Abstract
The aim was to investigate the implications of androgen receptor (AR) expression levels on outcomes for estrogen receptor (ER)-positive tumors. Immunohistochemically AR levels were determined from tissue microarrays of 614 ER-positive patients who received adjuvant endocrine with or without chemotherapy between November 1999 and August 2005. Characteristics and survival were analyzed using a Chi-square test, Kaplan–Meier methods, and Cox’s models. AR levels were categorized into 3 subgroups as follows: low, AR < 10%; intermediate, 10% ≤ AR < 50%; high, AR ≥ 50%. Low, intermediate, and high AR levels were observed in 29.0, 44.0, and 27.0% of patients, respectively. High AR was associated with smaller size, nodal uninvolvement, grade I/II tumor, higher progesterone receptor expression, and lower proliferation index. With a median follow-up of 70.9 months, the high AR subgroup showed better survival, and these associations were maintained in 119 patients who received endocrine therapy alone [hazard ratio (HR), 0.111; 95% CI, 0.013–0.961 for disease-free survival (DFS); HR, 0.135; 95% CI, 0.015–1.208 for overall survival (OS)]. No significant benefits from chemotherapy were demonstrated in the high AR subgroup; however, the benefit from chemotherapy was significant among 448 AR-intermediate or -low patients (HR, 2.679; 95% CI, 1.452–4.944 for DFS; HR, 3.371; 95% CI, 1.611–7.052 for OS). High AR is an independent prognostic factor and a significant predictor for better endocrine-responsiveness in ER-positive tumors. AR-low or -intermediate levels could give an additional indication for use of chemotherapy in ER-positive tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adjuvant endocrine therapy for early stage estrogen receptor (ER)-positive breast cancer has been known to be effective in delaying recurrence and prolonging survival [1]. However, despite well-documented predictive and prognostic roles of ER in breast cancer patients, up to 50% of ER-positive tumors eventually do not respond to endocrine therapies, either due to intrinsic de novo resistance or acquired resistance following prolonged use of those treatments [2, 3]. In addition to ER status, responsiveness to adjuvant endocrine therapy is significantly associated with the level of ER expression, progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2)-positivity, and Ki-67 proliferative index in patients with hormone receptor-positive breast cancer [3–5]. Recently, gene expression profiling such as Oncotype DX™ (Genomic Health, Inc., Redwood City, CA) and MammaPrint™ (Agendia, Amsterdam, The Netherlands) has been suggested for better prediction of relapse and survival outcome among those patients [6, 7].
Since breast cancer is a highly hormone-dependent tumor, endocrine therapy is the mainstay of adjuvant treatment. The additional use of cytotoxic chemotherapy in patients with ER-positive breast cancer is still being debated. In the adjuvant setting, less absolute benefit from additional chemotherapy was found in patients with ER-positive tumors compared to those with ER-negative diseases [8, 9]. This trend was also confirmed in the neoadjuvant chemotherapy trials, that ER-positive tumors were less likely to achieve a pathological complete response than ER-negative tumors [10]. In the current clinical practice, additional use of chemotherapy is based on the assessment of disease-relapse risk including traditional histopathological prognostic factors and gene signatures in patients with ER-positive tumors [11, 12].
With increasing results supporting the prognostic value of androgen receptor (AR) in both ER-positive and ER-negative tumors, we and others report the emerging roles of AR in breast cancer patients [13–16]. A significant number of primary breast cancers express AR [17]. Antiestrogen tamoxifen combined with androgen fluoxymesterone provided higher advantages of response rate, time to progression, and survival over tamoxifen alone for postmenopausal patients with metastatic breast cancer, especially those whose age was ≥65 years and whose ER level was ≥10 fmol/mg [18]. AR expression is significantly associated with ER-positive tumors and antiproliferative effect of aromatase inhibitors (AIs) may be partly potentiated by the inhibitory effect of androgen via AR [17, 19]. These data suggest that AR expression could be a significant factor for the prediction of therapeutic response to systemic therapies in ER-positive breast cancers. Recently, we reported significant impacts of AR on survival outcomes in ER-positive subgroup [13]. However, the in vitro study of de Amicis et al. [20] demonstrated that AR over-expression was associated with tamoxifen resistance by affecting tamoxifen-induced ER signaling pathways. The predictive value of AR expression levels for adjuvant endocrine and chemotherapy is not yet clearly established in patients with ER-positive breast cancer.
