Abstract
Purpose
This analysis describes the impact of hysterectomy on incidence rates and trends in endometrioid endometrial cancer in the United States among women of reproductive age.
Methods
Hysterectomy prevalence for states containing Surveillance, Epidemiology, and End Results (SEER) registry was estimated using data from the Behavioral Risk Factor Surveillance System (BRFSS) between 1992 and 2010. The population was adjusted for age, race, and calendar year strata. Age-adjusted incidence rates and trends of endometrial cancer among women age 20–49 corrected for hysterectomy were estimated.
Results
Hysterectomy prevalence varied by age, race, and ethnicity. Increasing incidence trends were observed, and were attenuated after correcting for hysterectomy. Among all women, the incidence was increasing 1.6% annually (95% CI 0.9, 2.3) and this increase was no longer significant after correction for hysterectomy (+ 0.7; 95% CI − 0.1, 1.5). Stage at diagnosis was similar with and without correction for hysterectomy. The largest increase in incidence over time was among Hispanic women; even after correction for hysterectomy, incidence was increasing (1.8%; 95% CI 0.2, 3.4) annually.
Conclusion
Overall, endometrioid endometrial cancer incidence rates in the US remain stable among women of reproductive age. Routine reporting of endometrial cancer incidence does not accurately measure incidence among racial and ethnic minorities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hysterectomy is one of the most frequently performed surgical procedures among women of reproductive age in the United States, second only to cesarean delivery. The majority of hysterectomies are performed for benign indications [1,2,3]. Approximately 428,523 hysterectomies for benign, non-obstetrical indications were performed yearly in the United States between 1998 and 2011 [4]. Recently, however, hysterectomy rates have been declining—a 39% decrease occurred from 2000 to 2014 (from 631 to 385 per 100 000) [5]. This decline corresponds to concerns about overuse of hysterectomy and increased use of alternatives to surgery as primary management of the conditions such as menorrhagia and pelvic pain [6]. These include hormonal management, operative hysteroscopy, endometrial ablation, uterine artery embolization, and use of the levonorgestrel intrauterine device (IUD). An inadvertent consequence of these trends towards conservative surgical management of the female genital tract may be an apparent increase in the incidence of gynecologic malignancies specifically cancers of the uterine corpus [7].
Over 61,030 women in the US are expected to be diagnosed with cancers of the uterine corpus in 2017, making it the most common gynecologic malignancy [8]. Incidence rates for endometrial cancer have climbed over the last decade, and the increase is projected to continue [8,9,10,11]. Women who have undergone a hysterectomy are no longer at risk for endometrial cancer, and failure to remove them from the denominator of the population at risk results in underestimation of rates [12,13,14,15]. Additionally, because hysterectomy frequency varies across racial and ethnic groups, correcting incidence rates for hysterectomy can markedly change the rates within underrepresented populations [12,13,14, 16,17,18,19,20,21]. In an analysis based on SEER data from 1992 to 2008 for women age 50 and older, correction of the denominator resulted in a 73% increase in incidence rate among white women and a 90% increase among Black women [22].
Previously, publications have assessed the impact of hysterectomy on incidence of endometrial cancer in the population as a whole, but the impact of hysterectomy correction on younger women has not previously been described. Endometrial cancer is generally thought of as a disease of postmenopausal women; however, 15% of women with this disease will be diagnosed during their reproductive years [12, 23]. A characteristic clinical profile is associated with younger patients with endometrial carcinoma typically. Most patients have an identifiable source of excess estrogen and endometrioid histologic subtypes are overwhelmingly more common [24,25,26,27,28]. Obesity is part of the distinct clinical profile of the young patient with endometrial cancer with a 1.59 increase in the relative risk of endometrial cancer per 5 kg/m2 increase in BMI [24, 28]. Over half of women under age 50 with endometrial cancer have been reported as obese at diagnosis [24, 25, 27]. Recognition of the obesity epidemic and delayed childbearing in the US has led to concerns of an increasing incidence of endometrial cancer among younger women [29]. We therefore evaluated rates of endometrioid endometrial cancer among women < 50 years of age in the US corrected for hysterectomy prevalence.
