Abstract
Purpose
Endometrial cancer accounts for 3.9% of all female cancers globally, and its incidence appears to be increasing in women under 40 years of age. This paper investigated ethnic-specific trends in endometrial cancer across different age groups in New Zealand.
Methods
Women who were diagnosed with endometrial cancer between 1996 and 2012 were identified from the New Zealand Cancer Registry. Annual age-standardized incidence and mortality rates were calculated for each ethnicity (Māori, Pacific, and non-Māori non-Pacific) in four age groups (< 40, 40–49, 50–74, and 75 +). The estimates were adjusted for hysterectomy. Joinpoint regression analysis was used to assess trends over time and annual percentage changes (APCs) were estimated.
Results
Between 1996 and 2012, age-standardized incidence rates increased in all women and significantly in the < 40, 40–49, and 50–74 age groups (APC 9.22, 3.56, and 1.65 respectively). Incidence rates were highest in Pacific women and increased most rapidly in those under 50 years of age (APC 9.36). Conversely, age-standardized mortality rates decreased in all women and significantly in the 50–74 and 75 + age groups (APC − 5.25 and − 5.06 respectively), with the highest rate observed in Pacific women.
Conclusion
Pacific women had the highest incidence of endometrial cancer and the trend was increasing, particularly in young women. This could be attributed, at least in part, to a high and increasing rate of obesity in these women and should be explored in future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometrial cancer (EC) is an increasingly problematic gynecological tumor, with its occurrence most common in postmenopausal women [1]. In 2012, EC was the 5th most common cancer among women internationally, and the 14th most deadly [2]. Incidence rates are highest in well-developed, western nations such as Australia, New Zealand, and the United States, with age-standardized incidence rates ranging between 12 and 20 cases per 100,000 women annually [3]. Moreover, incidence rates have largely been increasing, with statistically significant and positive annual percentage changes (APCs) noted in Australia and New Zealand between 1996 and 2007 [4].
The most prominent risk factors associated with EC include obesity, nulliparity, and natural aging, with obesity being the strongest modifiable risk factor [1]. As many as 40% of cases have been estimated to be attributed to obesity, with a recent meta-analysis noting a 1.59-fold (95% CI [1.50, 1.68], p < 0.0001) increased risk of EC associated with every 5 kg/m2 increase in BMI [5, 6]. The relationship between obesity and EC risk was found to be non-linear, however, with a much larger risk associated with every 5 kg/m2 increase in BMI for women with a BMI above 28 kg/m2 (relative risk (RR) = 3.04, 95% CI [2.31, 4.01]). Conversely, the most widely cited protective factors for EC throughout the literature include oral contraceptive use, smoking, and physical activity.
In New Zealand, EC was the 5th most common cancer among women in 2012, and the 9th most deadly [7]. There is marked ethnic variation in the incidence and mortality of EC in New Zealand, with Pacific women having higher rates than that of other ethnicities (and higher incidence rates than that of any other ethnic group in the world) [8, 9]. Furthermore, incidence rates have been increasing at a faster rate in Pacific women in comparison to their Māori and New Zealand European counterparts. In a study assessing incidence rates in New Zealand women between 1981 and 2004, the RR of EC in Pacific women increased from 1.96 to 3.78 relative to New Zealand European/other women, with a much higher pooled RR for women aged between 25 and 44 [10].
The aim of this study is to investigate trends in the incidence and mortality of endometrial cancer in New Zealand between 1996 and 2012. Contrary to previous New Zealand studies, rates will be adjusted for hysterectomy to more accurately reflect rates among the population at risk. Rates will be calculated for different age and ethnic groups, and underlying reasons for the observed trends in different age and ethnic groups will be explored.
