deming LS, Deming WE, Teller E (1940) On a theory of the van der waals adsorption of gases. J Am Chem Soc 62(7):1723–1732
Charter WF, E. Association (2006) AOA manufacturers, 4th ed. EM Association and JAM Association
Gary JH, Handwerk GE, Kaiser MJ (2007) Petroleum refining: technology and economics. CRC Press, Boca Raton
Jaimes L, Ferreira ML, de Lasa H (2009) Thiophene conversion under mild conditions over a ZSM-5 catalyst. Chem Eng Sci 64(11):2539–2561
Rodríguez-González L, Hermes F, Bertmer M, Rodríguez-Castellón E, Jiménez-López A, Simon U (2007) The acid properties of H-ZSM-5 as studied by NH3-TPD and 27 AL-MAS-NMR spectroscopy. Appl Catal A 328(2):174–182
Sahu UK, Sahu S, Mahapatra SS, Patel RK (2017) Cigarette soot activated carbon modified with fe3o4 nanoparticles as an effective adsorbent for as (iii) and as (v): material preparation, characterization and adsorption mechanism study. J Mol Liq 243:395–405
Sahu S, Sahu UK, Patel RK (2018) Synthesis of thorium–ethanolamine nanocomposite by the co-precipitation method and its application for Cr (vi) removal. New J Chem 42(7):5556–5569
Sahu S, Kar P, Bishoyi N, Mallik L, Patel RK (2019a) Synthesis of polypyrrole-modified layered double hydroxides for efficient removal of cr (VI). J Chem Eng Data 64(10):4357–4368
Sahu S, Sahu UK, Patel RK (2019b) Modified thorium oxide polyaniline core–shell nanocomposite and its application for the efficient removal of Cr (vi). J Chem Eng Data 64(3):1294–1304
Sahu UK, Sahu S, Mahapatra SS, Patel RK (2019c) Synthesis and characterization of magnetic bio-adsorbent developed from aegle marmelos leaves for removal of as (v) from aqueous solutions. Environ Sci Pollut Res 26(1):946–958
Shafi R, Hutchings GJ (2000) Hydrodesulfurization of hindered dibenzothiophenes: an overview. Catal Today 59(3):423–442
Sharifi K, Halladj R, Royaee SJ, Nasr MRJ (2018a) A new approach for gasoline upgrading: coupling octane enhancement and desulfurization of heavy straight-run naphtha over NI/HZSM-5 catalyst. Catal Commun 115:31–35
Sharifi K, Halladj R, Royaee SJ, Nasr MRJ (2018b) Synthesis of w/hzsm-5 catalyst for simultaneous octane enhancement-desulfurization process of gasoline production. Powder Technol 338:638–644
Sharifi K, Halladj R, Royaee SJ, Nasr MRJ (2019) Investigation of the effect of HZSM-5 over hds and reforming processes for clean gasoline production. Int J Environ Sci Technol 16(2):865–874
Shirazi L, Jamshidi E, Ghasemi MJCR (2008) The effect of Si/Al ratio of zsm-5 zeolite on its morphology, acidity and crystal size. J Exp Ind Crystallogr 43(12):1300–1306
Song C (2003) An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel. Catal Today 86(1):211–263
Wang L, Cai H, Li S, Mominou N (2013) Ultra-deep removal of thiophene compounds in diesel oil over catalyst tio 2/NI-ZSM-5 assisted by ultraviolet irradiating. Fuel 105:752–756
Yin C, Liu C (2004) Hydrodesulfurization of cracked naphtha over zeolite-supported Ni–Mo-s catalysts. Appl Catal A 273(1):177–184
Acknowledgements
This study was done at Islamic Azad University Science and Research Branch of Tehran. The authors would also like to appreciate all the insights and expertise provided by the Research Institute of Petroleum Industry for its valuable assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this manuscript. In addition, the ethical issues, including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, and redundancy, have been completely observed by the authors.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Ahmadvand, M.S., Askari, S. & Sharifi, K. Synthesis and performance of ZSM-5 and HZSM-5 in desulfurization of naphtha. Int. J. Environ. Sci. Technol. 17, 3541–3548 (2020). https://doi.org/10.1007/s13762-020-02639-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-020-02639-7