Materials and methods
Cancer incidence data for non-Hispanic (NH) white, NH black, and Hispanic women were obtained from 12 of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registries. These registries cover about 13% of the US population and include the following state and regional cancer registries: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Utah, San Francisco-Oakland, Seattle-Puget Sound, Los Angeles, San Jose-Monterey, and rural Georgia. Population-based data for non-Hispanic white, non-Hispanic black, and Hispanic women aged 20 to 49 diagnosed from 1992 to 2010 with cancer occurring in the corpus uterus (C54) and uterus (not otherwise specified) (C55) as defined by or converted to the World Health Organization International Classification of Disease for Oncology, 3rd edition (ICD-O-3), were identified. Asian/Pacific Islander or Alaskan Native/American Indian women were not included because of sparse data in the SEER registry in women under age 50.
The case definition was further refined to only include women with endometrioid endometrial cancer including non-specific adenocarcinoma. Endometrioid endometrial cancer represents the majority of endometrial cancer, particularly in women under 50 years of age and includes the following ICD-O-3 codes: Endometrioid adenocarcinoma, NOS (8380), adenocarcinoma, NOS (8140), mixed cell adenocarcinoma (8323), adenocarcinoma with squamous metaplasia (8570), adenosquamous carcinoma (8560), other (8050, 8141, 8143, 8210–8211, 8260–8263, 8340, 8381, 8440, 8470–8471, 8480–8481, 8490, 8550, 8571–8573), and non-specific adenocarcinoma (8010, 8020).
Other uterine cancers, such as sarcomas, clear cell or serous adenocarcinomas, and carcinosarcomas, have different biologic behavior and incidence trends. These histological subtypes are rare in young women and were excluded from this analysis because there were not sufficient data to analyze them separately.
FIGO stage was derived using the two different staging systems used by SEER registries during the study period. Cases diagnosed between 1992 and 2003 were staged using SEER Extent of Disease, 3rd edition, and cases diagnosed between 2004 and 2010 were staged using the Collaborative Stage Data Collection System [30]. The tumor extension codes from each system were harmonized and converted to a FIGO stage that was consistent over time (“Appendix”).
Incidence rates were estimated using SEER*Stat stratified by age (20–29, 30–34, 35–39, 40–44, 45–49), race (NH white, NH black, and Hispanic), and by 3-year calendar year intervals (1992–1994, 1995–1997, …, 2007–2010) [31]. All rates are the number of new cases per 100,0000 women. Since the 2000 US standard population is reported in 5-year age groups, incidence rates for the 20–29 age group were age-adjusted. Age-specific rates were reported for all other age groups. Rates reported by stage for ages 20–49 combined were age-adjusted.
Weighted estimates of hysterectomy prevalence were obtained from the Behavioral Risk Factor Surveillance Study (BRFSS) conducted by the Centers for Disease Control and Prevention [32]. The BRFSS is a cross-sectional state-specific telephone survey administered to adults 18 years and older living in households. We used BRFSS data from the states that contributed cancer incidence data (California, Connecticut, Georgia, Hawaii, Iowa, Michigan, New Mexico, Utah, Washington) in order for hysterectomy prevalence to represent the same geographic area as the cancer registries. BRFSS obtained information on hysterectomy in these states each year from 1992 to 2000 and then every other year. Data were not available for the state of Hawaii in 2004, and so this year was excluded from the analysis. Hysterectomy estimates were stratified by the same race/ethnicity, age, and calendar year intervals as the incidence rates. One caveat is that since BRFSS data were not collected every year, some 3-year intervals only use 1 or 2 years of survey data. For example, the hysterectomy prevalence estimate for 2004–2006 only contains survey data collected in 2006 since that was the only year available.
Smoothed hysterectomy prevalence was estimated using a logistic regression model with coefficients for age, year, and their interaction and weighted using the BRFSS weights. Separate models were estimated for each race/ethnicity group. The age and year-specific prevalence was estimated from the model and used to correct the SEER population-at-risk by subtracting the number of women who reported hysterectomy for each race/ethnicity group. The rates for the most recent 5-year period, 2006–2010, were calculated before and after correction to the population.