The aims of this study were to investigate the clinical implications of AR levels on survival outcomes, to determine the role of AR levels for predicting responsiveness to endocrine therapy, and to explore subgroup gaining clinical benefits from chemotherapy according to AR levels in ER-positive breast cancers. All patients had ER-positive tumor and received definite local therapies and endocrine therapy with or without chemotherapy.
Materials and methods
Study population
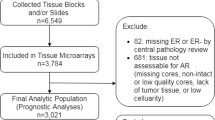
Tumor samples were collected between November 1999 and August 2005, formalin-fixed, and paraffin-embedded. Archival hematoxylin and eosin (H&E)-stained slides for each case were reviewed by breast pathologists. Immunohistochemistry (IHC) was interpreted in a blind fashion, without any information regarding clinical parameters or outcomes. Among the initial study population of 1,153, the exclusion criteria were as follows: unreadable AR expression (n = 13), pure in situ carcinoma (n = 43), metastatic disease (n = 12), ER-negative tumor (n = 251), patient receiving neoadjuvant chemotherapy (n = 66), and ER-positive patient who did not receive endocrine therapy (n = 72). Invasive carcinomas (n = 82) that did not present invasive focus upon review of archival H&E-stained slides were also excluded since they represented only extensive intraductal components. As a result, 614 patients were enrolled for analysis. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (4-2010-0136). Our study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [21].
Patient characteristics and survival were retrospectively obtained from medical records. Patients were treated with either mastectomy or breast-conservation surgery and sentinel lymph node biopsy or axillary lymph node dissection. After surgery, local radiotherapy or adjuvant treatments were administered if the patient was able to tolerate them. Clinical follow-up included history-taking, physical examinations, laboratory tests, and radiologic imaging every 6–12 months for detection of relapse. Tumor stage was based on the 6th American Joint Committee on Cancer criteria [22]. Histological grade was assessed by the modified Bloom-Richardson classification [23]. Disease-free survival (DFS) time was measured from the date of the first curative surgery to the date of the first locoregional or systemic recurrence, or death without any type of relapse. Overall survival (OS) time was measured from the date of the first operation to the date of the last follow-up or death from any cause.
Tissue microarray and immunohistochemistry
Tissue microarray (TMA) blocks were constructed using formalin-fixed, paraffin-embedded tumor samples as detailed in procedure descriptions from a previous study [13]. IHC staining was carried out using TMA. In brief, 5 μm-thick sections were deparaffinized and rehydrated. After treatment with 3% hydrogen peroxide solution for 10 min to block endogenous peroxidase, sections were pretreated in 10 mM citrate buffer for antigen retrieval in a microwave oven for 20 min. After incubation with primary antibodies against AR (AR 441, 1:100; Thermo Scientific, Fremont, CA), ER (SP1, 1:100; Thermo Scientific), PR (PgR 636, 1:50; DAKO, Glostrup, Denmark), HER2 (polyclonal, 1:1,500; DAKO), and Ki-67 (MIB-1, 1:100; DAKO), immunodetection was performed with biotinylated anti-mouse/rabbit immunoglobulin, followed by peroxidase-labeled streptavidin using a labeled streptavidin biotin kit with 3,3′-diaminobenzidine chromogen as the substrate. The slides were counterstained with Harris hematoxylin.
The level of nuclear AR expression was arbitrarily categorized into 3 groups as follows: low, AR < 10%; intermediate, 10% ≤ AR < 50%; high, AR ≥ 50% that is upper quartile of our study population. Tumors with ≥1% nuclear-stained cells were considered positive for ER and PR as the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [24]. Steroid hormone receptors were based on manual and absolute counts. Arbitrary cut-off score of 10% was used for Ki-67 expression. HER2 status was evaluated using the HercepTest™ (DAKO) and was interpreted as 0, 1+, 2+, or 3+ according to the ASCO/CAP guidelines [25]. HER2 was considered positive in cases with 3+ IHC score or gene amplification by fluorescence in situ hybridization (FISH) regardless of HER2 IHC result using the diagnostic criteria described below.