Methods
Data sources
Data on EC incidence and mortality between 1996 and 2012 were extracted from the New Zealand Cancer Registry (NZCR), and subsequently stratified by three ethnic groups (Māori, Pacific, and non-Māori non-Pacific). In the NZCR, ethnicity is recorded at level two of the Ministry of Health’s (MoH) classification, and is derived from hospital discharge, mortality, and National Health Index data. Up to three ethnic groups are recorded per individual, and patients with more than one recorded ethnicity are allocated to a single ethnic group in order of priority: Māori, Pacific, and non-Māori non-Pacific [11]. Māori are the indigenous Polynesian people and constitute approximately 14% of New Zealand women, while Pacific are immigrants or descended from immigrants from the Pacific Islands and constitute approximately 7% of New Zealand women. The non-Māori non-Pacific group predominantly includes women who are of European origin, but also includes Asian women and other ethnic groups. Population estimates were then extracted from the New Zealand census.
The NZCR is a population-based register of all primary malignant diseases diagnosed in New Zealand [12]. The primary source of incidence data for the NZCR comes from laboratories, while mortality data come from the Department of Internal Affairs register of Births, Deaths, and Marriages. Since the enactment of the Cancer Registry Act 1993 and Cancer Regulations 1994, data quality and coverage of the registry has significantly improved.
Hysterectomy prevalence figures were obtained from the MoH between 1999 and 2003, including data for all women and by ethnicity (2016 email from B. Rendle; unreferenced). It was assumed that there were no particularly pertinent sociocultural factors operating in New Zealand that would significantly alter these figures between 1996 and 2012. Therefore, the same prevalence figures were used to estimate the prevalence of hysterectomies between 1996 and 2012.
EC cases were identified using the WHO’s ICD-10-AM codes [12], with the majority of C54 (malignant neoplasm of corpus uteri) cancers included, and all C55 (malignant neoplasm of uterus, part unspecified) cancers included. Malignant neoplasms of the myometrium (C542 codes) fall outside the scope of EC, and were therefore excluded from the analysis. Tumor morphology (histology type and behavior code) was then checked using the WHO International Classification of Diseases for Oncology (ICD-O) codes (Supplementary Table 1).
Statistical analysis
Statistical analysis was undertaken to determine incidence and mortality rates for all women between 1996 and 2012, as well as stratifying by ethnicity and cancer type. Age-standardized incidence rates were calculated based on four age groups, these being < 40, 40–49, 50–74, and 75 +. Due to the relatively low number of deaths in the younger age groups, the < 40 and 40–49 age groups were combined for calculating age-standardized mortality rates, resulting in < 50, 50–74, and 75 + age groups. For similar reasons, age groups were again combined when analyzing the incidence and mortality data by ethnicity, with two age groups formed, these being < 50 and 50+. All rates were age-standardized using the WHO’s standard population [13].
Undergoing a hysterectomy removes the risk of EC, so analysis was carried out to determine incidence and mortality rates in the cohort of women subject to EC exclusively. Hysterectomy corrections were made on all women, as well for each ethnic group. The following results have all been adjusted for hysterectomies and rates are presented on a log-linear (base 10) scale. When adjusting for hysterectomies, the denominator (population estimates) was adjusted on an annual basis by subtracting the proportion of hysterectomies in each age group for each ethnicity from the original population estimates.
Annual percentage changes were then calculated by fitting a least squares regression line to the natural logarithm of the rates and determining the slope of the line. The formula \(({e^{{\text{slope}}}} - 1) \times 100\) is then taken as the annual percentage change. For the purposes of this research, this process was carried out using the Joinpoint regression program [14].
Results
In the 17 years analyzed between 1996 and 2012, the total number of cases across all women was 5,486 and the total number of deaths attributable to EC was 1920. Of the cases, there were 4,228 non-Māori non-Pacific, 655 Māori, and 603 Pacific. The average age-standardized incidence rate across all women was 14.5 per 100,000, and the average age-standardized mortality rate was 4.7 per 100,000 (Table 1, both adjusted for hysterectomy). When stratifying by age group, average age-standardized incidence rates were 0.9, 10.7, 58.1, and 53.0 per 100,000 women for the < 40, 40–49, 50–74, and 75 + age groups respectively, while average age-standardized mortality rates were 0.4, 17.6, and 36.2 for the < 50, 50–74, and 75 + age groups respectively (all rates adjusted for hysterectomy).
