Abstract
Purpose
To describe the association between diabetes and colon cancer recurrence.
Methods
We conducted a cohort study at two integrated health care delivery systems in the United States. Using tumor registry data, we identified patients aged ≥ 18 years when diagnosed with stage I–IIIA adenocarcinomas of the colon during 1995–2014. Pre-existing diabetes was ascertained via diagnosis codes. Medical records were reviewed for eligibility and to abstract recurrence and covariate information. Recurrence was ascertained beginning 90 days after the end of colon cancer treatment (i.e., cohort entry). Recurrence of any cancer or a new primary cancer at any site was a secondary outcome. We used multivariable Cox proportional hazards models to estimate hazard ratios (HR) and 95% confidence intervals (CIs) for the associations between diabetes at cohort entry and study outcomes.
Results
Among the 1,923 eligible patients, 393 (16.7%) had diabetes at cohort entry. Diabetes was not associated with recurrence (HR 0.87; 95% CI 0.56–1.33) or with any subsequent cancer (HR 1.09; 95% CI 0.85–1.40). When the definition of recurrence included second primary colorectal cancer, risk was non-significantly higher in patients with diabetes than without diabetes.
Conclusions
The risk of colon cancer recurrence appears to be similar in patients with and without diabetes at diagnosis.
Impact
Future studies should evaluate the association between diabetes and colorectal cancer outcomes, especially second primary colon cancers, in larger populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus affects 23 million people in the United States [1] and is present in approximately 9–23% of new colorectal cancer cases [2,3,4,5]. Diabetes shares risk factors with, and may increase the risk of, colorectal cancer [6,7,8,9,10,11,12,13]. People with diabetes have a higher risk of death due to colorectal cancer than persons without diabetes [13,14,15,16,17,18,19]. Hyperglycemia, hyperinsulinemia, and certain treatments for diabetes (e.g., sulfonylureas and insulin) may play roles in colorectal cancer development [6]. In contrast, metformin, the first-line oral agent for diabetes treatment, is hypothesized to have anticancer effects primarily by lowering levels of circulating insulin and by inhibiting a major protein synthesis/cell growth pathway [20, 21], though other mechanisms are also hypothesized [22].
The association between diabetes and colorectal cancer recurrence is unclear. A 2013 meta-analysis [23] of three studies [24,25,26] that included 429 diabetic colorectal cancer patients reported a pooled hazard ratio (HR) of 1.24 (95% confidence interval [CI] 0.99–1.55) for the association between pre-existing diabetes and colorectal cancer recurrence. A subsequent study reported a non-significant association between diabetes and recurrence among 2,183 colon cancer patients, of whom 288 had diabetes (HR 1.32; 95% CI 0.95–1.76) [27] while two other similarly sized studies reported point estimates near 1.0 [5, 28]. A 2017 systematic review did not identify additional studies [29]. To our knowledge, there are no published studies on diabetes and colon cancer recurrence in population-based cohorts in the United States. Thus, we undertook an analysis to evaluate the association between pre-existing diabetes and colon cancer outcomes (recurrences and subsequent cancers overall) in a population of early-stage colon cancer survivors.
Materials and methods
Setting and overview
We conducted a cohort study (Recurrence of Colon cancer in Relation to Drug use [RECORD]) at two health care systems within the Health Care Systems Research Network’s (HCSRN) Cancer Research Network [30]: Kaiser Permanente Washington (KPWA) and Kaiser Permanente Colorado (KPCO). All study procedures were approved by the KPWA institutional review board, to which KPCO ceded.
Both KPWA and KPCO store administrative and clinical information locally in a common data model, the HCSRN Virtual Data Warehouse (VDW) [31]. The VDW is a decentralized data model with mutually agreed upon variable definitions across HCSRN sites. The VDW includes data on enrollment in the health care system, diagnoses and procedures [International Classification of Diseases (ICD) and Current Procedural Terminology (CPT) codes], outpatient prescription medication fills, laboratory test results, vital signs, and deaths. As part of the VDW specifications, both sites also maintain a table of incident cancer diagnoses populated with data from the system’s own cancer registry (KPCO) or the local Surveillance, Epidemiology, and End Results program (SEER) registry (KPWA).