Fluorescence in situ hybridization
FISH using PathVysion HER2 DNA Probe Kit (Abott, Abott Park, IL) was performed manually in all patients. In brief, consecutive sections from TMA were deparaffinized and rehydrated. They were then boiled for 10 min in pretreatment solution, incubated with pepsin solution for 10 min, dehydrated in ethanol for 6 min, and finally air-dried. For hybridization, the buffered probe (HER2/neu and centromere 17) was introduced onto the slide and protected by a coverslip that was sealed with rubber cement. For denaturation, slides were heated to 82°C and incubated overnight at 45°C in a dark humidified chamber. The rubber cement and coverslip were then removed, and the slides were transferred to stringent wash buffer for 10 min at 65°C. Afterward, they were dehydrated in ethanol for 6 min and air-dried. Finally, they were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Evaluation of signals was carried out using an epifluorescence microscope (Olympus, Tokyo, Japan) equipped with a fluorescein, Cy3, and DAPI filter set and a 100-W mercury lamp. Counting was carried out according to the manufacturer’s manual. Signals were counted in at least 60 tumor nuclei each TMA cores. As the ASCO/CAP guidelines [25], an absolute HER2 gene copy number >6 or HER2 gene/chromosome 17 copy number ratio >2.2 was considered HER2-positive. Lymphocytes, fibroblasts, and normal ductal epithelial cells were used as internal controls.
Statistical analysis
The differences between the groups were evaluated by a Chi-square test. Fisher’s exact test was used when appropriate. Continuous variables were compared using one-way analysis of variance (ANOVA) with Bonferroni multiple comparison test. Survival curves were plotted using the Kaplan–Meier method and group differences in survival time were investigated by a log-rank test. A Cox’s hazards model was used to identify the variables that were independently associated with survival. All statistical tests were two-sided and a P-value < 0.05 was considered statistically significant. SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL) was used for all statistical analysis.
Results
Patient characteristics
The mean age at the time of diagnosis was 49.6 years (range, 24–86) and the median follow-up period was 70.9 months (range, 5.8–118.1) for whole study population. Clinicopathological characteristics according to adjuvant treatments are summarized in Table 1. The median proportion of ER expression was 80% among our study population, which was used as cut-off value of ER expression: low, 1% ≤ ER ≤ 80%; high, ER > 80%. Most patients (71.8%, n = 441) treated with selective estrogen receptor modulators (SERMs) for 5 years and 155 (25.2%) received AIs after being treated with SERMs for 2–3 or 5 years. Only 18 (2.9%) postmenopausal women received upfront AIs. Systemic chemotherapies were administered to 495 (80.6%) patients. Among these, 298 (60.2%) patients treated with cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) regimens and 90 (18.2%) received anthracycline-based (FAC, FEC, or AC) regimens. Adriamycin plus cyclophosphamide followed by paclitaxel was administered to 101 (20.4%) patients with axillary nodal metastasis. Six (1.2%) patients received oral doxifluridine.
AR levels and survival outcomes
Low, intermediate, and high AR expression was observed in 178 (29.0%), 270 (44.0%), and 166 (27.0%) patients, respectively. The association of clinicopathological parameters with AR levels is presented in Table 2. A high AR expression was significantly associated with smaller tumor size, nodal uninvolvement, grade I/II tumor, higher PR expression, and lower proliferation index. Although there was no statistical significance for this, a lower AR expression was associated with younger age at diagnosis, lower ER expression, and higher HER2-positivity. The 5-year DFS of patients with low, intermediate, and high AR expression was 81.3, 87.3, and 94.9%, respectively (P = 0.001). The 5-year OS according to AR levels was 89.8, 95.2, and 97.3%, respectively (P = 0.006). Survival curves according to AR levels are shown in Fig. 1. Higher AR expression was positively associated with better survival among whole study population. When other clinicopathological parameters entered Cox’s models, higher AR expression was a significant prognostic factor for survival by two or three categories analyses of AR levels (Table 3).
