Abstract
Purpose
Risk factors for breast cancer vary according to breast cancer subtype. This study analyzes the impact of potential risk factors in breast cancer by androgen receptor (AR) status.
Methods
A total of 17,035 women were followed in the population-based prospective Malmö Diet and Cancer Study. Baseline data included lifestyle factors including anthropometry, reproductive history, and exogenous hormone use. During follow-up (mean: 12.8 years), 747 invasive breast cancers were diagnosed. Expression of AR was determined by immunohistochemistry in tumor tissue microarrays.
Results
AR status was assessable in 516 of 747 tumors (69%). Among these, 467 tumors (90.5%) were AR positive (AR+) and 49 tumors (9.5%) were AR negative (AR-). AR negativity was significantly associated with estrogen receptor (ER) and progesterone receptor negativity, higher grade and proliferation (Ki67). Cox regression analyses stratified by AR status showed significant associations between reproductive factors and AR- breast cancer. The older the woman at first childbirth the higher the risk of AR- breast cancer; adjusted HR≤20yrs = 0.35, HR>20–≤25yrs = 0.62, HRnulliparous = 1.00, HR>25–≤30yrs = 1.29, HR>30yrs = 1.92, p trend = 0.001. No such association was seen for AR+ tumors. Similarly, ever oral contraceptive use increased the risk of AR- breast cancer [Adj. HR = 2.59, 95% CI (1.26–5.34)] compared to never use, but not for AR+ breast cancer.
Conclusions
Advanced age at first child birth and use of oral contraceptives were associated with increased risk of AR− breast cancer. This study may contribute to enhanced understanding of the role of the AR in breast carcinogenesis and improve risk stratification tools for personalized breast cancer prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproductive factors that increase the woman’s lifetime exposure to hormones, such as early menarche and late menopause, are established risk factors for breast cancer. Studies of breast cancer risk by receptor subtype have shown inconsistent results [1]. The importance of the androgen receptor (AR) as a prognostic factor and a possible treatment target in breast cancer is currently debated [2, 3]. Immunohistochemical (IHC) expression of AR is reported in 70–90% of all invasive breast cancers [4, 5]. Several studies have indicated that AR is an independent positive prognostic marker in breast cancer, as well as a predictive factor for response to endocrine treatment [4, 6–10]. AR has frequently been co-expressed with the estrogen receptor alpha (ER) and the progesterone receptor (PR) [8, 11, 12]. The AR acts as a transcription factor and has been shown to bind to ER-regulated genes to inhibit ER-dependent cell proliferation [6]. Recently, in vitro studies have suggested that activated AR up-regulates estrogen receptor beta gene expression, which inhibits breast cancer cell growth [13]. AR has also been suggested to interact with cell cycle checkpoint protein p21, which is involved in epidermal growth factor signaling [14]. Further, AR expression may be associated with response to tamoxifen (TAM) [15]. The significance of AR expression in ER negative (ER-) breast cancer and triple-negative breast cancer (TNBC) has been contradictory. Some studies show no prognostic value of AR in ER- and/or TNBC, whereas others report either improved or worsened outcomes for breast cancer patients with AR positive (AR+) in ER- and/or TNBC as reviewed by McNamara et al. [16]. However, a clinical phase II trial recently showed promising results for the anti-androgen bicalutamide in AR+/ER-/PR- metastatic breast cancer, high-lighting AR as a promising predictive marker in breast cancer treatment [17]. Another anti-androgen, enzalutamide, is currently being tested in an ongoing clinical trial (ClinicalTrials.gov identifier: NCT01597193). It has also recently been shown that the ER/AR ratio could be an important predictor of response to endocrine treatment [18].
Androgens exert stimulating effects directly on breast cancer cells through binding to the AR, as well as indirect effects through androgen aromatization into estrogens, which in turn bind to the ER [19–21]. The levels of circulating androgens are most likely affected by hormonal factors, such as reproductive history and anthropometric measures [22, 23]. However, the potential impact of lifestyle factors including anthropometry, reproductive history, and exogenous hormone use on AR expression in breast cancer has not yet been addressed. We hypothesized that lifestyle factors and body constitution associated with endogenous androgen levels may affect the risk of developing AR-defined breast cancer as they do for ER-defined breast cancer risk [1, 24].