Trends in endometrial cancer for women age < 50 were estimated using the Joinpoint regression software with no joinpoints allowed in the model [33]. Without joinpoints, the software fits a linear-weighted least squares regression model to the log-transformed incidence rates. The trends were summarized by the annual percent change (APC) based on the fitted trend.
Results
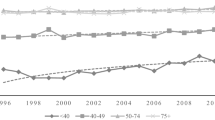
The prevalence of hysterectomy among women younger than age 50 is shown by race and age in Fig. 1. Hysterectomy prevalence increased with age and specifically among women over age 40. Prevalence among women younger than 40 years was generally low, < 10% in all race/ethnic groups. The prevalence of hysterectomy in women aged 45–49 years was 50% for NH black women, 28% of NH white women, and 22% of Hispanic women.
9,219 of 11,217 (82.1%) women under age 50 were diagnosed with endometrioid endometrial cancer during the study period and were included in this analysis. The excluded cancers included 1334 (12.9%) sarcomas, 400 (3.6%) serous and clear cell cancers, and 264 (2.3%) unclassified malignancies. These histologic subtypes were excluded because of their distinct etiologies, risk factors, and incidence patterns compared to endometrioid cancers [22]. Incidence rates of included cancers by age at diagnosis and race/ethnicity for the most recent 5-year period, 2006–2010, are shown in Table 1. The incidence rate was lowest for women aged 20–29: 0.4 for NH white women, 0.7 for NH Black women, and 1.5 for Hispanic women; the corrected and uncorrected for hysterectomy incidence rates are similar as the prevalence of hysterectomy is low in this age group. The incidence rates in each racial and ethnic group beyond age 29 increased with age, as did the difference between uncorrected and corrected rates. Among women aged 40–49, NH Black women have the lowest rates even after correction for hysterectomy. The corrected rate of endometrial cancer among NH Black women aged 45–49 was 12.7 compared to 29.1 among NH White women and 22.4 among Hispanic women. The majority of women under 50, over 87%, were diagnosed with local (Stage I/II) disease (Table 2). The 5-year incidence rate, corrected and uncorrected, increased with age for women of all stages. The largest difference between corrected and uncorrected rates is among women aged 45–49 with early-stage disease (17.5 vs. 22.7, respectively).
Both corrected and uncorrected trends in age-adjusted incidence rates for endometrioid endometrial cancer among women under age 50 are depicted in Fig. 2. Increasing uncorrected incidence trends were observed among NH Black and Hispanic women (Table 3). However, correction for hysterectomy attenuated the incidence trends especially among NH Black women. Specifically, the overall annual percent change in incidence among NH Black women was 4.1% annually (95% CI 1.3, 6.9) but this increase was no longer statistically significant after correction for hysterectomy (1.1; 95% CI − 1.6, 3.9). Even after correction for hysterectomy, the incidence rate among Hispanic women remained statistically significantly increasing 1.8% annually (95% CI 0.2, 3.4). The incidence rate was stable among NH White women both before and after correction for hysterectomy. When corrected for hysterectomy, the increasing incidence trend among women with both Stage I/II disease and Stage III/IV disease was no longer statistically significant.
Discussion
Our results demonstrate that for women younger than 50 years of age, reporting incidence rates of endometrial cancer without hysterectomy adjustment underestimates the true burden of disease. Additionally, incidence rates of endometrial cancer that are not corrected for hysterectomy distort patterns among racial and ethnic groups [12, 14, 15, 22].
Racial and ethnic variation in endometrial cancer incidence are well described—incidence of disease is lower among Black women but overall survival is consistently reported as worse [14, 16, 22]. Because rates of hysterectomy are higher among black women, correcting for hysterectomy reduces the disparity in incidence between White and Black women [22]. In fact, when incidence rates corrected for hysterectomy among women of all ages and all histologic subtypes are evaluated, the incidence among Black women equals or surpasses that of White women [12, 22].