Data on the prevalence of hysterectomy for each ethnic group were analyzed in 5-year age bands, with no women undergoing a hysterectomy before the age of 30. In each 5-year age band, the prevalence of hysterectomy was invariably highest in the non-Māori non-Pacific ethnic group, followed by Māori, and then Pacific. Among all women, the prevalence of hysterectomy was 7.8% in non-Māori non-Pacific, 2.0% in Māori, and 1.0% in Pacific. Therefore, when incidence rates were adjusted for hysterectomy, the largest increase was for non-Māori non-Pacific (24.8%), followed by Māori (10.1%), and then Pacific (4.9%) (Table 2). Following adjustment for hysterectomy, corrected incidence rates were highest in Pacific women (40.9 per 100,000), followed by Māori (19.6 per 100,000), and then non-Māori non-Pacific (12.6 per 100,000). In reference to Pacific women, the RR of EC in Māori women increased from 0.46 to 0.48 following adjustment for hysterectomy, while the RR of EC in non-Māori non-Pacific women increased from 0.26 to 0.31.
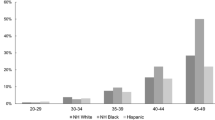
When analyzing incidence trends between 1996 and 2012, age-standardized incidence rates increased and the increase was statistically significant for the < 40, 40–49, and 50–74 age groups (Table 1; Fig. 1). Annual percentage changes were 9.22, 95% CI [6.10, 12.50], 3.56, 95% CI [1.70, 5.50], and 1.65, 95% CI [1.00, 2.30] for each respective age group. When stratifying incidence trends by ethnicity, age-standardized incidence rates in the < 50 age group increased and the increase was statistically significant for the non-Māori non-Pacific and Pacific ethnic groups (Table 1; Fig. 2). Annual percentage changes were 2.82, 95% CI [1.00, 4.60] and 9.36, 95% CI [5.00, 13.90] for the non-Māori non-Pacific and Pacific ethnicities, respectively. The trend in incidence in those of Pacific ethnicity under the age of 50 is particularly striking, increasing from 2 per 100,000 to 24 per 100,000 over the course of the study period. In the 50 + age group, age-standardized incidence rates increased and the increase was statistically significant for the non-Māori non-Pacific and Māori ethnic groups (Table 1). Annual percentage changes were 1.01, 95% CI [0.50, 1.60] and 2.42, 95% CI [0.70, 4.20] for the non-Māori non-Pacific and Māori ethnicities, respectively.
In contrast to incidence trends, age-standardized mortality rates decreased and the decrease was statistically significant for the 50–74 and 75 + age groups (Table 1). Annual percentage changes were − 5.25, 95% CI [− 6.60, − 3.80] and − 5.06, 95% CI [− 6.80, − 3.30] for each respective age group.
Discussion
The incidence rate of EC in Pacific women under the age of 50 in New Zealand is high and rapidly increasing, with an annual percentage change of 9.36 between 1996 and 2012. In contrast, the APC for non-Māori non-Pacific women under the age of 50 was 2.82, and 2.37 for Māori women. Among women under the age of 50, the average hysterectomy-corrected incidence rate in Pacific women was 11.4 per 100,000, compared with 4.1 for Māori women, and 1.6 for non-Māori non-Pacific women. The high and increasing incidence rate of EC in young Pacific women is largely consistent with trends found elsewhere; however, information on age-specific trends has been limited. In New Zealand, high and increasing incidence rates in Pacific women have been reported in the previous literature [8, 10]. Consistent with these studies, the current study indicates that the high and rapidly increasing incidence rate of EC was largely exclusive to young Pacific women.