The aim of this study was to evaluate AR expression in relation to clinically established tumor markers and to analyze the association between lifestyle factors and AR-defined breast cancer risk using the Malmö Diet and Cancer Study (MDCS). The MDCS is a large prospective population-based study consisting of 17,035 women, among whom a total of 747 have been diagnosed with incident invasive breast cancer.
Materials and methods
Malmö Diet and Cancer Study
MDCS is a population-based prospective cohort study of women living in Malmö, Sweden. Women born between 1923 and 1950 were eligible for inclusion, and enrollment took place between 1991 and 1996. At baseline, participants underwent anthropometric measures, answered questionnaires (multiple-choice and open-ended questions) regarding demographics, lifestyle, medical history, and reproductive history including exogenous hormone use. Exclusion criteria included only language incapacities and mental disabilities that prevented the respondent from answering the extensive questionnaire. Further details concerning the study design have been described previously [25]. In order to retrieve information on incident cancer and vital status among participants, the MDCS has been linked annually to the Swedish Cancer Registry, the Regional Tumor Registry for Southern Sweden, and the Swedish Cause of Death Registry. Ethical permission for this study was obtained from the Ethical Committee at Lund University (Dnr 472/2007).
The study cohort and follow-up
The study population consisted of 17,035 women enrolled in the MDCS. In this study, women with a history of breast cancer at baseline were excluded (n = 576), resulting in a total study cohort of 16,459 women. By the end of the follow-up period, on the 31 December 2007, a total of 826 women had been diagnosed with breast cancer. Review of tumor data revealed a total of 79 ductal carcinoma in situ (DCIS) tumors and 747 invasive breast cancers (Fig. 1).
Histopathological analyses
Tumor material was collected from formalin-fixed paraffin-embedded breast cancer blocks. All tumor samples obtained from women diagnosed from 1991 to 2004 were stained with hematoxylin and eosin (H&E), mounted on slides, and reviewed by one breast pathologist for confirmation of the histopathological diagnosis, i.e., the histological type according to WHO classification guidelines [26] and tumor grade. Tumor grading was performed according to Elston and Ellis [27] and included tubular formation, nuclear atypia, and mitotic index. For women diagnosed 2004–2007, the histological type and grade were collected from hospital records including pathology reports. Data on size and lymph node status were retrieved from pathological reports. In this study, tumor size was dichotomized into ≤20 or >20 mm groups and axillary lymph node involvement (ALNI) was recorded as negative or positive (≥1 metastatic nodes).
Tissue microarray and tumor markers
Representative areas of invasive cancer from the donating tumor block were selected for tissue microarray (TMA) construction as described in detail previously [28]. From each tumor, two cores of either 0.6 mm (1991–2004) or 1.0 mm (2005–2007) were mounted in a recipient block and TMA slides of 4 µm were cut and prepared for IHC analyses. The previously studied IHC markers included ER, PR, the proliferation marker Ki67, and human epidermal growth factor 2 (HER2) (1994–2004) [28]. Cut-off values in accordance with current clinical practice (ER, PR) and previous MDCS studies (Ki67) were used [28], and expression levels were defined as negative/low with ≤10% of positively stained nuclei and as positive/high with >10% of positively stained nuclei. HER2 was assessed either through IHC score, ranging from 0 to 3+ according to the HercepTest [29] (1991–2004), or by regional and national cancer registries and hospital records (2005–2007) including both IHC and in situ hybridization (ISH) analysis data. When the score of the IHC evaluation was applied, a score of 0 and 1+ was considered negative and a score of 3+ was considered positive. Tumors with an IHC score of 2+ were excluded as missing, if not confirmed as either amplified or normal in ISH analysis. An ISH analysis result was used when available. AR was assessed using the monoclonal antibody Ab-1 (clone AR441, dilution 1:200, Thermo Scientific, Fremont, CA, USA). Scoring of the AR was performed semi-quantitatively, using fractions of 0, 1–10, 11–50, 51–75, and 76–100% positive nuclei. In this study, a dichotomized variable with a cut-off at 10% was used. All arrays were evaluated independently twice (KE), and in case of discrepancy, a third examination was performed (SiB), followed by a final decision. In the case of heterogeneity of AR expression between the two duplicate cores, the decision was based on visual evaluation of the total tumor area of the two cores pooled together. A valid AR score was obtained for 516 of the 747 invasive breast cancer tumors. Among the 231 cases with missing AR status, there were 96 cases with no tissue available for histopathology. In 52 cases, there was lack of tissue cores in the array due to prior sectioning for marker analysis, and in 17 cases, tumor tissue cores displayed no invasive tumor foci. In the remaining 66 cases, the tissue samples in the cores were damaged during processing such that the tissue structure was destroyed/melted or dislocated on the TMA and could not be assessed for AR expression.