Consistent with previous reports, correction for hysterectomy prevalence in our study modified racial patterns in endometrial cancer incidence. However, Black women of reproductive age had consistently lower corrected rates of endometrial cancer than White and Hispanic women, and these rates changed less over time. This finding was counterintuitive and differed from previous reports looking at all uterine cancers as endometrial carcinoma risk factors, obesity, and diabetes are more prevalent among black than among white non-Hispanic women, regardless of hysterectomy status [14, 34]. The reason for this difference in rates between Black and White women may relate to other population level disparities related to access to specialized care for the diagnosis to be made. Additional differences between racial and ethnic groups may impact the motivation of young women to seek care for irregular bleeding or heavy menses, the most common presenting symptom of this disease among reproductive aged women.
Increasing rates of endometrial cancer among Hispanic women remained significant after correction for hysterectomy in this analysis. An increasing incidence of endometrial cancer patients of Hispanic origin in the US has been previously described [35]. The uncorrected incidence rate among Hispanic women in the US increased by 2.3% per year for women aged younger than 50 years between 2000 and 2012 [36]. Demographic characteristics of the diverse group of Americans classified as Hispanic within the SEER registry may differ from non-Hispanic women. Low education and socioeconomic levels are known to affect access to care. Medical comorbidities that alter uterine cancer risk including diabetes, metabolic syndrome, and obesity are more common among Hispanic women than other racial and ethnic groups. Diabetes incidence and prevalence has leveled off nationally, but continues to increase among Hispanics [37]. The prevalence of metabolic syndrome in Hispanic Americans is estimated at 38.6% [38]. Forty-six percent of Hispanic women are estimated to be obese compared to 35% of non-Hispanic women in the United States [39].
Several limitations of our study should be considered. First, the BRFSS is limited to those with working telephones, and the median response rate for the states in our study has been declining from 67% in 1992 (range 57.4–80.7%) to 55% in 2010 (range 43–65%) [40, 41]. Lower response rates have been associated with the underrepresentation of racial and ethnic minorities [42]. Although self-reports of hysterectomy in BRFSS are fairly accurate, hysterectomy rates from self-report may be less accurate in non-native English or lower English proficiency populations [43]. Also, state-level hysterectomy prevalence was used for the metropolitan areas in SEER. If the hysterectomy rates for the state were not representative of the SEER catchment area, there may have been an over- or underestimate of the corrected population. Pathology review for confirmation of the diagnosis and histology was not possible. Finally, rates and trends corrected for hysterectomy could not be calculated for Asian/Pacific Islander or Alaskan Native/American Indian women because of sparse data in both the BRFSS and SEER data in women under age 50.
Large datasets, such SEER data, are valuable tools that allow an understanding of variation in cancer outcomes among subpopulations and have been used to expose health-related disparities. However, population level narratives may also inadvertently propagate stereotypes by reinforcing differences between racial and ethnic groups. Although rates of medical comorbidities and biologic variation may impact differences in cancer outcomes among underserved and minority populations, the contribution of structural racism and implicit bias to health disparities is recently being acknowledged [44, 45]. The lack of correction for hysterectomy rates is an example of a structural bias in our reporting systems as the impact on White incidents rates is small, but the impact on Black women is much larger. A 40% Black-White mortality gap in endometrial cancer can be attributed to unequal surgical treatment and stage distribution [46]. An accurate accounting of the role of modifiable social determinants of health may uncover opportunities to reduce or prevent health systems level inequities which might be otherwise inaccurately ascribed to innate differences between individuals.
Correction for hysterectomy allows for more accurate reporting of incidence rates of endometrial cancer even among young women and among racial and ethnic groups. In conclusion, despite an obesity epidemic, corrected incidence rates of endometrial cancer in women aged < 50 years old appear to be stable over time among the US population as a whole, but increasing among Hispanic women.