Given EC’s underlying etiology and the results presented above, it may be expected that the prevalence of obesity is increasing more rapidly in young Pacific women than that of other ethnicities in New Zealand. Unfortunately, however, information on age-specific obesity trends in different ethnic groups is not available in New Zealand. Nevertheless, according to the New Zealand Health Survey [15], the prevalence of obesity (BMI of 30 or more) in Pacific women increased from 56.2 to 68.9% between 1997 and 2011/2012 (Ptrend < 0.05) (Supplementary Table 2). The increase was also statistically significant for European/other women, increasing from 16.9 to 24.8%. Although information on age-specific obesity trends is not available by ethnicity or gender, when obesity rates were stratified by age, most of the significant increases between 1997 and 2011/2012 were observed for individuals in the < 45 age groups.
As mentioned previously, a meta-analysis examining the strength of the association between increased BMI and EC risk found the relationship to be non-linear, with a much larger risk associated with a 5 kg/m2 increase for women with a BMI above 28 kg/m2 [6]. When estimates of ‘extreme’ obesity (“BMI ≥ 35 with comorbidities, or BMI ≥ 40, excluding those with BMI > 55”) were constructed by the Middlemore Public Health Department (Auckland, New Zealand), the percentage change was far greater for Pacific women that that of any other ethnic group between 2003 and 2007. The estimated prevalence of extreme obesity increased from 24.0 to 35.0% for Pacific women in this time period, compared with increases of 12.0 to 15.0% for Māori women, and 5.8 to 7.8% for non-Māori non-Pacific women (Supplementary Table 3). These increases may have contributed to the rapidly increasing incidence rate of EC in young Pacific women in New Zealand. Supporting this hypothesis, a recent USA study projecting future EC incidence rates found levels of extreme obesity (BMI above 40 kg/m2) to be a better predictor of past incidence rates than regular levels of obesity (BMI between 30 kg/m2 and 40 kg/m2) in their multivariate linear regression models [16].
In a USA-based study assessing EC trends between 1992 and 2009, it was noted that incidence rates were increasing over the entire time period for women aged between 20 and 49, but not for women aged between 50 and 74 [17]. Among women in the postmenopausal group, Joinpoint analyses identified an inflection point between the 1992–2002 and 2003–2009 time periods, with the rate of change significantly increasing after 2002. When the data were stratified by ethnicity, the increased APC after 2002 in the postmenopausal group was found to be accelerating more so in black and Asian/Pacific Island women in comparison to their white counterparts.
The significantly higher APC of EC observed in young women relative to older women has been postulated to be due to both rapidly increasing rates of obesity in young women, as well as decreased use of hormone replacement therapy (HRT) in postmenopausal women following the publication of results from the Women’s Health Initiative (WHI) trial in 2002 [18,19,20,21]. Following the decrease in HRT use, postmenopausal women who had previously used combined therapy were no longer afforded a protective effect on EC, and incidence rates increased. No protective effect would have been afforded to premenopausal women at any point however, and accordingly, EC incidence rates increased over the entire study period in this group. Therefore, because the analysis of incidence rates between 1996 and 2012 (Table 1) includes a considerable length of time in which many postmenopausal women would have been using HRT (note that the prevalence of HRT use among NZ women over the course of the study period was not known), the APCs for young women are much higher than that of the APCs for women in the postmenopausal age groups.
In the USA, where black women have long experienced higher obesity rates than white women [22], white women have been observed to have higher EC incidence rates than that of black women [4]. This difference, however, seems to be attenuated when adjusting for hysterectomy [23,24,25]. Because of the very high incidence rates of EC experienced by Pacific women in the current study, the disparity in incidence rates between Pacific and non-Māori non-Pacific women is attenuated to only a small degree after adjusting for hysterectomy (Table 2). Therefore, the disparity in EC incidence rates observed between Pacific and non-Māori non-Pacific women in this study cannot be explained by the differing prevalence of hysterectomy between ethnic groups. This would seem to give even more credence to the hypothesis of extreme obesity having a causal influence on the rapidly increasing EC incidence rates in Pacific women as above.
