Abstract
Purpose
High mammographic breast density is a strong, well-established breast cancer risk factor. Whether stem cells may explain high breast cancer risk in dense breasts is unknown. We investigated the association between breast density and breast cancer risk by the status of stem cell markers CD44, CD24, and ALDH1A1 in the tumor.
Methods
We included 223 women with primary invasive or in situ breast cancer and 399 age-matched controls from Mayo Clinic Mammography Study. Percent breast density (PD), absolute dense area (DA), and non-dense area (NDA) were assessed using computer-assisted thresholding technique. Immunohistochemical analysis of the markers was performed on tumor tissue microarrays according to a standard protocol. We used polytomous logistic regression to quantify the associations of breast density measures with breast cancer risk across marker-defined tumor subtypes.
Results
Of the 223 cancers in the study, 182 were positive for CD44, 83 for CD24 and 52 for ALDH1A1. Associations of PD were not significantly different across t marker-defined subtypes (51% + vs. 11–25%: OR 2.83, 95% CI 1.49–5.37 for CD44+ vs. OR 1.87, 95% CI 0.47–7.51 for CD44−, p-heterogeneity = 0.66; OR 2.80, 95% CI 1.27–6.18 for CD24+ vs. OR 2.44, 95% CI 1.14–5.22 for CD24−, p-heterogeneity = 0.61; OR 3.04, 95% CI 1.14–8.10 for ALDH1A1+ vs. OR 2.57. 95% CI 1.30–5.08 for ALDH1A1−, p-heterogeneity = 0.94). Positive associations of DA and inverse associations of NDA with breast cancer risk were similar across marker-defined subtypes.
Conclusions
We found no evidence of differential associations of breast density with breast cancer risk by the status of stem cell markers. Further studies in larger study populations are warranted to confirm these associations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Mammographic breast density is a well-established and strong breast cancer risk factor that reflects the amount of adipose, connective, and epithelial tissue in the breast [1]. Although several risk factors were examined in relation to the risk of high breast density [2,3,4,5,6], the mechanisms by which breast density alters breast cancer risk remain poorly understood. Breast tissue undergoes significant structural changes throughout a woman’s life [7]. The breast tissue architecture is maintained by a population of stem cells with self-renewal capacity, which are essential for tissue repair and remodeling throughout the woman’s life [8]. The mammary stem cells are a rapidly cycling cell population in the normal adult [9]. According to the stem cell hypothesis of breast carcinogenesis, larger mammary gland mass is expected to have a larger pool of mammary cells and be correlated with the number of mammary stem cells [10] and subsequent breast cancer risk [11], which could also be reflected in the degree of mammographic breast density.
In previous studies, well-characterized stem cell markers CD44, CD24, and aldehyde dehydrogenase family 1 member A1 (ALDH1A1) have been linked to younger age at diagnosis, higher odds of unfavorable tumor characteristics, including triple-negative receptor status (estrogen, progesterone, and HER2), poor prognosis, chemotherapy resistance, and distant metastasis [12,13,14,15,16,17]. Our recent study using a unique source of breast core biopsy tissue from healthy women compared, for the first time, the expression of stem cell markers CD44, CD24, and ALDH1A1 in areas of dense and non-dense tissue within the same woman [18]. For all three markers, we found higher levels of staining extent in dense as compared to non-dense tissue, though for CD24 and ALDH1A1 the difference did not reach statistical significance (CD44: 6.3% vs. 2.0%, p < 0.001; CD24: 8.0% vs. 5.6%, p = 0.10; and ALDH1A1: 0.5% vs. 0.3%, p = 0.12). Thus, our findings suggested an increased presence and/or activity of stem cells in dense as compared to non-dense breast tissue. To further examine the potential interrelationship of stem cell markers with density-associated increase in breast cancer risk, we investigated the association of breast density with the risk of breast cancer subtypes defined by the status of stem cell markers CD44, CD24, and ALDH1A1.