We identified potentially eligible incident colon cancer cases through the VDW; medical records abstractors then verified eligibility and abstracted detailed information on cancer treatment, patient risk factors (e.g., smoking and over-the-counter medication use), and study outcomes. Abstractors underwent extensive training that involved double reviews of selected records to achieve accuracy and consistency. Every 6 months, abstractors participated in inter- and intra-rater reliability activities for quality assurance. Medical records abstraction occurred from June 2014 through August 2016.
Cohort identification and eligibility
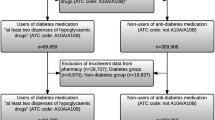
We used VDW data to identify patients aged ≥ 18 years when diagnosed with stage I–IIIA [American Joint Committee on Cancer (AJCC) 6th edition] malignant adenocarcinomas of the colon or rectosigmoid junction during 1995–2014 (Fig. 1). Using VDW data, we excluded patients who were not continuously enrolled in the health plan for at least 12 months before and 3 months after the cancer diagnosis; received a total colectomy prior to diagnosis; were previously or concurrently diagnosed with additional colorectal tumors or metastatic non-colorectal tumors; were diagnosed with additional primary tumors or index cancer recurrence within 90 days of the index diagnosis; or were marked by tumor registrars as “never disease free” after index diagnosis.
Study inclusion and exclusion criteria. Figure shows the steps taken to create a study cohort of stage I–IIIA incident colon cancer adenocarcinoma cases at Kaiser Permanente Washington and Colorado, 1995–2014. AJCC American Joint Committee on Cancer, EOT end of treatment, KPCO Kaiser Permanente Colorado, RECORD Recurrence of Colon Cancer in Relation to Drug use, VDW Virtual Data Warehouse
Subjects who were potentially eligible after VDW-based exclusions then underwent medical records review. We further excluded patients with incomplete medical records; whose chart-reviewed diagnosis date fell outside the 1995–2014 study window or differed from the tumor registry date by > 1 month; who were previously diagnosed with colorectal cancer, metastatic non-colorectal cancer, or familial adenomatous polyposis; whose records indicated that the index tumor actually occurred in the rectum or was metastatic; who did not have surgery to treat the index colon cancer; or who had positive surgical margins or tumor deposits. We looked for evidence of tumor progression during the 90 days after end of treatment for the index cancer and excluded those who had evidence of progression of the index cancer or indeterminate imaging results, died or disenrolled, or were otherwise determined to be not cancer-free within that timeframe. Because diabetes diagnosis data were not available on KPCO subjects before 1998, KPCO patients diagnosed with colon cancer before 1 January 1999 were excluded.
Outcome ascertainment
The primary outcome of this study was recurrence of the index colon cancer more than 90 days after treatment completion. Treatment completion was defined as the last date of chemotherapy, radiation, or surgery for the index cancer. Recurrence was defined by a clinical diagnosis in the medical record. At KPCO, information on recurrence was preloaded into the medical records abstraction instrument from tumor registry data, then supplemented and/or confirmed by study abstractors. At KPWA, recurrences are not recorded in the SEER registry; therefore, all recurrence information came from medical records abstraction. Both sites collected date of recurrence, pathological consistency with the index cancer (yes/no), and recurrence location [local = anastomosis or site of primary tumor or incision; elsewhere in the colon (including appendix or rectum); regional = in nearby nodes or on the outside of adjacent organs; distant = distant nodes, peritoneum, or inside other organs; or unknown]. For this study, recurrences were ascertained beginning 90 days after the end of treatment for the incident cancer (i.e., the start of follow-up). End of medical records abstraction (and thus end of follow-up) occurred at the earliest of death, disenrollment from health plan, or date of medical records abstraction.
Medical record abstractors also collected data on recurrences of other cancers and supplemented tumor registry data on second primary colorectal cancer diagnoses. Our secondary outcome was any cancer event, defined by a recurrence of any cancer or new primary cancer at any site.
In sensitivity analyses, we varied our definition of recurrence. Post hoc, we re-reviewed medical records of persons who died of colon cancer without a documented recurrence or second primary colon cancer to ensure that no study outcomes were missed. Because many of these cases had a cancer of unknown type shortly before death, we recorded these events to include in a broader definition of recurrence in sensitivity analyses. We also conducted a sensitivity analysis that included second primary colorectal cancer in the definition of recurrence to facilitate comparisons with other studies [24, 25, 28].
