Abstract
Purpose
Cancer genetic services (counseling/testing) are recommended for women diagnosed with breast cancer younger than 45 years old (young breast cancer survivors—YBCS) and at-risk relatives. We present recruitment of YBCS, identification and recruitment of at-risk relatives, and YBCS willingness to contact their cancer-free, female relatives.
Methods
A random sample of 3,000 YBCS, stratified by race (Black vs. White/Other), was identified through a population-based cancer registry and recruited in a randomized trial designed to increase use of cancer genetic services. Baseline demographic, clinical, and family characteristics, and variables associated with the Theory of Planned Behavior (TPB) were assessed as predictors of YBCS’ willingness to contact at-risk relatives.
Results
The 883 YBCS (33.2% response rate; 40% Black) who returned a survey had 1,875 at-risk relatives and were willing to contact 1,360 (72.5%). From 853 invited at-risk relatives (up to two relatives per YBCS), 442 responded (51.6% response rate). YBCS with larger families, with a previous diagnosis of depression, and motivated to comply with recommendations from family members were likely to contact a greater number of relatives. Black YBCS were more likely to contact younger relatives and those living further than 50 miles compared to White/Other YBCS.
Conclusion
It is feasible to recruit diverse families at risk for hereditary cancer from a population-based cancer registry. This recruitment approach can be used as a paradigm for harmonizing processes and increasing internal and external validity of large-scale public health genomic initiatives in the era of precision medicine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Public health action leveraging family history is needed to reach a large number of individuals at risk for hereditary cancer [1]. An important public health intervention is the promotion of cancer genetic services (counseling and testing) in families at risk for hereditary breast and ovarian cancer syndrome [2, 3]. Germline genetic testing identifies mutation carriers and enables personalized cancer risk management. Cascade genetic testing among at-risk relatives can also confirm the non-inheritance of a well-characterized mutation and prevent unnecessary early onset screening and healthcare costs among “true negative” relatives [4]. Clinical guidelines recommend genetic assessment for all first- and second-degree relatives of women diagnosed with breast cancer younger than 50 years [5].
Reaching a large number of at-risk individuals, let alone at-risk families, has been a long-standing challenge for researchers. Using cancer registries has been one way to address this challenge. Studies in the US [6–9] and internationally [10–13] have used population-based cancer registries, hospital-based registries [14, 15], or both [16] often with random sampling for patients who met eligibility criteria [7, 13–15, 17]. Response rates from hospital-based registries varied, depending on clinicians’ time and motivation to recruit patients [7, 9, 16]. Recruitment from population-based registries yielded more participants, but without targeted sampling [13, 14, 18], minority, rural, and hard-to-reach patients were underrepresented due to low response rates.
Breast [6, 7, 11–13, 15, 19] and colorectal cancer patients [8, 16, 17] have often been recruited from cancer registries. Identifying cancer survivors with the early onset disease is critical for identifying at-risk relatives and promoting genetic testing and cancer screening due to possible hereditability of a cancer syndrome [7, 8, 11, 15, 16, 18]. Survivors have often been asked to allow the research team to contact at-risk relatives directly, which ensures that the latter receive the invitation [6, 8, 11, 13, 16]. One study asked cancer survivors whether they would like to recruit at-risk relatives themselves, or they would rather allow the research team to do this on their behalf, but did not report response rates for either recruitment method [17].
It is unclear to what extent women affected by breast cancer at a young age are willing to contact at-risk relatives for promoting screening and cancer genetic services, and factors associated with their willingness to do so. Moreover, little is known about using cancer registries to recruit at-risk families affected by the early onset breast cancer. Young breast cancer survivors (YBCS) is a growing clinical population for which there is paucity of information; recruiting at-risk relatives who could benefit from advances in genomic medicine is also an emerging priority research area. To address this gap, in our knowledge, this paper presents methodological details about using a population-based cancer registry to identify and recruit young breast cancer survivors (YBCS) and at-risk relatives. The study also explored predictors of YBCS’ willingness to contact at-risk relatives.