Materials and methods
Study design and population
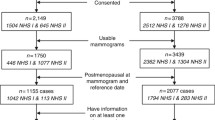
The participants for this investigation were selected from Mayo Clinic Mammography Study (MCMAM), a retrospective case–control study nested within the Mayo Clinic mammography screening practice in Rochester, Minnesota [19, 20]. Briefly, breast cancer cases were women 50 years or older diagnosed with primary invasive or in situ cancer between 1997 and 2001 who had at least two prior screening mammograms 2 years before diagnosis and lived within a 120-mile radius of the clinic. Two controls with no prior history of breast cancer from the screening practice were matched to each case on age (within 5 years), final screening exam date, menopausal status at final exam date (pre or post), time between baseline and final mammogram, number of prior screening mammograms, and county of residence [19, 20]. We included in this study invasive cases who had tumor tissue samples available for tissue microarray (TMA) construction (74% of invasive cases). Cases with and without TMAs were similar with respect to the distribution of breast cancer risk factors. The final study sample included 223 cases and 399 matched controls. This study was approved by the Mayo Clinic and University of Florida Institutional Review Boards.
Assessment of mammographic breast density
The earliest pre-diagnostic screening mammogram during the preceding 10-year period, but at least 2 years before breast cancer or corresponding exam date for matched controls, was used for breast density assessment. The average time between mammogram and reference date was 7.0 years (range 2.1–10.4) and was similar for cases and control. Left and right mediolateral oblique (MLO) and craniocaudal (CC)] views of all mammograms were digitized on a Lumiscan 75 scanner with 12-bit grayscale depth and 0.13 × 0.13 mm2 pixel size. Percent breast density (percentage of the breast area occupied by fibroglandular elements), absolute dense and non-dense areas (in cm2) were estimated using computer-assisted thresholding technique [21,22,23]. As previously described, first the cases and controls were randomly grouped into batches, order of woman was randomly assigned, and then mammograms with different views and sides were randomly ordered within each woman. Each batch contained 5% repeat set of images to assess reliability of the readings. In this reproducibility study, reliability in breast density assessment was very high (r > 0.93) [19].
Tissue microarray construction and immunohistochemistry
Two breast tumor TMAs were constructed using 2 or 3 cores per woman as well as control samples from benign breast tissue, placenta, liver, ovary and tonsils (total of 714 tumor cores and 42 controls). Immunohistochemical analysis of the markers TMAs was performed at the University of Florida Pathology Core Lab according to the previously described standard protocol and using commercially available antibodies (DAKO AutostainerPlus, CD44 [DAKO] 1:25 dillution; CD24 [Abcam] 1:200 dillution and ALDH1A1 [Abcam] 1:300 dillution) [18]. Staining for each of the tissue markers was performed on a separate TMA. Briefly, slides were de-paraffinized with xylene and re-hydrated through decreasing concentrations of ethanol to water, including an intermediary step to quench endogenous peroxidase activity (3% hydrogen peroxide in methanol). Slides were transferred to 1X TBS-T (Tris-buffered saline-Tween). For heat-induced antigen retrieval, sections were heated in a steamer while being submerged in Citra (Biogenex, Fremont, CA) or Trilogy (Cell Marque, Rocklin, CA) for 30 min. Slides were subsequently rinsed in 1XTBS-T and incubated with a universal protein blocker Sniper (Biocare Medical, Walnut Creek, CA), for 15 min and then rinsed in 1XTBS-T and co-incubated in primary antibody ALDH1A1 or CD24 or CD44 for 1 h. Next, slides were rinsed in 1XTBS-T followed by application of conjugated secondary antibody: Mach 2 goat anti-rabbit horse (or mouse) radish peroxidase-conjugated (Biocare Medical, Walnut Creek, CA) for 30 min. Detection of antibodies was achieved by incubating slides in 3′3′ diaminobenzidine (Vector Laboratories Inc., Burlingame, CA) for 4 min. Slides were counterstained with hematoxylin (Biocare Medical, Walnut Creek, CA) 1:10 for 3 min and mounted with Cytoseal XYL (Richard-Allen Scientific, Kalamazoo, MI).