Exposure ascertainment
In the main analysis, we defined diabetes status based on diagnosis codes (ICD-9: 249.x, 250.x, 357.2, 362.0x, 366.41, 648.0x; ICD-10: E08x, E09x, E10x, E11x, E13x, O24x) recorded in the 12 months prior to colon cancer diagnosis through 90 days after the end of treatment (i.e., cohort entry). We classified a patient as having diabetes if they had (1) at least one inpatient/emergency department diabetes diagnosis; or (2) two or more outpatient ambulatory visit diabetes diagnoses within a 6-month period. The first date when patients satisfied this criterion was defined as their date of diabetes diagnosis. In secondary analyses, diabetes was a time-varying exposure that was ascertained through the end of follow-up. We did not distinguish between type 1 and type 2 diabetes.
In a secondary analysis, we analyzed persons with advanced diabetes, defined by use of insulin at cohort entry or most recent hemoglobin A1c (HbA1c) value (from 1 year before colon cancer diagnosis to cohort entry) being ≥ 9 mg/dL. Insulin prescriptions, which were assumed to run out after 6 months, were ascertained from the VDW pharmacy dispensings table. HbA1c values were ascertained from the VDW laboratory results table and/or legacy local data sources available beginning in 1995 at KPWA and 2000 at KPCO. Duration of diabetes was not available.
Covariates
The VDW served as the data source for demographic characteristics (i.e., sex, race, and ethnicity) and for year and stage of the index colon cancer diagnosis. Diagnosis codes were used to calculate Charlson comorbidity scores in the year before colon cancer diagnosis [32]. Persons were classified as having hypertension or hypercholesterolemia as of their first diagnosis code between 12 months before colon cancer diagnosis and the end of follow-up (Fig. 2). Height, weight at diagnosis, cancer treatments, aspirin use, and use of other non-steroidal anti-inflammatory medications were extracted from the VDW and supplemented by medical record review. Medical record reviewers also abstracted patients’ smoking status at and after diagnosis.
For descriptive purposes, we also extracted information on diabetes medication dispensings from the VDW pharmacy table. The study pharmacist (DMB) identified the generic names of diabetes medications in each of the following drug classes: metformin, sulfonylureas, insulins, thiazolidinediones, meglitinides, DPP inhibitors, GLP-1 agonists, alpha-glucosidase inhibitors, pramlintide, and combination medications. We extracted all outpatient dispensings for these medications beginning 12 months before colon cancer diagnosis through the end of follow-up. We collected data on statin dispensings in the same way.
Statistical analyses
We compared descriptive statistics for potential confounders by diabetes status at cohort entry and used a Poisson model to compute unadjusted rates of outcomes and 95% CIs stratified by diabetes status. We used Cox proportional hazards models to estimate hazard ratios for outcomes in relation to diabetes at cohort entry. The time scale for these analyses was time since end of treatment plus 90 days. In our primary analysis, estimates were adjusted for sex (male, female), age at index colon cancer diagnosis (natural cubic splines with knots at tertiles), health care system (KPWA or KPCO), diagnosis year (natural cubic splines with knots at tertiles), AJCC 6th edition tumor stage at diagnosis (I, IIA, IIB, IIIA), race (Black, non-Black, other/unknown), body mass index (BMI) at diagnosis (< 25.0, 25.0 to < 30.0, ≥ 30.0 kg/m2), and smoking history at and after diagnosis (time-varying: ever, never). In sensitivity analyses, we also adjusted in a time-varying fashion for the following covariates at or after cohort entry: statin use (any, none), aspirin use (any, none), hypertension diagnosis, and hypercholesterolemia diagnosis. These variables could have been recorded before or after a patient’s diabetes diagnosis in the medical record. We did not include these variables in our main model because of the possibility that they might be in the causal pathway between diabetes and recurrence. For comparison, minimally adjusted models included only age, diagnosis year, study site, and sex.
In analyses of colon cancer recurrence (the primary outcome), subjects were censored at the earliest of disenrollment from the health care system, death, second primary cancer at any site (including colon), recurrence of a non-colon cancer, or date of medical records abstraction. In the analysis of the secondary outcome, which was the composite endpoint of any cancer event, subjects were censored at disenrollment from health care system, death, or date of medical records abstraction.