Hysterectomy rates and indications change over time and vary by ethnic group [23], and overseas studies have noted that hysterectomy rates have been declining in the USA and UK [23, 26]. Therefore, the assumption of hysterectomy rates remaining stable over time in the current study may have been unwarranted. In the USA, hysterectomy rates have been declining in white women, whereas hysterectomy rates in blacks have remained relatively stable. By analogy, hysterectomy rates may have declined faster in non-Māori non-Pacific women relative to Pacific women in New Zealand. If this were the case, the disparity in incidence rates between Pacific and non-Māori non-Pacific women would be attenuated for even less after adjustment for hysterectomy, and the resulting lower risk of EC among non-Māori non-Pacific women would be even lower than that observed in Table 2. Again, there would have to be other mechanisms in place in order to explain the disparity in EC risk between ethnicities, with high and increasing rates of extreme obesity in Pacific women a seemingly likely contributor.
During the latter part of the study period, both fertility rates (that is, total fertility rates) and levels of physical activity decreased in Pacific women, and increased in non-Māori non-Pacific women [15, 27]. As both of these factors are protective for EC [28, 29], these trends, coupled with obesity, could therefore partly explain the trends in incidence rates observed in this study. On the other hand, the prevalence of cigarette smoking (another protective factor for EC [30]), increased in Pacific women, and decreased in non-Māori non-Pacific women during the course of the study period [27].
In contrast to incidence rates, mortality rates for all women decreased in all age groups between 1996 and 2012, with statistically significant APCs of − 5.25 and − 5.06 in the 50–74 and 75 + age groups respectively (Table 1). These decreases can predominantly be attributed to advances in diagnostic services and subsequent treatment [31], although largely seem to be confined to type 1 EC exclusively. When mortality trends were analyzed in Joinpoint for all women in this study, the APC of type 1 mortality was a statistically significant − 6.97, while the APC of type 2 mortality was a non-significant + 2.04. Consistent with this, type 2 EC mortality rates were found to be increasing significantly among all women in the USA between 2006 and 2011 [32]. These trends are unsurprising given the increasing incidence of type 2 EC and its relatively poor prognosis in comparison to type 1 EC [33], and while type 2 EC comprised just 14.2% of the cases in this study, it contributed to 25.7% of the total deaths.
The primary strength of this study is that it is population based and captured both EC incidence and mortality in all New Zealand women between 1996 and 2012. Because the Cancer Registry Act and Cancer Regulations were both implemented prior to the study period, it can be assured that the registry’s data we used were of high quality. Furthermore, we confined our study to endometrial carcinoma exclusively, and excluded other malignant neoplasms of the uterus. Contrary to previous New Zealand studies, we also adjusted rates for hysterectomy to more accurately reflect rates among the population at risk. In doing so, we assumed that the hysterectomy prevalence estimates we used were applicable over the entire study period, although this assumption may have been unwarranted. Another major limitation of this study relates to how ethnicity is classified in the NZCR and census. When calculating incidence rates, the NZCR (numerator) uses ethnicity information collected from health user or discharge information in hospitals, while the census (denominator) uses ethnicity information collected from a standardized questionnaire [34, 35]. Discordance has consistently been reported between these two datasets, and historically, both Māori and Pacific have been undercounted in cancer registration data relative to census data, while non-Māori non-Pacific have been overcounted [36]. As such, incidence rates presented previously may be slightly lower than their actual rates for Māori and Pacific, and slightly higher than their actual rates for non-Māori non-Pacific, although recent evidence suggests that this problem was less pronounced in the latter part of our study period [37]. Finally, it is also worth noting that the New Zealand census is a five-year survey, and population estimates must be made in between census dates [38]. Therefore, when calculating incidence and mortality rates, it is important to acknowledge that these estimates are subject to error.