Participants’ baseline characteristics
Educational level was categorized into O-level college (9 years of school attendance), A-level college (another 3 years of education), and university level (education following A-levels). Type of occupation was recorded as manual worker, nonmanual worker, or as employer/self-employed. Alcohol use was divided into three categories: (1) nothing last year, (2) something last year but not last month, or (3) something last month. Smoking habits were categorized into never, current, and former smokers. Anthropometric measures such as weight (multiples of 0.1 kg) and height (to the nearest 0.005 m) were measured by a trained nurse, and body mass index (BMI) was calculated as kg/m2. BMI categories used were <25, ≥25–<30, or ≥30 kg/m2 [30]. Waist circumference was measured at the midpoint between the lower ribs and the iliac crest (to the nearest 0.01 m) and categorized as ≤80, >80–≤88, and >88 cm [31]. Hip circumference was measured at the level of greatest lateral extension (to the nearest 0.01 m). Waist and hip measurements were used to construct a waist–hip ratio (WHR) (m/m), and categories used were ≤0.80, >0.80–≤0.85, and >0.85 [31, 32]. Body fat percentage was calculated from measurements of bio-impedance (BIA 103, RLJ-Systems, Detroit, MI, USA).
Information on reproductive history included age at menarche, parity, age at first childbirth, breast-feeding (<1, 1–<12 or ≥12 months), age at menopause, and exposure to oral contraceptives (OC) (ever/never). Menopausal status at baseline was defined as pre-, peri-, or postmenopausal as described in detail previously [28]. Use of hormonal replacement therapy (HRT) was categorized as current use or nonuse and as type of HRT, coded according to the Anatomic Therapeutic Chemical classification (ATC) system [28]. The present study grouped use of HRT into estrogen only (eHRT) and combined estrogen plus progestin HRT (cHRT). Progestin-only HRT use was uncommon (n = 106, 0.65%) and thus excluded from analysis of HRT.
Statistical analysis
Statistical analyses were performed using the statistical software SPSS Statistics 19 (IBM, Chicago, IL, USA). Variables were categorized either as described earlier or as tertiles based upon the distribution in the entire study cohort, excluding prevalent cases. Age at baseline was not normally distributed due to participant recruitment; thus, age was presented as median and interquartile range and analyzed as a categorized variable of four equally sized groups. Height (normally distributed) and the natural logarithm of weight (skewed distribution) were used as continuous variables in the multivariable analyses. Hazard ratios (HR) stratified for AR status were calculated using Cox proportional hazards model and presented as age-adjusted HR with 95% confidence intervals (CI). Multivariable analyses were adjusted for age at baseline (categorized into quartiles), height (continuous), weight (natural logarithm), occupation type, age at first childbirth (categorized, including nulliparous women), ever OC use, and current HRT use and type, if not otherwise specified. Univariable analyses were carried out using log-rank test and presented as Kaplan–Meier curves for both AR- and ER-defined breast cancer. Each woman in the study cohort was followed from inclusion until the event of breast cancer, death, emigration, or end of the follow-up period by the 31 December 2007. In the subanalyses of AR+ breast cancer, time of follow-up was censored at the time of AR negative (AR-) breast cancer, cancer in situ, or breast cancer with missing AR status. Subanalyses for AR- breast cancer were performed correspondingly.