Methods
Design, setting, sample, and procedures
Identifying and recruiting YBCS and at-risk relatives were examined with baseline data from a prospective randomized trial designed to increase cancer surveillance and use of genetic services in families with the early breast cancer onset [20]. All appropriate Institutional Review and Scientific Advisory Boards approved the study protocol.
The Michigan Cancer Surveillance Program is a population-based cancer registry and was searched for YBCS cases from 1998 onward. Eligible YBCS were (1) female, 25–64 years old; (2) diagnosed with unilateral or bilateral invasive breast cancer or ductal carcinoma in situ (DCIS) between 20 and 45 years old; (3) lived in Michigan when diagnosed; and (4) were not pregnant, incarcerated, or institutionalized during recruitment. A random sample of 3,000 YBCS was identified out of 7,866 cases included in the registry. Most people (93%) in Michigan are self-identified as Black or White. To ensure adequate representation and have enough Black participants in the final sample of the randomized trial, YBCS were stratified according to registry-recorded race (1,500 Black vs. 1,500 White/Other) [21]. The 7% of YBCS of other ethnic/racial background were grouped with White YBCS, because they could not be included as a separate stratum. The cancer registry cross-referenced mortality data for deceased YBCS [20].
The Michigan Cancer Surveillance Program mailed the reporting facility and the physician of record requesting, for any reason, the YBCS should not be contacted for the study. A non-response within 30 days was taken as an agreement for contacting the YBCS. This contact method is consistent with patient preferences [22] and provides the opportunity for physician input, while eliminating barriers of requesting permission for patient contact [23]. The cancer registry mailed an invitation letter, an informed consent form, and the self-administered baseline survey to YBCS. Contact information for Institutional Review Boards, the Director of the cancer registry, the Principal Investigator, and the Cancer-Genomics Program at the Michigan Department of Health and Human Services were also provided.
A staff person from the cancer registry (50% FTE for six months) carried out contacting duties, starting with the letter to the facility and physician of record and recruitment letters. YBCS were identified between February and April 2012; letters to facilities/ physicians were sent in June 2012. YBCS were sent up to three recruitment letters between August and November 2012. A search through Accurint, a LexisNexis database with coverage across the US, obtained new addresses for YBCS not reached with the initial letters. A new series of letters was sent if an alternate address was located. Less than 20 YBCS contacted the registry with questions about being included in the database. YBCS accepting participation returned a signed consent and the baseline survey to the cancer registry and received a $10 gift card as a token of appreciation.
Identifiable information for YBCS was not released from the cancer registry to the research team until the YBCS accepted participation. Two board-certified genetic counselors from the Cancer-Genomics Program at the Michigan Department of Health and Human Services reviewed surveys to ascertain YBCS eligibility. If the YBCS reported a known mutation, such as BRCA1, BRCA2, or a known hereditary cancer syndrome, such as Lynch, PTEN Hamartoma Tumor, Li-Fraumeni, or Peutz-Jeghers syndromes, the genetic counselors made three attempts to contact her and verify the response. YBCS with a confirmed hereditary cancer syndrome were excluded from the randomized trial, because the intervention was not applicable to them. Their relatives were not recruited in the study; instead, YBCS were mailed printed material from the National Cancer Institute, the Centers for Disease Control and Prevention, and from FORCE (Facing Our Risk of Cancer Empowered) to distribute to them.
The genetic counselors used YBCS’ baseline surveys to identify relatives who were eligible to participate in the study. Identification of eligible relatives followed a protocol that involved a pedigree-based algorithm. YBCS were asked (1) to list anonymously all their first- and second-degree female relatives, e.g., sister, maternal aunt, etc.; (2) who were unaffected by cancer; (3) the relative’s age; (4) whether she lived in Michigan; (5) whether she lived within 50 miles from the YBCS; and (6) whether the YBCS was willing to contact her. The study recruited only relatives that the YBCS was willing to contact. Relatives eligible to participate had to be female, between 25 and 64 years old, unaffected by any type of cancer, first-degree relative or second-degree relative of the YBCS, US resident, able to read English and provide informed consent, and not currently pregnant, incarcerated or institutionalized. Recruitment priority was given to first- vs. second-degree relatives, and to younger vs. older women. Genetic counselors could contact YBCS for additional information regarding eligibility of relatives.