The percentage of staining extent for each marker was assessed by a single pathologist using Olympus BX43 microscope. Any cellular staining pattern (e.g., nuclear, cytoplasmic or membranous) was considered positive. Staining extent was quantified as percentage of the area occupied by positively stained cells out of the total tissue area in the slide. Cases with one or more percent of positive staining on any of the cores were considered marker-positive. We also calculated the average marker expression across all available cores for a woman. The composition of the breast tissue was evaluated as proportion of epithelial, stromal, and adipose tissue elements.
Covariates
Weight, height, and use of postmenopausal hormones (PMH) at the time of the index mammogram were abstracted from the Mayo Clinic medical records. Weight and height were used to calculate body mass index (BMI) in kg/m2. Information on all other covariates was obtained from a clinical database of self-reported information collected at each visit.
Statistical analysis
Mammographic percent breast density was categorized as 0–10%, 11–25% (reference), 26–50%, and ≥ 51%, consistent with previous analyses [24, 25]. Absolute dense and non-dense areas were defined as quartiles based on the distribution in controls (absolute dense area: 0.0–18.9; 2nd: 19.0–33.9; 3rd: 34.0–54.6; 4th: > 54.7 cm2; non-dense area: 1st: 4.2–75.5; 2nd: 75.6–132.2; 3rd: 132.3–200.4; 4th: ≥ 200.5 cm2). Density measures were also modeled as continuous variables. We used polytomous logistic regression to describe the associations of breast density measures with breast cancer risk by the status of each of the stem cell markers. The risk estimates were adjusted for age (continuous) and body mass index (continuous). We further examined the associations by the number of markers that stained positively for each woman (i.e., none, one, or two–three). Finally, in additional analysis, we modeled each of the density variables a binary using the median in controls as a cut-point (21%, 34 cm2, and 132.5 cm2 for percent density, absolute dense, and non-dense areas, respectively).
Contrasts were used to construct a test of association of breast density with breast cancer risk by marker status (p-heterogeneity) within the polytomous regression framework to investigate whether there was statistical evidence of differences in associations for marker-defined tumor subtypes. For these heterogeneity tests, breast density was modeled using an ordinal trend across quartiles in order to increase power.
Spearman correlation coefficient was used to examine correlations of stem cell marker expression with clinico-histological tumor characteristics (tumor grade, size, nodal involvement, and statuses of estrogen receptor, progesterone receptor, and HER2). Analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC, USA). For all analyses, the level of statistical significance was assessed at 0.05 level. All tests were two-sided.
Results
Characteristics of breast cancer cases and controls in this study are presented in Table 1. Compared to controls, cases had a higher percent density (29.8% vs. 24.0%, p < 0.001) and a larger dense area (53.2 cm2 vs. 40.5 cm2, p < 0.001). The mean age at mammogram was similar in cases and controls (61.8 and 61.6 years, respectively, p = 0.81). As compared to controls, cases appeared to have slightly greater BMI (27.9 vs. 27.1 kg/cm2, p = 0.05). Distributions of other risk factors were similar in cases and controls. The average expression of stem cell markers in the tumor samples is presented in Table 2.