Tumor characteristics, such as tumor size, ALNI, histological grade, ER, PR, HER2, and Ki67 were categorized and analyzed in relation to AR status with X 2-analyses or Fishers’ exact test. Odds ratios (ORs) with 95% confidence intervals (CIs) and p values are presented. Logistic regression was used to compare AR status in relation to combined ER/PR status and histological grade. ORs and 95% CIs are presented. Three two-way interaction terms were created between the following three variables: age at first childbirth (≤20, >20–≤25, nulliparous, >25–≤30, >30) and ever OC use (yes/no) or cHRT (yes/no), and finally, ever OC use (yes/no) and cHRT (yes/no). p values of less than 0.05 were considered significant. All p values were two-tailed. Nominal p values without adjustment for multiple testing are presented.
Results
Tumor characteristics
A total of 467 tumors (90.5%) were AR+, and 49 tumors (9.5%) were AR- (Table 1). AR negativity in the tumors was significantly associated with higher tumor grade and higher proliferation rates. ER- and PR- expression showed highly significant associations with AR- expression, mainly driven by co-expression between AR and ER. No significant association with AR was observed for tumor size and ALNI. In analyses using a variable combining HER2 and hormone receptor status, AR negativity was associated with the triple-negative phenotype rather than the HER2+/ ER- /PR- phenotype. In order to address potential selection bias, tumors with missing AR data were analyzed in line with tumors with known AR. Risk factors for women with missing AR status are presented separately and show no major differences compared to women with known AR status (Table 1). Furthermore, sensitivity analysis was performed for tumor characteristics with regard to missing AR status. In the analysis that considered all tumors with missing AR status to be AR+, all results remained statistically significant (data not shown). When missing tumors were all assumed to be AR-, the results changed; this is in accordance with nearly 90% of invasive breast cancer tumors being AR+. The distribution of the baseline characteristics among all participants is described in Supplementary Table 1.
Risk factors for AR-defined breast cancer
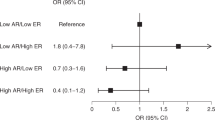
The higher the age at first childbirth, the higher the risk of AR- breast cancer. This trend became somewhat stronger in the multivariable analysis. In contrast, age at first childbirth did not affect the risk of AR+ breast cancer or the overall risk of invasive breast cancer (Table 2; Fig. 2). Breast-feeding did not affect the risk of developing AR-defined breast cancer. Ever users of OC had a significantly increased risk of AR- breast cancer compared with never users of OC (Table 2; Fig. 2). Ever use of OC was not associated with risk of invasive breast cancer or AR+ breast cancer. Results remained significant in multivariable analyses. The use of cHRT was significantly associated with an increased breast cancer risk, irrespective of AR status, compared with nonusers of HRT (Table 2; Fig. 2) and irrespective of OC use prior to cHRT. The results remained essentially the same in multivariable analyses. There were no significant interactions between OC use and cHRT use, OC use and age at first childbirth, or age at first childbirth and cHRT use (p ≥ 0.17). As for anthropometric measures, the mid-category of waist measurements, >80–≤ 88 cm, showed a significantly decreased risk of AR- breast cancer (Table 3). The result remained significant in multivariable analysis. In analyses stratified for menopausal status, the result only remained significant among postmenopausal women (age-adjusted HRpost_mp 0.24 (0.057–1.01) p = 0.052). No associations between waist circumference and overall breast cancer risk or AR+ breast cancer risk were seen, neither in the entire cohort nor in analyses stratified according to menopausal status. No associations between hip measurements and risk of invasive or AR-defined breast cancer were seen, neither for the entire cohort nor when stratified according to menopausal status. WHR was not associated with risk of invasive breast cancer in general or AR- breast cancer in particular. When stratified according to menopausal status, no significant differences were seen for WHR measurements among invasive or AR- breast cancer. The mid-category of WHR, >0.80–≤0.85, showed an increased risk of AR+ breast cancer (Table 3). The result remained significant in multivariable analysis and was most prominent among the postmenopausal women in analysis stratified for menopausal status (age-adjusted HRpost_mp 1.31 (1.02–1.68) p = 0.034). Height and weight were significant risk factors for invasive breast cancer in general; however, they did not differ according to AR status (Table 3). Education level, smoking, and alcohol use were not associated with either invasive or AR-defined breast cancer risk. Nonmanual workers had an increased risk of invasive breast cancer in general compared to manual workers (age-adjusted HR 1.26 (1.07–1.47) p = 0.004). However, the association was not related to AR-defined breast cancer risk.