Each participating YBCS was mailed a letter suggesting up to two of her female relative(s) to invite in the study, along with consent forms, surveys, and postage-paid envelopes to pass on to them. A Project Navigator was available by phone to discuss concerns about this procedure. The research team did not have direct contact with relatives until they returned a signed consent accepting participation. Approximately 25% of at-risk relatives did not respond within six to eight weeks to the initial invitation to the study. In these cases, the Project Navigator contacted the YBCS to select an alternate relative. Relatives’ baseline surveys were returned to the Cancer-Genomics Program and were reviewed by two genetic counselors to ascertain eligibility. Identification, recruitment, and collection of data from relatives occurred between November 2012 and May 2013.
Measures
YBCS’ and relatives’ surveys included identical scales, except for scales assessing YBCS’ responses to breast cancer (e.g., fear of cancer recurrence, self-efficacy dealing with cancer diagnosis, etc). These scales did not apply to relatives who were unaffected by cancer. The behavior of interest in this paper is the number of relatives that YBCS was willing to contact. Predictors of intention to perform a behavior were chosen according to the Theory of Planned Behavior (TPB), i.e., knowledge and attitudes, subjective norms, and perceived control [24]. Family support facilitates decisions for genetic testing [25] and was added to the model. Measures were reliable (Cronbach’s alpha >0.71) and publicly available.
Predictors of willingness to invite at-risk relatives included YBCS’s characteristics, relatives’ characteristics as reported in the YBCS survey, and predictors related to the adapted TPB. Specifically, these predictors were (1) YBCS’ demographics (age; race; education; income; insurance; marital status; lives alone; has a routine source of care; cannot access care due to high out-of-pocket costs); (2) YBCS’ clinical characteristics (years since diagnosis; invasive breast cancer vs. DCIS; unilateral or bilateral breast surgery; family history of cancer; having genetic counseling and/or testing; number of relatives with breast cancer; and the previous diagnoses of depression and anxiety); (3) Relatives’ characteristics assessed in YBCS’ surveys (first- or second-degree relatives; age; lives in Michigan; lives within 50 miles from YBCS). Predictors from the adapted TPB were (4) knowledge and attitudes (perceived breast cancer risk; fear of cancer recurrence; knowledge of breast cancer risk factors; and breast cancer genetics); (5) subjective norms (family members expect engagement in preventive behaviors; healthcare providers expect engagement in preventive behaviors; motivation to comply with family members’ suggestions; and motivation to comply with healthcare providers’ suggestions); (6) perceived control (breast cancer self-efficacy); and (7) family support (communication; support in illness; and hardiness); and number of female relatives as a proxy of YBCS’ family size. Most predictors from the adapted TPB were assessed on 7-point Likert scales ranging from one “Strongly Disagree” to seven “Strongly Agree.” Perceived breast cancer risk was assessed with one item asking participants to rate their chances of getting breast cancer on a 10-point Likert scale with verbal anchors (“Definitely will not” to “Definitely will”). Items assessing knowledge of breast cancer risk factors and knowledge of breast cancer genetics could be answered as “True”, “False”, or “Do not Know.”
Data analyses
Data analyses were performed in the R software (Version 3.2.2, R Core Team, 2015).
Means, standard deviations, and frequencies were used to describe the sample. A Poisson regression was used to model predictors of number of relatives YBCS were willing to contact based on the distribution of the dependent variable. Power analysis determined that a sample of n = 352 would achieve 80% power for the Poisson regression with two tailed alpha set at p < .05. Missing values were less than 18% of the survey data, and involved primarily values in multi-item scales assessing concepts of the adapted TPB. Missing values were addressed with multiple imputations using the R software mipackage [26, 27]. For each copy of the imputed data set (m = 10), we conducted a variable selection based on the LASSO method [28] using the R software glmnet package [27]. A ten-fold cross-validation determined the value of the tuning parameter λ in LASSO. The final Poisson regression model was identified using the λ value with the smallest cross-validation error. The final model included predictors with non-zero coefficients in the fitted LASSO model. The means and standard errors of estimates for each imputed data set were then pooled to create a combined estimate based on Rubin’s rules [29].