To explore the implications of AR levels according to systemic treatments, survival analysis was performed among the 119 patients who received endocrine therapy alone. High AR expression was significantly associated with better DFS (Fig. 2a). Borderline significance was determined regarding OS (Fig. 2b). Multivariate analysis revealed an independent significance of AR levels for DFS, but not for OS among 119 patients treated with endocrine agents alone (Table 4). Survival according to AR levels is presented in Fig. 2 among the 495 patients who received both endocrine and chemotherapy. A positive association of AR levels with survival outcomes was demonstrated in the univariate analysis (Fig. 2c for DFS and Fig. 2d for OS). However, no statistical significance of AR levels was shown in the multivariate analysis (Table 4). When AR level was categorized into two groups [low versus (vs.) intermediate/high], it did not reach a statistical significance yet regardless of adjuvant therapeutic modalities (Table 4).
Clinical benefits from chemotherapy by AR levels
The implications of AR levels on survival outcomes remained significant in the multivariate analysis of patients treated with endocrine agents alone, but disappeared in those who received both endocrine and chemotherapy. These findings suggested that use of chemotherapy might provide different clinical benefits depending on AR levels. A favorable association with statistical significance was determined in AR-high subgroup, so, we divided the study population into two groups as follows: AR-high vs. AR-intermediate/low.
Among 166 patients with high AR expression, those who received chemotherapy (n = 131) were significantly associated with larger tumor size (P < 0.001), nodal involvements (P < 0.001), and grade II/III tumors (P = 0.004). In the intermediate/low AR subgroup (n = 448), a similar association was shown in patients who received chemotherapy (n = 364). Survival according to use of chemotherapy are presented in Fig. 3 among patients with high (Fig. 3a, b) or intermediate/low (Fig. 3c, d) AR expression. Patients with high AR expression showed better outcomes irrespective of the administration of chemotherapy. On the other hand, among patients with intermediate/low AR expression, those who received chemotherapy demonstrated trends of favorable survival.
Use of chemotherapy did not support survival benefits to patients with high AR expression in Cox’s model adjusted for age at diagnosis, tumor and node stage, grade, ER levels, PR expression, HER2 positivity, and Ki-67 proliferative index. On the contrary, among patients with intermediate/low AR expression, no use of chemotherapy was significantly associated with elevated risk for DFS and OS (Table 4). When AR level was categorized into low versus intermediate/high groups, additional use of chemotherapy provided survival benefit to both groups, which suggested that AR-intermediate/low expression might be an indicator for determining use of adjuvant chemotherapy in ER-positive breast cancers. However, formal interaction tests between use of chemotherapy and AR levels (AR-high vs. AR-intermediate/low) were not significant for DFS (P = 0.283) and OS (P = 0.559); therefore, statistical interpretation should be done with caution.
Discussion
Adjuvant treatments including endocrine and chemotherapy have improved survival of breast cancer patients [1]. However, those therapies also cause unavoidable exposure to various degrees of toxicity. Expression of ER in breast cancers is not only a good prognostic marker but a predictive indicator of responsiveness to endocrine therapy [26, 27]. For an accurate prediction of responsiveness to tamoxifen, ER expression levels, presence of PR expression, receptor tyrosine kinase pathways including HER2, gene signatures, and genetic polymorphisms of tamoxifen-metabolizing enzymes, particularly CYP2D6, have been suggested [3–5, 26, 27], however, other endocrine-related pathway such as androgen and AR signaling has not been extensively investigated.