Risk factors for ER-defined breast cancer
In order to assess whether the AR-defined breast cancer risk factors add independent information in addition to ER-defined risk factors, the analyses were repeated stratified according to ER status. Neither use of OC nor age at first childbirth was associated with ER-defined breast cancer risk (Supplementary Fig. 1).
Discussion
Here, we present data from the large MDCS showing that advanced age at first childbirth and ever use of OC increased the risk of AR- breast cancer, without influencing the risk of AR+ breast cancer. Use of cHRT was associated with increased risk of breast cancer irrespective of AR status.
The distribution of tumor characteristics in relation to AR status was consistent with similar studies, markedly for ER status, ALNI, size, and grade [4, 12, 33]. No association was seen between AR expression and HER2 status alone. When HER2 status was combined with ER and PR, AR negativity was associated with the triple-negative phenotype rather than the HER2+/ER-/PR- phenotype. Similar findings on AR and HER2 status have been described previously [12] although a review has reported heterogeneous results regarding AR and HER2 status [16]. Ni et al. [34] reported that targeting the AR in ER-/HER2+ breast tumors effectively inhibits tumor growth. Thus, androgen receptor blocking drugs (e.g., bicalutamide [17] or enzalutamide (ClinicalTrials.gov identifier: NCT01597193)) may be a treatment option for breast cancer patients with ER−AR+ tumors [18].
In this study, risk assessment analyses for ER-defined breast cancer differed from AR-defined breast cancer risk. Consequently, we suggest that risk factors for AR-defined breast cancer differ from those of ER-defined breast cancer and that AR status can add information independent from ER. We therefore consider risk assessment in relation to AR to be of interest, especially as we show that these risk estimates add information to the risk defined by ER stratification.
The main limitations of this study are the limited number of women who developed AR- tumors and the number of tumors with missing AR data; both factors may impact the robustness of the results. However, in accordance with the results of the sensitivity analysis on missing AR status, missing tissue was not considered to exert a risk of selection bias in this study. Some additional methodological concerns should be addressed. The participation rate in the MDCS was 40%, and a previous study on the background population has shown a selection of higher socioeconomic status in participants [35]. The participating women in the MDCS were often postmenopausal; hence, the risk factors elucidated from this study may primarily cover postmenopausal breast cancer. The information on menopausal status was obtained at baseline and not at diagnosis, making adjustment for menopausal status in this study difficult. We did, however, stratify the findings of anthropometry according to menopausal status at baseline, since it is well documented that the association of breast cancer with BMI varies according to menopausal status. Furthermore, the vast majority of participants were of Swedish ethnicity, thereby possibly limiting the applicability of our results to women in general. Information on exposures was obtained from baseline questionnaires, and it is possible for these exposures to have changed over time since women were 44 years or older at inclusion. HRT use may be underestimated as participants may have initiated HRT use after inclusion in the study. However, age at first childbirth and ever use of OC are likely to be correctly reported since few women would give birth or initiate OC use after age 44. The nulliparous women might be a heterogeneous group with some women postponing pregnancy for social reasons, whereas others may experience fertility problems. Thus, the hormonal influence may differ between such subgroups, which may have an impact on the interpretation of the results. However, this study was not able to address this issue in depth as information on infertility was not available. Since the aim of this study was to compare AR expression in relation to lifestyle factors including anthropometry, reproductive history, and exogenous hormone use, we consider it possible to make internal comparisons between the subjects in order to obtain relative risks. Moreover, all multivariable analyses were adjusted for potential confounders and showed similar results as compared to age-adjusted analyses.