Results
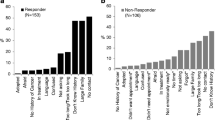
Overall, 883 YBCS accepted participation (33.2%); 353 were identified from the cancer registry as Black (27.5%) and 530 as White/Other (38.6%) (Fig. 1 ). Most YBCS (n = 778, 88.1%) resided in Michigan and 11.9% in 23 other states (data available upon request). The most common reason for no participation was lack of current address for YBCS (n = 252), most commonly for Black YBCS (69% of invalid addresses). Accurint enabled locating 91.6% of the initial YBCS cohort. Other known reasons for non-participation were: YBCS was deceased (n = 66); the reporting physician advised against contact (n = 22); YBCS was incarcerated (n = 3). From 883 participating YBCS, 24 were ineligible, because they were not diagnosed younger than 45 years old (n = 10), were pregnant (n = 9), and returned their survey too late (n = 5). There was “no response” from 832 Black (55%) and 663 White/Other YBCS (44%).
YBCS race was recorded based on registry data, since this was a criterion for sample stratification before random selection, while race of relatives was based on self-report. Thus, the race of family members is not the same in 20 family units. YBCS were on average 11 ± 4 year post-diagnosis. They were approximately 10 years older than relatives, less educated, and had fewer barriers to access healthcare services compared to relatives. About one in four YBCS reported multiple breast cancers and/or other cancers, such as ovarian. Most had a family history of breast cancer (Tables 1, 2).
Among 859 eligible YBCS, approximately one in three reported having genetic counseling and/or genetic testing. The majority of these YBCS reported having a negative test result, meaning that a mutation connected to cancer was not identified. Only 58 YBCS reported a BRCA1 (n = 19) or BRCA2 (n = 14), or other mutation (n = 7); having a family member with a known mutation (n = 6); having a known hereditary cancer syndrome (e.g., Li Fraumeni) (n = 12). These 58 YBCS were excluded from the randomized trial and their data are not included in the Poisson regression, because their willingness to contact at-risk relatives may be different from other YBCS. There were 801 eligible YBCS whose data are included in the Poisson regression.
Responses from YBCS’ baseline surveys helped to identify 1,875 at-risk relatives; YBCS were willing to contact 1,360 relatives (72.5%). We examined that characteristics of relatives YBCS were not willing to contact. There was no difference between first- vs. second-degree relatives and whether the relative resided in Michigan. Black YBCS were significantly more likely to not be willing to invite older relatives. The mean age for no-contact relatives from Black YBCS was 47.6 ± 11.7 vs. 44.0 ± 13 from White/Other (p = .001). Black YBCS were also significantly more likely to not be willing to contact relatives that lived within 50 miles (n = 124) vs. White/Other (n = 99) (p = .027).
To have comparable family units in terms of participating members, the study invited up to two relatives per YBCS (total n = 853 at-risk relatives) (Fig. 2). Greater emphasis was placed on recruiting first-degree relatives (440 sisters; 231 daughters; 25 mothers) compared to second-degree relatives (89 nieces; 36 half-sisters; 32 aunts). Overall, 442 relatives accepted participation (51.5%); some were ineligible due to pregnancy (n = 5) or survey returned too late (n = 6). A total of 431 relatives enrolled 231 sisters (53.6%); 123 daughters (28.5%); 9 mothers (2.1%); 41 nieces (9.5%); 14 half-sisters (3.2%); and 13 aunts (3.0%). Most relatives (n = 313, 72.6%) resided in Michigan; 27.4% resided in 27 other states (data available upon request).