Among 628 cores for CD44, our results show the mean staining extent of 8.5% (range 0–90%), among 632 cores for CD24 the mean staining extent of 8.3% (range 0–90%), and among 635 cores for ALDH1A1 the mean expression of 1.0% (range 0–40%). Comparison of the staining for each marker across all cores for a woman (2 to 3) showed high correlation across the cores (intraclass correlation [ICC] = 0.82 [95% CI 0.78–0.86], 0.78 [95% CI 0.72–0.83] and 0.58 [95% CI 0.48–0.67] for CD44, CD24, and ALDH1A1, respectively). Of the 223 cancers in the study, 182 (82%) were positive for CD44, 83 (37%) for CD24 and 52 (23%) for ALDH1A1. As expected, percent breast density and absolute dense area were positively associated with breast cancer in this subset of the case–control study (OR 2.72, 95% CI 1.47–5.05 for > 51% vs. 11–25% and OR 1.78, 95% CI 1.12–2.83 for absolute dense area fourth vs. second quartile) (Table 3). Association of breast density with stem cell marker-defined breast cancer subtypes is presented in Table 4. Percent breast density was positively associated with all three marker-defined breast cancer subtypes. Associations of percent breast density with CD24 and ALDH1A1-defined subtypes were similar in marker-positive and marker-negative tumors (percent density ≥ 51% vs. 11–25%: OR 2.80, 95% CI 1.27–6.18 for CD24+ vs. OR 2.44, 95% CI 1.14–5.22 for CD24−, p-heterogeneity = 0.61; OR 3.04, 95% CI 1.14–8.10 for ALDH1A1 vs. OR 2.57, 95% CI 1.30–5.08 for ALDH1A1−, p-heterogeneity = 0.94). Even though the difference in the association of percent breast density with breast cancer risk in CD44+ and CD44− tumors was not statistically significant, the risk estimates appeared stronger in CD44+ subtypes (percent density ≥ 51% vs. 11–25%: OR 2.83, 95% CI 1.49–5.37 vs. OR 1.87, 95% CI 0.47–7.51, respectively, p-heterogeneity = 0.66).
Absolute dense area was also positively associated with all marker-defined breast cancer subtypes (Table 4). Association of absolute dense area appeared to be stronger in CD44+ as compared to CD44− tumors but the difference did not reach statistical significance (fourth vs. second quartile: OR 1.95, 95% CI 1.18–3.21 for CD44+ vs. OR 1.22, 95% CI 0.51–2.93 for CD44−, p-heterogeneity = 0.36). The association of the absolute dense area by CD24 and ALDH1A1 status was similar in marker-positive and marker-negative tumors (fourth vs. second quartile: OR 1.75, 95% CI 0.90–3.38 for CD24+ vs. OR 1.82, 95% CI 1.05–3.15 for CD24−, p-heterogeneity = 0.99; OR 1.86, 95% CI 0.83–4.16 for ALDH1+ vs. OR 1.77, 95% CI 1.06–2.94 for ALDH1A1−, p-heterogeneity = 0.82).
Absolute non-dense area was inversely associated with breast cancer risk in most of the marker-defined tumor subtypes. We found no significant differences in the associations of non-dense area with breast cancer risk by the status of any of the three markers (p-heterogeneity = 0.29, 0.69, and 0.49 for CD44, CD24, and ALDH1A1, respectively) (Table 4).
Correlations between stem cell marker expression and selected clinico-histological tumor characteristics, though significant, were negligible in its magnitude (Supplementary Tables 1 and 2).
When considering combined expression of the markers, we did not find any differences in associations in those with one or two–three positive markers as compared to no positivity (Supplementary Table 3). Similarly, we found no differences in associations of density with breast cancer risk across marker-defined subtypes when density measures were modeled as binary (Supplementary Table 4).
Discussion
We present the first study to examine breast density with breast cancer subtypes defined by stem cell markers. This preliminary investigation found no evidence of differential associations of breast density measures with breast cancer risk by the status of stem cell markers.
Breast tissue is very dynamic and undergoes significant remodeling and structural changes during puberty, pregnancy, lactation, and involution [7]. Breast stem cells are essential for the continuous tissue remodeling and maintenance of the breast tissue architecture. The association between stem cells and breast density is supported by findings from previous studies that demonstrate consistent associations of epidemiologic risk factors that influence breast stem cell activity (birthweight, early-life body size, and parity) with breast density [2, 6, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. In our recent investigation using paired core biopsy samples from healthy women, we found greater expression of stem cell markers in areas of dense as compared to non-dense breast tissue [18]. Previous literature suggests that stem cell markers are expressed predominantly in epithelium and stroma [42] and as expected, the observed differences were attenuated after additional adjustment for proportion of epithelial or stromal elements and further attenuated after adjustment for combined area of for epithelial and stromal tissue. In our study, expression of all three markers was more apparent in tissue sections with a larger proportion of epithelium though the staining for all three markers was detected even in some sections with no epithelium, but large proportion of stromal tissue suggesting that expression in the stroma is also important. These findings support the hypothesis that breast density-associated increase in breast cancer could potentially result from higher presence of cells with stem-like properties in dense breast tissue. Findings from our current exploratory study, though not significant, suggest that the magnitude of the association of breast density with breast cancer risk may be greater for stem cell marker–positive tumors as compared to marker-negative subtypes. The association of percent density with the risk of tumor subtypes also appears to be driven by the absolute dense area, consistent with predominant expression of these markers in epithelial and stromal tissue. However, given the small sample in this pilot investigation, these results should be interpreted with caution and warrant confirmation in future larger studies.