The breast epithelium is considered most sensitive to hormonal stimuli during the period between menarche and first childbirth. Further, the breast tissue matures during breast-feeding, and it may take multiple pregnancies before the breast tissue is fully matured [36]. In line with this, advanced age at first childbirth has been shown both to increase the risk of breast cancer in general [37] and to affect histological types and ER/PR-defined breast cancer differently [1, 38]. To our knowledge, analyses stratified according to AR status have not been performed.
Ma et al. [24] hypothesized that the protective effect of parity could be explained by reduced circulating hormone levels, increased levels of sex hormone binding globulin SHBG, or by increasing the breast epithelium differentiation to a less susceptible state concerning the effects of estrogen and progestin. Previous results from the MDCS have suggested that advanced age at first childbirth and parity correlate with more aggressive breast cancer subtypes [39], which is in agreement with the current findings of an association between advanced age at first childbirth and risk of AR- breast cancer. A recently published case study showed increasing frequency of TNBC among women with an older age at first child birth. However, AR expression was not investigated in the study [40].
In this study, a higher risk of AR- breast cancer among ever users of OC compared to never users was found, whereas no effect was seen on AR+ breast cancer. The Collaborative Group on Hormonal Factors in Breast Cancer reported a small increase in breast cancer risk, especially for women younger than 20 years using combined OC and up to 10 years afterward [41]. Results of analyses on OC use by receptor subtype, although inconsistent, have shown an increased risk of ER- breast cancers over ER+ breast cancers [1], which would be in line with our results of increased AR- tumors among ever users of OC.
With regard to HRT use, the Collaborative Group reported an obvious risk increase, lasting up to 5 years after cessation of therapy [42]. Previously, we have shown an increased risk of breast cancer among cHRT users in the MDCS [28]. The same pattern was seen in this study, the risk increment for women using HRT being irrespective of AR status. In relation to breast cancer subtypes, HRT use has been associated with ER+/PR- breast cancers and the progestin component of cHRT has been suggested to be of considerable importance [43, 44]. The importance of a stable testosterone/estrogen ratio in order to avoid breast cancer development has been stressed [45]. Use of either OC or HRT is capable of distressing this ratio by reducing free androgens, thus leaving the estrogenic stimulation on the breast unopposed [45]. It has been postulated that the synthetic progestins disrupt androgen signaling that normally exerts protective effects on the breast [46].
Furthermore, OC users have been shown to have lower testosterone levels compared to nonusers of OCs [23]. Androgens have been suggested to protect breast tissue from excessive hormone-induced proliferation, contrasted by findings indicating that androgens might increase breast cancer risk [45]. Preclinical studies implied that androgens promote apoptosis in human breast cancer cell lines [47]. Other preclinical data showed that AR blocks ER-stimulated growth in breast cancer cells [6] and that overexpression of AR decreased ER transcriptional activity [48]. Whether different AR genotypes are reflected in AR expression in breast tumors is unknown, but we have recently reported on AR genotypes predicting response to TAM treatment [49]. The treatment predictive value of tumor-specific AR expression has been addressed in endocrine-treated cohorts, demonstrating high AR expression to be a predictive factor for response to endocrine treatment [10]. Low or intermediate AR expression might be used as an indication to supplement endocrine treatment with chemotherapy or not [10].
Summary and future perspectives
In summary, age at first childbirth and use of OC increase the risk of AR- breast cancer without influencing the risk of AR+ breast cancer. This information may contribute to the comprehension of AR’s role in breast carcinogenesis and potentially play a role in breast cancer prevention by improving risk stratification. Further, considering the future potential for AR as a treatment target in breast cancer, more studies elucidating AR function are needed.