References
Cohen SL, Vitonis AF, Einarsson JI (2014) Updated hysterectomy surveillance and factors associated with minimally invasive hysterectomy. J Soc Laparoendosc Surg 18:e2014.00096
Whiteman MK, Hillis SD, Jamieson DJ, Morrow B, Podgornik MN, Brett KM et al (2008) Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol 198:34.e1–34.e7
Merrill RM (2008) Hysterectomy Surveillance in the United States, 1997 through 2005. Med Sci Monit 14:24–31
Mikhail E, Salemi JL, Mogos MF, Hart S, Salihu HM, Imudia AN (2015) National trends of adnexal surgeries at the time of hysterectomy for benign indication, United States, 1998–2011. Am J Obstet Gynecol 213:713.e1–713.e13
Doll KM, Dusetzina SB, Robinson W (2016) Trends in inpatient and outpatient hysterectomy and oophorectomy rates among commercially insured women in the United States, 2000–2014. JAMA Surg 151:876–877
Sutton C (2010) Past, present, and future of hysterectomy. J Minim Invasive Gynecol 17:421–435
Temkin SM, Minasian L, Noone A-M (2016) The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol 6:89
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B (2013) Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol 37:374–377
Sheikh MA, Althouse AD, Freese KE, Soisson S, Edwards RP, Welburn S et al (2014) USA endometrial cancer projections to 2030: should we be concerned? Future Oncol 10:2561–2568
Duncan ME, Seagroatt V, Goldacre MJ (2012) Cancer of the body of the uterus: trends in mortality and incidence in England, 1985–2008. BJOG Int J Obstet Gynaecol 119:333–339
Siegel RL, Devesa SS, Cokkinides V, Ma J, Jemal A (2013) State-level uterine corpus cancer incidence rates corrected for hysterectomy prevalence, 2004 to 2008. Cancer Epidemiol Biomark Prev 22:25–31
Stang A, Hawk H, Knowlton R, Gershman ST, Kuss O (2014) Hysterectomy-corrected incidence rates of cervical and uterine cancers in Massachusetts, 1995 to 2010. Ann Epidemiol 24:849–854
Sherman ME, Carreon JD, Lacey JV, Devesa SS (2005) Impact of hysterectomy on endometrial carcinoma rates in the United States. J Natl Cancer Inst 97:1700–1702
Merrill RM (2006) Impact of hysterectomy and bilateral oophorectomy on race-specific rates of corpus, cervical, and ovarian cancers in the United States. Ann Epidemiol 16:880–887
Redburn JC, Murphy MFG (2001) Hysterectomy prevalence and adjusted cervical and uterine cancer rates in England and Wales. BJOG Int J Obstet Gynaecol 108:388–395
Luoto R, Raitanen J, Pukkala E, Anttila A (2004) Effect of hysterectomy on incidence trends of endometrial and cervical cancer in Finland 1953–2010. Br J Cancer 90:1756–1759
Wong C, Jim M, King J, Tom-Orme L, Henderson J, Saraiya M et al (2011) Impact of hysterectomy and bilateral oophorectomy prevalence on rates of cervical, uterine, and ovarian cancer among American Indian and Alaska Native women, 1999–2004. Cancer Causes Control 22:1681–1689
Stang A (2012) Impact of hysterectomy on the age-specific incidence of cervical and uterine cancer in Germany and other countries. Eur J Pub Health 23:879–883
Stang A, Merrill R, Kuss O (2012) Prevalence-corrected hysterectomy rates by age and indication in Germany 2005–2006. Arch Gynecol Obstet 286:1193–1200
Hammer A, Rositch AF, Kahlert J, Gravitt PE, Blaakaer J, Søgaard M (2015) Global epidemiology of hysterectomy: possible impact on gynecological cancer rates. Am J Obstet Gynecol 213:23–29
Jamison PM, Noone A-M, Ries LAG, Lee NC, Edwards BK (2013) Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomark Prev 22:233–241
DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL et al (2014) Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 64:252–271
Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM et al (2005) Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 105:575–580
Topuz S, Sozen H, Vatansever D, Iyibozkurt AC, Ozgor BY, Bastu E et al (2016) Do obesity and age effect the clinicopathological features and survival outcomes in premenopausal women with endometrial cancer? Eur J Gynaecol Oncol 37:320–326
Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF (2001) Endometrial cancer in women 40 years old or younger. Gynecol Oncol 83:388–393
Garg K, Soslow RA (2014) Endometrial carcinoma in women aged 40 years and younger. Arch Pathol Lab Med 138:335–342
Goodwin PJ, Stambolic V (2015) Impact of the obesity epidemic on cancer. Annu Rev Med 66:281–296
Lambe M, Wuu J, Weiderpass E, Hsieh C-C (1999) Childbearing at older age and endometrial cancer risk (Sweden). Cancer Causes Control 10:43–49
Fritz A, Ries LAG (1988) Surveillance Research Program, National Cancer Institute. SEER Extent of Disease—1988: Codes and Coding Instructions, 3rd edn.