Out of the 19 predictors selected by LASSO, five predictors were significantly associated with the number of relatives YBCS who were willing to contact in the Poisson regression (Table 3). The exponentiated coefficients indicate the multiplicative difference between willingness and unwillingness to contact relatives. YBCS with larger families were 26% more likely to contact at-risk relatives. YBCS reporting greater motivation to comply with suggestions from family members were 6% more likely to contact relatives. YBCS with a previous diagnosis of depression, those with a greater number of relatives diagnosed with breast cancer, and those reporting less motivation to comply with healthcare providers were approximately 16, 8, and 8% less likely to contact relatives.
Discussion
We present the feasibility of using a state cancer registry to identify and recruit at-risk families in a trial designed to increase surveillance and use of cancer genetic services. The study assessed methods to integrate unaffected relatives with a possible predisposition to hereditary cancer to an efficacy trial and circumvent typical barriers associated with collection of family history and participant recruitment. Although family history is typically assessed during medical visits in clinics or hospitals, lack of time, and shortage of personnel in these settings prevent collection of detailed family information and limit follow-up. Clinical sites are seldom large enough to target and reach at-risk populations. Cancer registries are an efficient alternate to implement family studies due to their greater outreach capacity. They contain data of reported malignancies within a particular population and can help establish family based registries.
Using the database of the Michigan Cancer Surveillance Program enabled targeting a large number of YBCS, a growing clinical population for which there is paucity of information. Response rate among YBCS was approximately 33%, similar to other studies that recruit participants via mailed invitation letters [12–15]. YBCS stratification enabled recruitment of a large number of Black YBCS. Response rate among Black YBCS was lower, often because the cancer registry did not have a current address and possibly because the sample had been diagnosed on average 11 years prior to attempted contact. Updating contact information on a regular basis should be considered. YBCS reported multiple signs in their personal history which could be indicative of hereditary cancer, e.g., multiple primary breast cancers, ovarian cancer, and male breast cancers. Using age of onset (≤45 years old) as the primary selection criterion identified survivors who should be evaluated genetically according to current guidelines [5]. This approach could be used for population-based recruitment for biobanks and family studies evaluating the penetrance of newly identified mutations [30].
The study involved YBCS to recruit relatives, with a response rate among relatives 51.5%, which supports the feasibility of this approach. Unique to this study was examining YBCS’ overall willingness to make contact effort. The only information for non-participating relatives was available in YBCS’ baseline survey and included their age, relationship with the YBCS, whether they live in Michigan, further than 50 miles from the YBCS, and whether the YBCS was willing to contact them. From data available in YBCS’ surveys, we identified racial differences in YBCS’ willingness to invite relatives, i.e., Black YBCS were more willing to invite younger vs. older relatives and those living further than 50 miles. YBCS with larger families were more willing to contact a greater number of relatives. There was a positive correlation between number of contacts an YBCS was willing to make and her family size, which was an expected finding. We considered using the proportion of relatives that YBCS were willing to contact (e.g., one relative out of five) as the dependent variable of the Poisson regression. However, this does not capture the “effort” needed by YBCS to contact each of her relatives. An YBCS who were willing to invite one out of two relatives shows 50% “effort.” The same is true for an YBCS willing to invite five out of ten relatives, although she is willing to do more contact “effort.”
YBCS with more relatives diagnosed with breast cancer were willing to contact fewer of them, most likely because there were not many cancer-free relatives in their family. YBCS with a previous diagnosis of depression and those reporting less motivation to comply with healthcare providers were also less willing to contact relatives. Some YBCS likely did not invite relatives for logistic reasons (e.g., beginning of school year for children), while lack of open communication may also play a role in relatives’ non-response to this invitation. In addition, response rates were lower among second-degree and Black relatives. Possibly, non-responding relatives perceived YBCS’ breast cancer diagnosis not a threat to their own health. These findings highlight the need for healthcare and public health professionals to take an active role in encouraging contact within members of at-risk families and for identifying YBCS with a diagnosis of depression, who may benefit from supportive interventions to reach out to family members. Targeted and culturally sensitive interventions designed to increase awareness of breast cancer genetics are needed for at-risk and vulnerable populations.