References
Althuis MD, Fergenbaum JH, Garcia-Closas M, Brinton LA, Madigan MP, Sherman ME (2004) Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res 13:1558–1568
Hickey TE, Robinson JL, Carroll JS, Tilley WD (2012) Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol (Baltimore, Md.) 26:1252–1267
Garay JP, Park BH (2012) Androgen receptor as a targeted therapy for breast cancer. Am J Cancer Res 2:434–445
Hu R, Dawood S, Holmes MD et al (2011) Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res Off J Am Assoc Cancer Res 17:1867–1874
Park S, Koo J, Park HS et al (2010) Expression of androgen receptors in primary breast cancer. Ann Oncol Off J Eur Soc Med Oncol/ESMO 21:488–492
Peters AA, Buchanan G, Ricciardelli C et al (2009) Androgen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancer. Cancer Res 69:6131–6140
Castellano I, Allia E, Accortanzo V et al (2010) Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 124:607–617
Park S, Koo JS, Kim MS et al (2011) Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol Off J Eur Soc Med Oncol/ESMO 22:1755–1762
Bryan RM, Mercer RJ, Bennett RC, Rennie GC, Lie TH, Morgan FJ (1984) Androgen receptors in breast cancer. Cancer 54:2436–2440
Park S, Park HS, Koo JS, Yang WI, Kim SI, Park BW (2012) Higher expression of androgen receptor is a significant predictor for better endocrine-responsiveness in estrogen receptor-positive breast cancers. Breast Cancer Res Treat 133:311–320
Kuenen-Boumeester V, Van der Kwast TH, Claassen CC et al (1996) The clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parameters. Eur J Cancer 32A:1560–1565
Ogawa Y, Hai E, Matsumoto K et al (2008) Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 13:431–435
Rizza P, Barone I, Zito D et al (2014) Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res 16:R21
Garay JP, Karakas B, Abukhdeir AM et al (2012) The growth response to androgen receptor signaling in ERalpha-negative human breast cells is dependent on p21 and mediated by MAPK activation. Breast Cancer Res 14:R27
Abukhdeir AM, Vitolo MI, Argani P et al (2008) Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci USA 105:288–293
McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H (2013) Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol 133:66–76
Gucalp A, Tolaney S, Isakoff SJ et al (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res 19:5505–5512
Cochrane DR, Bernales S, Jacobsen BM et al (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16:R7
Birrell SN, Hall RE, Tilley WD (1998) Role of the androgen receptor in human breast cancer. J Mammary Gland Biol Neoplasia 3:95–103
Somboonporn W, Davis SR (2004) Testosterone effects on the breast: implications for testosterone therapy for women. Endocr Rev 25:374–388
Labrie F, Luu-The V, Labrie C et al (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24:152–182
Bernstein L, Ross RK (1993) Endogenous hormones and breast cancer risk. Epidemiol Rev 15:48–65
Jernstrom HC, Olsson H, Borg A (1997) Reduced testosterone, 17 beta-oestradiol and sexual hormone binding globulin, and increased insulin-like growth factor-1 concentrations, in healthy nulligravid women aged 19–25 years who were first and/or second degree relatives to breast cancer patients. Eur J Cancer Prev 6:330–340
Ma H, Bernstein L, Pike MC, Ursin G (2006) Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res 8:R43
Berglund G, Elmstahl S, Janzon L, Larsson SA (1993) The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med 233:45–51
World Health Organization (1982) Histological typing of breast tumors, 2nd edn. World Health Organization. Geneva, 1981. Ann Pathol 2:91–105
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Borgquist S, Anagnostaki L, Jirstrom K, Landberg G, Manjer J (2007) Breast tumours following combined hormone replacement therapy express favourable prognostic factors. Int J Cancer 120:2202–2207
Dowsett M, Bartlett J, Ellis IO et al (2003) Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol 199:418–423
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894:i–xii, 1–253
World Health Organization W (2008) WHO expert consultation on waist circumference and waist-hip ratio. WHO