In terms of future research specific to New Zealand, the routine provision of age and ethnic-specific obesity trends would provide important information to Pacific (and other) women in relation to EC, as well a range of other health conditions. Similarly, information on other risk/protective factors over a life course, such as HRT and OC use, would provide necessary information to be able to more reliably interpret EC incidence trends over time.
It has also been suggested that clinicians should be more alert to EC symptoms in obese and/or Pacific women in their normal line of work. Currently, young obese women may not be fully investigated when they present with traditional EC symptoms; however, further investigation of symptoms is now considered mandatory in this group given their high-risk profile.
In conclusion, the incidence rate of EC increased between 1996 and 2012 in New Zealand women. The increase was much faster in younger women, particularly of Pacific ethnicity. The disparity in EC incidence between Pacific women and other ethnic groups was not attenuated when the rates were corrected for hysterectomy, and is likely to be due to high and increasing rates of obesity (and extreme obesity in particular) in Pacific women. Other potential contributors include declining fertility rates and levels of physical activity, although their effects are relatively modest in comparison to obesity’s influence. Given current obesity trends, more efforts are needed to tackle obesity through population level measures such as the adoption of healthy food policies. Such interventions may target young Pacific women to mitigate the rising incidence of EC in this group.
References
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387(10023):1094–1108
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):359–386
GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012 [Internet]. Lyon: International Agency for Research on Cancer; c2018. Accessed 15 Jan 2017 from http://globocan.iarc.fr/Default.aspx
CI5plus: Cancer incidence in five continents time trends [Internet]. Lyon: International Agency for Research on Cancer; c2018. Accessed 15 Jan 2017 from http://ci5.iarc.fr/CI5plus/Pages/online.aspx
Calle EE, Kaaks R (2004) Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4(8):579–591
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578
Ministry of Health (2015) Cancer: Historical summary 1948–2012. Ministry of Health, Wellington
Firestone RT, Ellison-Loschmann L, Shelling AN, Ekeroma A, Ikenasio-Thorpe BA, Pearce N et al (2012) Ethnic differences in disease presentation of uterine cancer in New Zealand women. J Fam Plann Reprod Health Care 38(4):239–245
CI5XI: Cancer incidence in five continents volume XI [Internet]. Lyon: International Agency for Research on Cancer; c2018. Accessed 14 Feb 2017 from http://ci5.iarc.fr/CI5-XI/Default.aspx
Meredith I, Sarfati D, Ikeda T, Atkinson J, Blakely T (2012) High rates of endometrial cancer among Pacific women in New Zealand: the role of diabetes, physical inactivity, and obesity. Cancer Causes Control 23(6):875–885
Ethnicity code tables [Internet]. Wellington: Ministry of Health; c2018. Accessed 29 Jan 2017 from https://www.health.govt.nz/nz-health-statistics/data-references/code-tables/common-code-tables/ethnicity-code-tables
New Zealand Cancer Registry (NZCR) [Internet]. Wellington: Ministry of Health; c2018. Accessed 15 Feb 2017 from https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/new-zealand-cancer-registry-nzcr
World Health Organization (2001) Age standardization of rates: a new WHO standard. World Health Organization, Geneva
Kim HJ, Fay MP, Feuer EJ, Midthune DN (2000) Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19(3):335–351
Ministry of Health (2012) The health of New Zealand adults 2011/12: key findings of the New Zealand Health Survey. Ministry of Health, Wellington