Strengths and limitations
The study did not use a direct measure of willingness to contact at-risk relatives for genetic screening per se, but willingness to contact relatives for research. We consider this a reasonable proxy for the behavior of interest. YBCS’ mutation status was based on self-report, although genetic counselors verified most of these reports. Self-reported genetic counseling and testing for breast cancer is generally accurate [31]. We examined YBCS overall willingness to contact relatives and not based on specific relative characteristics. Recruitment of at-risk relatives could have been compromised if the YBCS had not clearly understood instructions, or was hesitant to communicate information about breast cancer genetics. We did not recruit relatives from YBCS with an identified hereditary cancer syndrome, because we could not provide these families with appropriate intervention materials. In clinical practice, it would be important to contact these families and inform them about their cancer risk. The study “imposed” a recruitment scheme that involved only two relatives per YBCS (to have comparable family units), and favored first- vs. second-degree relatives and younger vs. older women, to reach relatives who could benefit the most from information about the possible hereditary nature of breast cancer in their family. The downside of this recruitment scheme was that although YBCS’ wishes were respected (invited only relatives she was willing to contact), YBCS were not really “free” to recruit the number and relatives of their choice to the study. Despite these limitations, the study recruited a large, random sample of at-risk families from a cancer registry database with an adequate representation of Black YBCS. Our recruitment method supports large-scale public health genomic studies.
Conclusions
Study findings are especially important in the era of the precision medicine initiative [32] which aims to develop a national cohort of one million or more U.S. participants and lead efforts in cancer-genomic research and other chronic diseases. The goal is to create a cohort reflecting the diversity of the U.S. population with participants from all age groups, health status, and from diverse social and racial/ethnic background, living in a variety of geographies, social environments, and economic circumstances. Our recruitment approach is supportive of such a large-scale public health genomic initiative. It can save resources and impact at-risk families by eliminating typical barriers associated with clinic-based recruitment. This method can also serve as a platform for standardizing nationwide recruitment efforts that support the internal and external validity of large-scale public health genomic efforts [33]. This recruitment method can also be used for studies aiming to increase genomic public health literacy [34], examine the effectiveness of genomic-based approaches in precision medicine [35], and using a “hybrid” approach bridging the gap between clinical and public health genomic research [36].
References
Bowen MS, Kolor K, Dotson WD, Ned RM, Khoury MJ (2012) Public health action in genomics is now needed beyond newborn screening. Public Health Genom 15(6):327–334. doi:10.1159/000341889
Balmana J, Diez O, Rubio IT, Cardoso F (2011) BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol 22(Suppl 6):vi31–34. doi:10.1093/annonc/mdr373
Couch FJ, Nathanson KL, Offit K (2014) Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343(6178):1466–1470. doi:10.1126/science.1251827
Nelson HD, Pappas M, Zakher B, Mitchell JP, Okinaka-Hu L, Fu R (2014) Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: a systematic review to update the U.S. Preventive Services Task Force recommendation. Ann Intern Med 160(4):255–266. doi:10.7326/M13-1684