Costa-Paiva L, Godoy CE Jr, Antunes A Jr, Caseiro JD, Arthuso M, Pinto-Neto AM (2011) Risk of malignancy in endometrial polyps in premenopausal and postmenopausal women according to clinicopathologic characteristics. Menopause 18:1278–1282
Byun JY, Kim SE, Choi BG, Ko GY, Jung SE, Choi KH (1999) Diffuse and focal adenomyosis: MR imaging findings. Radiographics, 19 Spec No:S161-70
Kim H-J, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19:335–351
Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS et al (2007) Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol 109:655–662
Cote ML, Ruterbusch JJ, Olson SH, Lu K, Ali-Fehmi R (2015) The growing burden of endometrial cancer: a major racial disparity affecting black women. Cancer Epidemiol Biomark Prev 24:1407–1415
Siegel RL, Fedewa SA, Miller KD, Goding-Sauer A, Pinheiro PS, Martinez-Tyson D et al (2015) Cancer statistics for Hispanics/Latinos, 2015. CA Cancer J Clin 65:457–480
Geiss LS, Wang J, Cheng YJ et al (2014) Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA 312:1218–1226
Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ (2015) Prevalence of the metabolic syndrome in the United States, 2003–2012. Jama 313:1973–1974
Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. In: Prevention CfDCa, editor. National Center for Health Statistics 2015
1992 BRFSS SUMMARY QUALITY CONTROL REPORT. 1992
Behavioral Risk Factor Surveillance System 2010 Summary Data Quality Report Centers for Disease Control 2010
Schneider KL, Clark MA, Rakowski W, Lapane KL (2012) Evaluating the impact of non-response bias in the Behavioral Risk Factor Surveillance System (BRFSS). J Epidemiol Community Health 66:290–295
Brett KM, Madans JH (1994) Hysterectomy use: the correspondence between self-reports and hospital records. Am J Public Health 84:1653–1655
Hardeman RR, Medina EM, Kozhimannil KB (2016) Structural racism and supporting black lives—the role of health professionals. N Engl J Med 375:2113–2115
Ansell DA, McDonald EK (2015) Bias, black lives, and academic medicine. N Engl J Med 372:1087–1089
Doll KM, Winn AN, Goff BA (2017) Untangling the Black-White mortality gap in endometrial cancer: a cohort simulation. Am J Obstet Gynecol 216:324–325
Acknowledgments
This work was presented in part at the Meeting of the International Gynecologic Cancer Society in Lisbon, Portugal, in October 2016. The authors thank T Gibson and S Scoppa from Information Management Systems, Inc, for their assistance with data management.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
FIGO stage | Extent of disease (1992–1997) | Extent of disease (1998–2003) | Collaborative stage (2004–2009) | Collaborative stage (2010) |
|---|---|---|---|---|
Stage 0 | 0 | 0 | 0 | |
Stage 1 | 10–15, 20–25, 30–35 | 10–14 | 100, 110, 115, 120, 123, 125, 130, 133, 135, 140, 160, 180, 400 | 100, 110, 114, 120, 123, 125–126, 130, 135, 140, 145, 160, 180, 400 |
Stage 2 | 40, 50 | 40, 50–52 | 500, 510, 520, 523, 525, 540, 545, 550 | 500, 520, 523, 525, 540 |
Stage 3 | 60 | 60–61, 64–66 | 600, 610, 635, 640, 645, 650, 660, 663, 670, 680, 688, 692 | 545, 550, 605, 630, 635, 640, 655, 660, 662, 665, 680 |
Stage 4 | 70, 80, 85 | 70, 80, 85 | 700, 705, 715, 800, 810, 820 | 682, 688, 693–694, 696, 708, 710, 715, 800, 810, 820 |
Rights and permissions
About this article
Cite this article
Temkin, S.M., Kohn, E.C., Penberthy, L. et al. Hysterectomy-corrected rates of endometrial cancer among women younger than age 50 in the United States. Cancer Causes Control 29, 427–433 (2018). https://doi.org/10.1007/s10552-018-1018-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-018-1018-z