Sheikh MA, Althouse AD, Freese KE, Soisson S, Edwards RP, Welburn S et al (2014) USA endometrial cancer projections to 2030: should we be concerned? Future Oncol 10(16):2561–2568
Wartko P, Sherman ME, Yang HP, Felix AS, Brinton LA, Trabert B (2013) Recent changes in endometrial cancer trends among menopausal-age US women. Cancer Epidemiol 37(4):374–377
Writing Group for the Women’s Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women’s health initiative randomized controlled trial. JAMA 288(3):321–333
Duong LM, Wilson RJ, Ajani UA, Singh SD, Eheman CR (2011) Trends in endometrial cancer incidence rates in the United States, 1999–2006. J Womens Health 20(8):1157–1163
Sjögren LL, Mørch LS, Løkkegaard E (2016) Hormone replacement therapy and the risk of endometrial cancer: a systematic review. Maturitas 91:25–35
Duncan ME, Seagroatt V, Goldacre MJ (2012) Cancer of the body of the uterus: trends in mortality and incidence in England, 1985–2008. BJOG 119(3):333–339
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM (2006) Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295(13):1549–1555
Temkin SM, Minasian L, Noone AM (2016) The end of the hysterectomy epidemic and endometrial cancer incidence: what are the unintended consequences of declining hysterectomy rates? Front Oncol 6:1–6
Siegel RL, Devesa SS, Cokkinides V, Ma J, Jemal A (2013) State-level uterine corpus cancer incidence rates corrected for hysterectomy prevalence, 2004 to 2008. Cancer Epidemiol Biomarkers Prev 22(1):25–31
Jamison PM, Noone A-M, Ries LAG, Lee NC, Edwards BK (2013) Trends in endometrial cancer incidence by race and histology with a correction for the prevalence of hysterectomy, SEER 1992 to 2008. Cancer Epidemiol Biomarkers Prev 22(2):233–241
Redburn JC, Murphy MFG (2001) Hysterectomy prevalence and adjusted cervical and uterine cancer rates in England and Wales. BJOG 108(4):388–395
Births tables [Internet]. Wellington: Statistics New Zealand; c2018. Accessed 27 Feb 2017 from http://archive.stats.govt.nz/browse_for_stats/population/births/births-tables.aspx
Wu QJ, Li YY, Tu C, Zhu J, Qian KQ, Feng TB et al (2015) Parity and endometrial cancer risk: a meta-analysis of epidemiological studies. Sci Rep 5:1–17
Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M (2015) A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol 30(5):397–412
Zhou B, Yang L, Sun Q, Cong R, Gu H, Tang N et al (2008) Cigarette smoking and the risk of endometrial cancer: a meta-analysis. Am J Med 121(6):501–508
Weiderpass E, Antoine J, Bray FI, Oh JK, Arbyn M (2014) Trends in corpus uteri cancer mortality in member states of the European Union. Eur J Cancer 50(9):1675–1684
Gaber C, Meza R, Ruterbusch JJ, Cote ML (2017) Endometrial cancer trends by race and histology in the USA: projecting the number of new cases from 2015 to 2014. J Racial Ethn Health Disparities 4(5):895–903
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17
Ministry of Health (2004) Ethnicity data protocols for the health and disability sector. Ministry of Health, Wellington
Bramley D, Latimer S (2007) The accuracy of ethnicity data in primary care. N Z Med J 120(1264):1–8
Shaw C, Atkinson J, Blakely T (2009) (Mis)classification of ethnicity on the New Zealand Cancer Registry: 1981–2004. N Z Med J 122(1294):10–22
Boyd M, Atkinson J, Blakely T (2016) Ethnic counts on mortality, New Zealand Cancer Registry and census data: 2006–2011. N Z Med J 129(1429):22–39
Statistics New Zealand (2016) How accurate are population estimates and projections? Statistics New Zealand, Wellington
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Formal consent
For this type of study, formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Scott, O.W., Tin Tin, S., Bigby, S.M. et al. Rapid increase in endometrial cancer incidence and ethnic differences in New Zealand. Cancer Causes Control 30, 121–127 (2019). https://doi.org/10.1007/s10552-019-1129-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-019-1129-1