NCCN (2016) Genetic/familal high-risk assessment: breast and ovarian. National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 25 Nov 2016
Bastani R, Maxwell AE, Bradford C, Das IP, Yan KX (1999) Tailored risk notification for women with a family history of breast cancer. Prev Med 29(5):355–364. doi:10.1006/pmed.1999.0556
Pal T, Rocchio E, Garcia A, Rivers D, Vadaparampil S (2011) Recruitment of black women for a study of inherited breast cancer using a cancer registry-based approach. Genet Test Mol Biomarkers 15(1–2):69–77. doi:10.1089/gtmb.2010.0098
Simmons RG, Lee YC, Stroup AM, Edwards SL, Rogers A, Johnson C, Wiggins CL, Hill DA, Cress RD, Lowery J, Walters ST, Jasperson K, Higginbotham JC, Williams MS, Burt RW, Schwartz MD, Kinney AY (2013) Examining the challenges of family recruitment to behavioral intervention trials: factors associated with participation and enrollment in a multi-state colonoscopy intervention trial. Trials 14:116. doi:10.1186/1745-6215-14-116
Smith T, Stein KD, Mehta CC, Kaw C, Kepner JL, Buskirk T, Stafford J, Baker F (2007) The rationale, design, and implementation of the American Cancer Society’s studies of cancer survivors. Cancer 109(1):1–12. doi:10.1002/cncr.22387
Courtney RJ, Paul CL, Carey ML, Sanson-Fisher RW, Macrae FA, D’Este C, Hill D, Barker D, Simmons J (2013) A population-based cross-sectional study of colorectal cancer screening practices of first- degree relatives of colorectal cancer patients. BMC Cancer 13(1):13
Dite GS, Jenkins MA, Southey MC, Hocking JS, Giles GG, McCredie MR, Venter DJ, Hopper JL (2003) Familial risks, early-onset breast cancer, and BRCA1 and BRCA2 germline mutations. J Natl Cancer Inst 95(6):448–457
Lijovic M, Davis SR, Fradkin P, La China M, Farrugia H, Wolfe R, Bell RJ (2008) Use of a cancer registry is preferable to a direct-to-community approach for recruitment to a cohort study of wellbeing in women newly diagnosed with invasive breast cancer. BMC Cancer 8:126. doi:10.1186/1471-2407-8-126
Sutherland HJ, Lacroix J, Knight J, Andrulis IL, Boyd NF, Ontario Cancer Genetics, N (2001) The Cooperative Familial Registry for Breast Cancer Studies: design and first year recruitment rates in Ontario. J Clin Epidemiol 54(1):93–98
Carpentier MY, Tiro JA, Savas LS, Bartholomew LK, Melhado TV, Coan SP, Argenbright KE, Vernon SW (2013) Are cancer registries a viable tool for cancer survivor outreach? A feasibility study. J Cancer Surviv 7(1):155–163. doi:10.1007/s11764-012-0259-1
Pakilit AT, Kahn BA, Petersen L, Abraham LS, Greendale GA, Ganz PA (2001) Making effective use of tumor registries for cancer survivorship research. Cancer 92(5):1305–1314
Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D, Colon Cancer Family R (2007) Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev 16(11):2331–2343. doi:10.1158/1055-9965.EPI-07-0648
Carey M, Sanson-Fisher R, Macrae F, Hill D, D’Este C, Paul C, Doran C (2012) Improving adherence to surveillance and screening recommendations for people with colorectal cancer and their first degree relatives: a randomized controlled trial. BMC Cancer 12. doi:10.1186/1471-2407-12-62, Artn 62
Anderson B, McLosky J, Wasilevich E, Lyon-Callo S, Duquette D, Copeland G (2012) Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol 2012:298745. doi:10.1155/2012/298745
Knight JA, Sutherland HJ, Glendon G, Boyd NF, Andrulis IL, Network OCG (2002) Characteristics associated with participation at various stages at the Ontario site of the Cooperative Family Registry for Breast Cancer Studies. Ann Epidemiol 12(1):27–33. doi:Doi:10.1016/S1047-2797(01)00253-8
Katapodi MC, Northouse LL, Schafenacker AM, Duquette D, Duffy SA, Ronis DL, Anderson B, Janz NK, McLosky J, Milliron KJ, Merajver SD, Duong LM, Copeland G (2013) Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer. 13. doi:10.1186/1471-2407-13-97, Artn 97
Wikipedia (2012) Stratified Sampling. WIKI. https://en.wikipedia.org/wiki/Stratified_sampling.