We examined, for the first time, the association of mammographic breast density with the risk of breast cancer subtypes defined by the status of CD44, CD24, and ALDH1A1 markers. Our case–control study had several strengths, including the close matching on important covariates, mammograms from at least 5 years before breast cancer for > 90% of cases, ascertainment of weight, height and data on breast cancer risk factors with medical records for all cases and controls generally at time of mammogram, and the quantitative assessment of three breast density phenotypes [19]. Our study had a few limitations. From the previous nested case–control study, TMAs for staining were available only for 74% of breast cancer cases. However, women with and without TMA were similar with respect to important characteristics, including age, BMI, reproductive variables, hormone use, and a family history of breast cancer. Marker staining was assessed by a single expert pathologist. Validation studies have demonstrated a very high inter-observer agreement in staining assessment by different experts as well as between automated and pathologist readings [43,44,45]. Consistently, in clinical practice, reading of the staining results for several markers is performed by only one pathologist and not by consensus of multiple pathologists. Further, use of a single reader eliminates inter-rater variability.
Even though this study used the clinic-based design and was lacking ethnical diversity in the study population, because of the restricting eligibility to the 120-mile radius and requiring serial mammograms on all women, the study population was closer to a community-based population rather than a referral or high-risk population [19]. Finally, in this preliminary investigation, we did not have sufficient power to examine associations by menopausal status or to define subtypes using combination of the markers.
Conclusions
We found no evidence of differential associations of breast density measures with breast cancer risk by the status of stem cell markers. However, our power was limited and further studies in larger study populations are warranted to examine these associations in a larger sample.
References
McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev 15:1159–1169
Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA (2000) Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States). Cancer Causes Control 11:653–662
Vachon CM, Sellers TA, Vierkant RA, Wu FF, Brandt KR (2002) Case–control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomark Prev 11:1382–1388
Titus-Ernstoff L, Tosteson AN, Kasales C, Weiss J, Goodrich M, Hatch EE, Carney PA (2006) Breast cancer risk factors in relation to breast density (United States). Cancer Causes Control 17:1281–1290
Tamimi RM, Hankinson SE, Colditz GA, Byrne C (2005) Endogenous sex hormone levels and mammographic density among postmenopausal women. Cancer Epidemiol Biomark Prev 14:2641–2647
Yaghjyan L, Colditz GA, Rosner B, Bertrand KA, Tamimi RM (2016) Reproductive factors related to childbearing and mammographic breast density. Breast Cancer Res Treat 158:351–359
Cobaleda C, Cruz JJ, Gonzalez-Sarmiento R, Sanchez-Garcia I, Perez-Losada J (2008) The emerging picture of human breast cancer as a stem cell-based disease. Stem Cell Rev 4:67–79
Smalley M, Ashworth A (2003) Stem cells and breast cancer: a field in transit. Nat Rev Cancer 3:832–844
Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ (2006) Purification and unique properties of mammary epithelial stem cells. Nature 439:993–997
Trichopoulos D, Adami HO, Ekbom A, Hsieh CC, Lagiou P (2008) Early life events and conditions and breast cancer risk: from epidemiology to etiology. Int J Cancer 122:481–485
Savarese TM, Low HP, Baik I, Strohsnitter WC, Hsieh CC (2006) Normal breast stem cells, malignant breast stem cells, and the perinatal origin of breast cancer. Stem Cell Rev 2:103–110