We tested the proportional hazards assumption in the fully adjusted models of all four outcomes (primary outcome, secondary outcome, and two sensitivity analyses in which we varied the definition of recurrence as described above) by including an interaction term between diabetes at cohort entry and the log of analysis time. The proportional hazards assumption was satisfied for the primary and secondary analyses but did not hold for the two sensitivity analyses. Thus, we visually examined stratified cumulative hazard plots over time to determine if hazards were approximately proportional within discrete intervals of follow-up time. We then fit separate Cox proportional hazards models to each of the two identified time periods and found that, consistent with the original analysis, neither HR was statistically significant. Thus, we report a single HR over the entire study period.
In exploratory analyses, diabetes status was analyzed as time-varying in a Cox proportional hazards model. Patients who had no evidence of diabetes at cohort entry (i.e., unexposed) could become exposed if they met the diabetes definition outlined above during follow-up. However, once a person was classified as having diabetes, they could never become unexposed. In our analysis of patients with advanced diabetes, patients without diabetes served as the reference group.
A statistically significant difference in all comparisons was defined as p value < 0.05. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Study population
We identified 3,326 patients aged ≥ 18 years when diagnosed with stage I–IIIA malignant adenocarcinomas of the colon or rectosigmoid junction during 1995–2014. We excluded 631 people based on VDW data and 656 people during chart review (Fig. 1). A total of 2,039 people remained eligible after medical records abstraction (Fig. 1). An additional 116 patients diagnosed before 1 January 1999 at KPCO were excluded, leaving 1,923 eligible for this analysis.
At cohort entry, 393 (16.7%) of patients had diabetes, and an additional 100 patients (for a total of 25.6% of the cohort) developed diabetes during study follow-up. Patients with diabetes at cohort entry were more likely to be male, Hispanic, and Black or of other/unknown race compared to patients without diabetes (Table 1). Compared to people without diabetes, patients with diabetes were more likely to have a higher BMI and a greater comorbidity burden at diagnosis. People without diabetes were more likely than those with diabetes to be diagnosed with colon cancer by screening (rather than symptoms) but had less imaging on average (not shown). Most cancer characteristics—including age at diagnosis, stage at diagnosis, tumor location, tumor grade, and having positive lymph nodes—did not differ markedly based on diabetes status. However, patients with diabetes tended to have larger tumors than patients without diabetes. Approximately 90% of patients were diagnosed with stage I or IIA colon cancer, with the majority having grade I or II cancer, tumors < 5 cm, and no positive regional lymph nodes. A similar proportion of patients with and without diabetes received chemotherapy and radiation, and time from diagnosis to cohort entry (mean 137 days, standard deviation 72 days) was similar in the two groups. Statin and aspirin use in the year before colon cancer diagnosis were both more common in patients with diabetes compared to patients without diabetes. Overall cohort characteristics by study site are shown in Online Appendix Table 1. Sixty-eight percent of diabetes patients were taking at least one type of diabetes medication between end of treatment and cohort entry, inclusive (Table 2). Most patients had HbA1c < 8. There were 135 patients with HbA1c ≥ 9 mg/dL in the year before colon cancer diagnosis to cohort entry or were using insulin in the 90 days prior to cohort entry.
Risk of recurrence and subsequent cancer events
Over a median 4.7 years of follow-up (interquartile range 2.1–8.4 years), there were 139 colon cancer recurrences (12.8 recurrences per 1,000 person-years, 95% CI 10.8–15.2). Diabetes at cohort entry was not associated with colon cancer recurrence (HR 0.87; 95% CI 0.56–1.33) or subsequent cancer events overall (HR 1.09; 95% CI 0.85–1.40) in multivariable models (Table 3). Of the 427 first subsequent cancer events, 139 were colon cancer recurrences, 36 were second primary colorectal cancers, 28 were non-colorectal cancer recurrences, 210 were non-colorectal primary cancers, and 14 were cancers of unknown type.
Our findings for recurrence were generally consistent across sensitivity analyses. When diabetes was modeled through the end of study follow-up in a time-varying manner, there was no associated increased recurrence risk (HR 0.84; 95% CI 0.56–1.23, Online Appendix Table 2). Advanced diabetes at cohort entry was not significantly associated with recurrence; however, the hazard ratio (HR 1.17; 95% CI 0.62–2.21) was higher than for diabetes overall.