Molarius A, Seidell JC, Sans S, Tuomilehto J, Kuulasmaa K (1999) Waist and hip circumferences, and waist-hip ratio in 19 populations of the WHO MONICA Project. Int J Obes Relat Metab Disord J Int Assoc Study Obes 23:116–125
Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM (2011) Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Modern Pathol Off J U S Can Acad Pathol Inc 24:924–931
Ni M, Chen Y, Lim E et al (2011) Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 20:119–131
Manjer J, Carlsson S, Elmstahl S et al (2001) The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev 10:489–499
Russo J, Moral R, Balogh GA, Mailo D, Russo IH (2005) The protective role of pregnancy in breast cancer. Breast Cancer Res 7:131–142
Parsa P, Parsa B (2009) Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pacific J Cancer Prev APJCP 10:545–550
Wohlfahrt J, Mouridsen H, Andersen PK, Melbye M (1999) Reproductive risk factors for breast cancer by receptor status, histology, laterality and location. Int J Cancer 81:49–55
Butt S, Borgquist S, Anagnostaki L, Landberg G, Manjer J (2009) Parity and age at first childbirth in relation to the risk of different breast cancer subgroups. Int J Cancer 125:1926–1934
Martinez ME, Wertheim BC, Natarajan L et al (2013) Reproductive factors, heterogeneity, and breast tumor subtypes in women of Mexican descent. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res 22:1853–1861
Collaborative Group on Hormonal Factors in Breast Cancer (1996) Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet 347:1713–1727
Collaborative Group on Hormonal Factors in Breast Cancer (1997) Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 350:1047–1059
Bao PP, Shu XO, Gao YT et al (2011) Association of hormone-related characteristics and breast cancer risk by estrogen receptor/progesterone receptor status in the shanghai breast cancer study. Am J Epidemiol 174:661–671
Fournier A, Fabre A, Mesrine S, Boutron-Ruault MC, Berrino F, Clavel-Chapelon F (2008) Use of different postmenopausal hormone therapies and risk of histology- and hormone receptor-defined invasive breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 26:1260–1268
Dimitrakakis C, Bondy C (2009) Androgens and the breast. Breast Cancer Res 11:212
Birrell SN, Butler LM, Harris JM, Buchanan G, Tilley WD (2007) Disruption of androgen receptor signaling by synthetic progestins may increase risk of developing breast cancer. FASEB J Off Publ Feder Am Soc Exp Biol 21:2285–2293
Kandouz M, Lombet A, Perrot JY et al (1999) Proapoptotic effects of antiestrogens, progestins and androgen in breast cancer cells. J Steroid Biochem Mol Biol 69:463–471
Lanzino M, De Amicis F, McPhaul MJ, Marsico S, Panno ML, Ando S (2005) Endogenous coactivator ARA70 interacts with estrogen receptor alpha (ERalpha) and modulates the functional ERalpha/androgen receptor interplay in MCF-7 cells. J Biol Chem 280:20421–20430
Lundin KB, Henningson M, Hietala M, Ingvar C, Rose C, Jernstrom H (2011) Androgen receptor genotypes predict response to endocrine treatment in breast cancer patients. Br J Cancer 105:1676–1683
Acknowledgments
We thank breast pathologist Dr. Lola Anagnostaki and Ms. Elise Nilsson for their excellent and dedicated assistance in selecting suitable histology sections and in the TMA construction process. We are also grateful to data manager Ms. Anna Hwasser for her skillful assistance. The study was funded by Governmental funding of Clinical Research within the National Health Services (Region Skåne ALF) and the Swedish Research Council.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10552_2014_394_MOESM1_ESM.pptx
Supplementary Fig. 1 Kaplan–Meier estimate of risk for ER negative or ER positive defined breast cancer in relation to A+B) age at first childbirth, C+D) ever use of OCs, and E+F) use of estrogen only or combined estrogen plus progestin HRT (PPTX 251 kb)
Rights and permissions
About this article
Cite this article
Elebro, K., Butt, S., Dorkhan, M. et al. Age at first childbirth and oral contraceptive use are associated with risk of androgen receptor-negative breast cancer: the Malmö Diet and Cancer Cohort. Cancer Causes Control 25, 945–957 (2014). https://doi.org/10.1007/s10552-014-0394-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0394-2