Giatromanolaki A, Sivridis E, Fiska A, Koukourakis M (2011) The CD44+/CD24− phenotype relates to ‘triple-negative’ state and unfavorable prognosis in breast cancer patients. Med Oncolgy 28(3):745–752
Abraham BK, Fritz P, Van der Kuip H, Buck M, Szabo Z, Athelogou M, and Brauch H (2005) Evaluation of CD44+/CD24−/low cells in breast cancer and relevance for distant metastasis. AACR Meeting Abstracts, p 481
Neumeister V, Agarwal S, Bordeaux J, Camp RL, Rimm DL (2010) In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am J Pathol 176:2131–2138
Eden JA (2010) Human breast cancer stem cells and sex hormones–a narrative review. Menopause 17:801–810
Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1:555–567
Liu Y, Lv DL, Duan JJ, Xu SL, Zhang JF, Yang XJ, Zhang X, Cui YH, Bian XW, Yu SC (2014) ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis. BMC Cancer 14:444
Yaghjyan L, Stoll E, Ghosh K, Scott CG, Jensen MR, Brandt KR, Visscher D, Vachon CM (2017) Tissue-based associations of mammographic breast density with breast stem cell markers. Breast Cancer Res 19:100
Vachon CM, Brandt KR, Ghosh K, Scott CG, Maloney SD, Carston MJ, Pankratz VS, Sellers TA (2007) Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiol Biomark Prev 16:43–49
Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Pankratz VS, Visscher D, Norman A, Couch F, Shepherd J, Fan B, Chen Y-Y, Ma L, Beck AH, Cummings SR, Kerlikowske K, Vachon CM (2013) Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res 15:R104
Ursin G, Ma H, Wu AH, Bernstein L, Salane M, Parisky YR, Astrahan M, Siozon CC, Pike MC (2003) Mammographic density and breast cancer in three ethnic groups. Cancer Epidemiol Biomark Prev 12:332–338
Byng JW, Boyd NF, Fishell E, Jong RA, Yaffe MJ (1994) The quantitative analysis of mammographic densities. Phys Med Biol 39:1629–1638
Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ (1995) Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 87:670–675
Phipps AI, Buist DS, Malone KE, Barlow WE, Porter PL, Kerlikowske K, O’Meara ES, Li CI (2012) Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol 22:340–348
Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K (2004) Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiol Biomark Prev 13:2090–2095
Cerhan JR, Sellers TA, Janney CA, Pankratz VS, Brandt KR, Vachon CM (2005) Prenatal and perinatal correlates of adult mammographic breast density. Cancer Epidemiol Biomark Prev 14:1502–1508
Ginestier C, Wicha MS (2007) Mammary stem cell number as a determinate of breast cancer risk. Breast Cancer Res 9:109
Xue F, Michels KB (2007) Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol 8:1088–1100
Rice MS, Tamimi RM, Connolly JL, Collins LC, Shen D, Pollak MN, Rosner B, Hankinson SE, Tworoger SS (2012) Insulin-like growth factor-1, insulin-like growth factor binding protein-3 and lobule type in the nurses’ health study II. Breast Cancer Res 14:1–7
Christopoulos PF, Msaouel P, Koutsilieris M (2015) The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 14:43
Rinaldi S, Peeters PHM, Berrino F, Dossus L, Biessy C, Olsen A, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Téhard B, Nagel G, Linseisen J, Boeing H, Lahmann PH, Trichopoulou A, Trichopoulos D, Koliva M, Palli D, Panico S, Tumino R, Sacerdote C, van Gils CH, van Noord P, Grobbee DE, Bueno-de-Mesquita HB, Gonzalez CA, Agudo A, Chirlaque MD, Barricarte A, Larrañaga N, Quiros JR, Bingham S, Khaw KT, Key T, Allen NE, Lukanova A, Slimani N, Saracci R, Riboli E, Kaaks R (2006) IGF-I, IGFBP-3 and breast cancer risk in women: the European prospective investigation into cancer and nutrition (EPIC). Endocr Relat Cancer 13:593–605
Baer HJ, Colditz GA, Willett WC, Dorgan JF (2007) Adiposity and sex hormones in girls. Cancer Epidemiol Biomark Prev 16:1880–1888