When second primary colorectal cancers were included in the definition of recurrence, we observed a diabetes-associated increased risk in the minimally adjusted model (HR 1.41, 95% CI 1.01–1.96). The increased risk was not significant in the fully adjusted or additionally adjusted models, but the point estimates were similar.
Discussion
In research published to date, diabetes is consistently associated with colorectal cancer incidence, and, in some studies, with colorectal cancer fatality [23, 33]. Thus, diabetes could plausibly increase the risk of colon cancer recurrence. A 2013 meta-analysis reported a near-significant pooled relative risk, and there was some suggestion (though not significant) of an increased risk of recurrence with diabetes among colon cancer patients in a Korean hospital (HR 1.32; 95% CI 0.98–1.76) [27]. However, the body of literature on this topic is relatively small, and no studies have been conducted in population-based (i.e., non-trial) settings in the United States, where diabetes severity and treatment may differ from other countries.
In our medical records-based cohort study, we did not observe an association between diabetes and colon cancer recurrence. Our results may provide some reassurance to patients with both diabetes and colon cancer. However, we cannot completely exclude an increased risk of colon cancer recurrence with diabetes. First, our confidence intervals were wide because recurrence is a relatively rare event. Second, though not significant, our point estimate for the association between diabetes and the composite outcome of colon cancer recurrence, second primary colorectal cancer, or cancer of unknown type (1.29)—though not significant—was similar to the estimates in the Mills et al. meta-analysis (1.27) and the Jeon et al. study (1.32). The consistency of this association across studies makes it difficult to rule out the possibility of an association.
Our study had several important strengths. We examined recurrence as an outcome in a population-based cohort in the United States, which can be challenging because recurrence is not routinely collected by population-based cancer registries [34]. We had access to information on recurrence in this study through medical records and were able to exclude people with signs of disease progression before and shortly after the end of treatment. Recall bias was not a concern because we used extensive medical records and administrative data to ascertain exposure, confounders, and outcomes. Participation bias was avoided because everyone meeting eligibility criteria based on medical records abstraction was included.
Several limitations are worth noting, however. We were unable to investigate associations between diabetes medications and colon cancer recurrence, as we had initially planned, due to limited power. We had extensive data from medical record review and controlled for the strongest predictors of recurrence (e.g., stage), but were not able to control for lifestyle factors. The degree of confounding by such factors is likely to be small after controlling for BMI, hypercholesterolemia, and hypertension, as we did. However, we may not have been able to completely control for aspirin use. We collected aspirin use from prescription fills and medical records abstraction, but we noted variation within and between medical records reviewers on this variable during our quality assurance assessment.
Our study does not suggest an increased risk of colon cancer recurrence in patients with diabetes. However, power was limited and given the results of our sensitivity analyses, and the literature on this topic as a whole, there is need for additional research on diabetes and colorectal cancer outcomes—especially in large populations in which the association with diabetes medication use can be studied and in which the risk of second primary colon cancers can be further investigated.
References
Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, Orchard TJ, Rolka DB, Imperatore G (2018) Prevalence of diagnosed diabetes in adults by diabetes type—United States, 2016. MMWR Morb Mortal Wkly Rep 67(12):359–361. https://doi.org/10.15585/mmwr.mm6712a2
Cossor FI, Adams-Campbell LL, Chlebowski RT, Gunter MJ, Johnson K, Martell RE, McTiernan A, Simon MS, Rohan T, Wallace RB, Paulus JK (2013) Diabetes, metformin use, and colorectal cancer survival in postmenopausal women. Cancer Epidemiol 37(5):742–749. https://doi.org/10.1016/j.canep.2013.04.015
Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT (2012) Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol 30(1):53–59. https://doi.org/10.1200/jco.2011.38.0303
Garrett CR, Hassabo HM, Bhadkamkar NA, Wen S, Baladandayuthapani V, Kee BK, Eng C, Hassan MM (2012) Survival advantage observed with the use of metformin in patients with type II diabetes and colorectal cancer. Br J Cancer 106(8):1374–1378. https://doi.org/10.1038/bjc.2012.71
Singh PP, Shi Q, Foster NR, Grothey A, Nair SG, Chan E, Shields AF, Goldberg RM, Gill S, Kahlenberg MS, Sinicrope FA, Sargent DJ, Alberts SR (2016) Relationship between metformin use and recurrence and survival in patients with resected stage iii colon cancer receiving adjuvant chemotherapy: results from North Central Cancer Treatment Group N0147 (Alliance). Oncologist 21(12):1509–1521. https://doi.org/10.1634/theoncologist.2016-0153
González N, Prieto I, del Puerto-Nevado L, Portal-Nuñez S, Ardura JA, Corton M, Fernández-Fernández B, Aguilera O, Gomez-Guerrero C, Mas S, Moreno JA, Ruiz-Ortega M, Sanz AB, Sanchez-Niño MD, Rojo F, Vivanco F, Esbrit P, Ayuso C, Alvarez-Llamas G, Egido J, García-Foncillas J, Ortiz A, Consortium DCC (2017) 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget 8(11):18456–18485. https://doi.org/10.18632/oncotarget.14472
Giovannucci E, Michaud D (2007) The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 132(6):2208–2225. https://doi.org/10.1053/j.gastro.2007.03.050
Cohen DH, Leroith D (2012) Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer 19(5):F27–F45. https://doi.org/10.1530/ERC-11-0374
Deng L, Gui Z, Zhao L, Wang J, Shen L (2012) Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci 57(6):1576–1585. https://doi.org/10.1007/s10620-012-2055-1
Onitilo AA, Engel JM, Glurich I, Stankowski RV, Williams GM, Doi SA (2012) Diabetes and cancer I: risk, survival, and implications for screening. Cancer Causes Control 23(6):967–981. https://doi.org/10.1007/s10552-012-9972-3
Chang CK, Ulrich CM (2003) Hyperinsulinaemia and hyperglycaemia: possible risk factors of colorectal cancer among diabetic patients. Diabetologia 46(5):595–607. https://doi.org/10.1007/s00125-003-1109-5
Luo S, Li JY, Zhao LN, Yu T, Zhong W, Xia ZS, Shan TD, Ouyang H, Yang HS, Chen QK (2016) Diabetes mellitus increases the risk of colorectal neoplasia: an updated meta-analysis. Clin Res Hepatol Gastroenterol 40(1):110–123. https://doi.org/10.1016/j.clinre.2015.05.021
Luo W, Cao Y, Liao C, Gao F (2012) Diabetes mellitus and the incidence and mortality of colorectal cancer: a meta-analysis of 24 cohort studies. Colorectal Dis 14(11):1307–1312. https://doi.org/10.1111/j.1463-1318.2012.02875.x
Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J (2011) Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 26(11):863–876. https://doi.org/10.1007/s10654-011-9617-y
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350:g7607. https://doi.org/10.1136/bmj.g7607
Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM (2012) Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care 35(9):1835–1844. https://doi.org/10.2337/dc12-0002
Larsson SC, Orsini N, Wolk A (2005) Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 97(22):1679–1687. https://doi.org/10.1093/jnci/dji375
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159(12):1160–1167. https://doi.org/10.1093/aje/kwh161
Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Emerging Risk Factors C (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364(9):829–841. https://doi.org/10.1056/NEJMoa1008862
Dowling RJ, Goodwin PJ, Stambolic V (2011) Understanding the benefit of metformin use in cancer treatment. BMC Med 9(1):33. https://doi.org/10.1186/1741-7015-9-33
Kourelis TV, Siegel RD (2012) Metformin and cancer: new applications for an old drug. Med Oncol 29(2):1314–1327. https://doi.org/10.1007/s12032-011-9846-7
Zhang HH, Guo XL (2016) Combinational strategies of metformin and chemotherapy in cancers. Cancer Chemother Pharmacol 78(1):13–26. https://doi.org/10.1007/s00280-016-3037-3
Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G (2013) Diabetes mellitus and colorectal cancer prognosis: a meta-analysis. Dis Colon Rectum 56(11):1304–1319. https://doi.org/10.1097/DCR.0b013e3182a479f9
Noh GY, Hwang DY, Choi YH, Lee YY (2010) Effect of diabetes mellitus on outcomes of colorectal cancer. J Korean Soc Coloproctol 26(6):424–428. https://doi.org/10.3393/jksc.2010.26.6.424
Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, Fuchs CS (2003) Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol 21(3):433–440. https://doi.org/10.1200/JCO.2003.07.125
Feng J, Zhou X, Mao W (2011) Prognostic analysis of colorectal cancer patients with diabetes mellitus in China—the experience of a single institution. Adv Clin Exp Med 20(4):473–480
Jeon JY, Jeong DH, Park MG, Lee JW, Chu SH, Park JH, Lee MK, Sato K, Ligibel JA, Meyerhardt JA, Kim NK (2013) Impact of diabetes on oncologic outcome of colorectal cancer patients: colon vs. rectal cancer. PLoS ONE 8(2):e55196. https://doi.org/10.1371/journal.pone.0055196
Bae S, Wong HL, Tie J, Desai J, Field K, Kosmider S, Fourlanos S, Jones I, Skinner I, Gibbs P (2015) Impact of diabetes status and medication on presentation, treatment, and outcome of stage II colon cancer patients. J Cancer Epidemiol 2015:189132. https://doi.org/10.1155/2015/189132
Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L (2017) The relationship between diabetes and colorectal cancer prognosis: a meta-analysis based on the cohort studies. PLoS ONE 12(4):e0176068. https://doi.org/10.1371/journal.pone.0176068
Chubak J, Ziebell R, Greenlee RT, Honda S, Hornbrook MC, Epstein M, Nekhlyudov L, Pawloski PA, Ritzwoller DP, Ghai NR, Feigelson HS, Clancy HA, Doria-Rose VP, Kushi LH (2016) The Cancer Research Network: a platform for epidemiologic and health services research on cancer prevention, care, and outcomes in large, stable populations. Cancer Causes Control 27(11):1315–1323. https://doi.org/10.1007/s10552-016-0808-4
Ross TR, Ng D, Brown JS, Pardee R, Hornbrook MC, Hart G, Steiner JF (2014) The HMO research network virtual data warehouse: a public data model to support collaboration. EGEMS (Washington, D.C.) 2(1):1049. https://doi.org/10.13063/2327-9214.1049
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45(6):613–619. https://doi.org/10.1016/0895-4356(92)90133-8
Bella F, Minicozzi P, Giacomin A, Crocetti E, Federico M, Ponz de Leon M, Fusco M, Tumino R, Mangone L, Giuliani O, Budroni M, Sant M (2013) Impact of diabetes on overall and cancer-specific mortality in colorectal cancer patients. J Cancer Res Clin Oncol 139(8):1303–1310. https://doi.org/10.1007/s00432-013-1439-8
Warren JL, Yabroff KR (2015) Challenges and opportunities in measuring cancer recurrence in the United States. J Natl Cancer Inst 107:8. https://doi.org/10.1093/jnci/djv134
Acknowledgments
We would like to thank Dr. Elizabeth Trice Loggers for assistance with designing the medical record abstraction instrument and providing clinical input, and Lee Barr, Kate Burniece, and Angela Plata for reviewing medical records. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R01CA172073 (Chubak) and R50CA211115 (Bowles). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The collection of cancer incidence data used in this study was supported by the Cancer Surveillance System of the Fred Hutchinson Cancer Research Center, which is funded by Contract No. N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute with additional support from the Fred Hutchinson Cancer Research Center and the State of Washington. Funders did not participate in the design, data collection, manuscript drafting, or decision to submit the manuscript for publication.
Funding
Monica Fujii reports receiving funding from a research contracts awarded to Kaiser Permanente Washington Health Research Institute from Allergan, BioDelivery Sciences, Collegium, Daiichi Sankyo, Depomed, Egalet, Endo, Janssen, Mallinckrodt, Pernix, Pfizer, Purdue, and West-Ward. Denise Boudreau reports receiving funding from research contracts awarded to Kaiser Permanente Washington Health Research Institute from Allergan, BioDelivery Sciences, Collegium, Daiichi Sankyo, Depomed, Egalet, Endo, Janssen, Mallinckrodt, Pernix, Pfizer, Purdue, and West-Ward. Onchee Yu reports receiving funding from a research grant awarded to the Kaiser Permanente Washington Health Research Institute from Bayer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
For this type of study, formal consent is not required.
Research involving animals
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chubak, J., Yu, O., Ziebell, R.A. et al. Risk of colon cancer recurrence in relation to diabetes. Cancer Causes Control 29, 1093–1103 (2018). https://doi.org/10.1007/s10552-018-1083-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-018-1083-3