Beskow LM, Sandler RS, Millikan RC, Weinberger M (2005) Patient perspectives on research recruitment through cancer registries. Cancer Causes Control 16(10):1171–1175. doi:10.1007/s10552-005-0407-2
Beskow LM, Millikan RC, Sandler RS, Godley PA, Weiner BJ, Weinberger M (2006) The effect of physician permission versus notification on research recruitment through cancer registries (United States). Cancer Causes Control 17(3):315–323. doi:10.1007/s10552-005-0521-1
Ajzen I (1991) The Theory of Planned Behavior. Organ Behav Hum Decis Process 50(2):179–211. doi:10.1016/0749-5978(91)90020-T
Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD (2013) Individual and family characteristics associated with BRCA1/2 genetic testing in high-risk families. Psychooncology 22(6):1336–1343. doi:10.1002/pon.3139
Su Y-S, Yajima M, Gelman AE, Hill J (2011) Multiple imputation with diagnostics (mi) in R: Opening windows into the black box. J Stat Softw 45(2):1–31
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33(1):1–22
Tibshirani R (1997) The lasso method for variable selection in the Cox model. Stat Med 16(4):385–395
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley series in probability and mathematical statistics applied probability and statistics. Wiley, New York
Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN (2014) Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw 12(9):1339–1346
Hamann HA, Tiro JA, Sanders JM, Melhado TV, Funk RK, Carpentier MY, Bartholomew LK, Argenbright KE, Vernon SW (2013) Validity of self-reported genetic counseling and genetic testing use among breast cancer survivors. J Cancer Surviv 7(4):624–629. doi:10.1007/s11764-013-0301-y
Collins FS, Varmus H (2015) A new initiative on precision medicine. N Engl J Med 372(9):793–795
Khoury MJ, Bowen S, Bradley LA, Coates R, Dowling NF, Gwinn M, Kolor K, Moore CA, St Pierre J, Valdez R, Yoon PW (2009) A decade of public health genomics in the United States: centers for disease control and prevention 1997–2007. Public Health Genom 12(1):20–29. doi:10.1159/000153427
Syurina EV, Brankovic I, Probst-Hensch N, Brand A (2011) Genome-based health literacy: a new challenge for public health genomics. Public Health Genom 14(4–5):201–210. doi:10.1159/000324238
Boccia S, Mc Kee M, Adany R, Boffetta P, Burton H, Cambon-Thomsen A, Cornel MC, Gray M, Jani A, Knoppers BM, Khoury MJ, Meslin EM, Van Duijn CM, Villari P, Zimmern R, Cesario A, Puggina A, Colotto M, Ricciardi W (2014) Beyond public health genomics: proposals from an international working group. Eur J Public Health 24(6):877–879. doi:10.1093/eurpub/cku142
Modell SM, Greendale K, Citrin T, Kardia SL Expert and advocacy group consensus findings on the horizon of public health genetic testing. In: Healthcare, 2016. vol 1. Multidisciplinary Digital Publishing Institute, p 14
Acknowledgments
Jenna McLoskly, CGC, Cancer-Genomics Program—Michigan Department of Health and Human Services for patient and at-risk relative identification, recruitment, and assessment of eligibility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was supported by the Centers for Disease Control and Prevention (CDC), 5U48DP001901-03 and by the Robert Wood Johnson Foundation (RWJF)—Nurse Faculty Scholars 68039 Award.
Conflict of interest
Maria C. Katapodi was the Principal Investigator of this study and received research support from DCD and RWJF. Debra Duquette declares that she has no conflict of interest James J. Yang declares that he has no conflict of interest. Kari Mendelsohn-Victor declares that she has no conflict of interest Beth Anderson declares that she has no conflict of interest. Christos Nikolaidis declares that he has no conflict of interest Emily Mancewicz declares that she has no conflict of interest Laurel L. Northouse declares that she has no conflict of interest Sonia Duffy declares that she has no conflict of interest. David Ronis declares that he has no conflict of interest Kara J. Milliron declares that she has no conflict of interest. Nicole Probst-Herbst declares that she has no conflict of interest Sofia D. Merajver declares that she has no conflict of interest. Nancy K. Janz declares that she has no conflict of interest. Glenn Copeland declares that he has no conflict of interest. J. Scott Roberts declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Katapodi, M.C., Duquette, D., Yang, J.J. et al. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: a methodological study. Cancer Causes Control 28, 191–201 (2017). https://doi.org/10.1007/s10552-017-0858-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-017-0858-2