Bertrand KA, Baer HJ, Orav EJ, Klifa C, Shepherd JA, Van Horn L, Snetselaar L, Stevens VJ, Hylton NM, Dorgan JF (2015) Body fatness during childhood and adolescence and breast density in young women: a prospective analysis. Breast Cancer Res 17:1–10
Poole EM, Tworoger SS, Hankinson SE, Schernhammer ES, Pollak MN, Baer HJ (2011) Body size in early life and adult levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3. Am J Epidemiol 174:642–651
Warner ET, Eliassen AH, Chen WY, Holmes MD, Willett WC, Tamimi RM (2013) Abstract 130: childhood and adolescent body size and risk of fatal breast cancer. Cancer Res 73:130
Baer HJ, Colditz GA, Rosner B, Michels KB, Rich-Edwards JW, Hunter DJ, Willett WC (2005) Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res 7:1–12
Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L (2007) A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomark Prev 16:1795–1802
Frazier AL, Rosenberg SM (2013) Pre-adolescent and adolescent risk factors for benign breast disease. J Adolesc Health 52:S36–S40
Warner ET, Hu R, Collins LC, Beck AH, Schnitt S, Rosner B, Eliassen AH, Michels KB, Willett WC, Tamimi RM (2016) Height and body size in childhood, adolescence, and young adulthood and breast cancer risk according to molecular Subtype in the nurses’ health studies. Cancer Prev Res 9:732–738
Woolcott CG, Koga K, Conroy SM, Byrne C, Nagata C, Ursin G, Vachon CM, Yaffe MJ, Pagano I, Maskarinec G (2012) Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case–control studies. Breast Cancer Res Treat 132:1163–1171
Yaghjyan L, Mahoney MC, Succop P, Wones R, Buckholz J, Pinney SM (2012) Relationship between breast cancer risk factors and mammographic breast density in the Fernald Community Cohort. Br J Cancer 106(5):996–1003
Anwar T, Kleer CG (2013) Tissue-based identification of stem cells and epithelial-to-mesenchymal transition in breast cancer. Hum Pathol 44:1457–1464
Collins LC, Marotti J, Baer HJ, Deitz AC, Colditz GA, Tamimi RM (2008) Comparison of estrogen receptor assay results from pathology reports with results from central laboratory testing: implications for population-based studies of breast cancer. J Natl Cancer Inst 100:218–221
Ma H, Wang Y, Sullivan-Halley J, Weiss L, Burkman RT, Simon MS, Malone KE, Strom BL, Ursin G, Marchbanks PA, McDonald JA, Spirtas R, Press MF, Bernstein L (2009) Breast cancer receptor status: do results from a centralized pathology laboratory agree with SEER registry reports? Cancer Epidemiol Biomark Prev 18:2214–2220
Ahern TP, Beck AH, Rosner BA, Glass B, Frieling G, Collins LC, Tamimi RM (2017) Continuous measurement of breast tumour hormone receptor expression: a comparison of two computational pathology platforms. J Clin Pathol 70(5):428–434
Acknowledgments
This study was funded by the University of Florida Cancer Center through the Florida Consortium of National Cancer Institute Centers Program at the University of Florida (Bridge Funding to L.Y.); and Mayo Clinic Breast SPORE (NCI P50 CA116201) and National Cancer Institute (R01 CA128931; R01 CA140286). The authors would like to thank Ms. Elaine Dooley for performing immunohistochemical analyses for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
This study was compliant with the U.S. Health Insurance Portability and Accountability Act and was approved by the Mayo Clinic Institutional Review Board (IRB) which issued a waiver of informed consent as well as the University of Florida IRBs. The Mayo Clinic patients provided a general authorization for use of medical record information for research purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yaghjyan, L., Esnakula, A.K., Scott, C.G. et al. Associations of mammographic breast density with breast stem cell marker-defined breast cancer subtypes. Cancer Causes Control 30, 1103–1111 (2019). https://doi.org/10.1007/s10552-019-01207-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-019-01207-w