Clinical significance of AR was previously proposed for the treatment of metastatic breast cancer patients, but, the prognostic and predictive values of AR have recently been highlighted in primary breast cancer patients [18, 28]. Our recent study suggests that AR expression is significantly associated with better survival and provides possible implications regarding the prediction of responses to endocrine therapy in ER-positive tumors [13]. To confirm the predictive role of AR, this retrospective study was carried out in the ER-positive subset of study cohorts, most of whom treated with SERMs. Higher levels of AR expression were significantly associated with favorable clinicopathological characteristics and better outcomes among study population as a whole and the subgroup who received endocrine therapy alone. These results are consistent with recent studies [14, 29], but they did not investigate survival according to AR levels. Although AR is not a definite target of SERMs and arbitrary cut-off values are used, our pioneering exploration suggests AR expression level could provide both prognostic and predictive information for ER-positive, luminal subtype tumors. A higher AR level may be associated with better responsiveness to endocrine therapy in patients with ER-positive breast cancer. Since the recent prospective GeparTrio trial of neoadjuvant setting that was different from our adjuvant setting revealed no role of AR on survival in luminal or HER2-like subtypes [30], it remains to validate in an independent dataset and to determine explanatory molecular mechanisms.
ER-positive tumors make up more than two-third of all breast cancer cases, usually demonstrate better survival, and respond well to endocrine agents having relative low toxicity [1]. It is not easy to accurately predict clinical benefits from cytotoxic chemotherapy in ER-positive subgroup. The current National Comprehensive Cancer Network (NCCN) guidelines, St. Gallen consensus meeting, Adjuvant Online (www.adjuvantonline.com), and CancerMath (www.lifemath.net/cancer) recommend that decision making regarding the additional use of chemotherapy is based on multiple prognostic factors including tumor size, nodal status, ER, PR, and HER2 status [11, 12, 31, 32]. Validation trials using 21-gene recurrence score (TAILORx trial) or 70-gene signature (MINDACT trial) are ongoing and the clinical significance of gene expression profiling will be confirmed in the near future.
So far, little is known about the possible implications of AR on the clinical benefits from chemotherapy in ER-positive breast cancer patients. Therefore, AR expression has not been significantly considered in the current clinical guidelines or risk prediction models. The present study has suggested possible promising results, although it has the limitations of retrospective nature and use of TMA blocks, not whole sections. There were no significant benefits from chemotherapy in patients with high AR level and borderline trends in those with lower AR level. Our risk models were adjusted for traditional prognostic parameters such as tumor and nodal stage, grade, ER levels, and HER2. Recent reports have demonstrated that certain subtypes or therapeutic regimens had a higher possibility of discriminating clinical benefits from investigation of AR expression [13, 14, 33]. Taken together, consideration of AR expression levels might be able to define more specific subgroup gaining benefits from additional adjuvant endocrine or chemotherapy. Furthermore, if gene signatures related to AR signal pathways are included in risk assessment models, it might be possible to predict more precise, improved, and individual outcomes for breast cancer patients.
In summary, AR expression level is positively associated with survival outcomes in ER-positive breast cancers. Higher AR expression is an independent prognostic factor and a significant predictor for better endocrine-responsiveness in the ER-positive subgroup. Patients with ER-positive, AR-high level tumors are less likely to gain clinical benefits from additional cytotoxic chemotherapy. Therefore, AR expression levels might be able to provide additional information of indication for adjuvant systemic chemotherapy in patients with ER-positive tumors.
Abbreviations
- AIs:
-
Aromatase inhibitors
- ANOVA:
-
Analysis of variance
- AR:
-
Androgen receptor
- ASCO/CAP:
-
American Society of Clinical Oncology/College of American Pathologists
- CMF:
-
Cyclophosphamide, methotrexate, and fluorouracil
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DFS:
-
Disease-free survival
- ER:
-
Estrogen receptor
- FISH:
-
Fluorescence in situ hybridization
- H&E:
-
Hematoxylin and eosin
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- SERMs:
-
Selective estrogen receptor modulators
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- TMA:
-
Tissue microarray
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. doi:10.1016/S0140-6736(05)66544-0
Bedard PL, Freedman OC, Howell A et al (2008) Overcoming endocrine resistance in breast cancer: are signal transduction inhibitors the answer? Breast Cancer Res Treat 108:307–317. doi:10.1007/s10549-007-9606-8
Ma CX, Sanchez CG, Ellis MJ (2009) Predicting endocrine therapy responsiveness in breast cancer. Oncology (Williston Park) 23:133–142
Harvey JM, Clark GM, Osborne CK et al (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Cui X, Schiff R, Arpino G et al (2005) Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol 23:7721–7735. doi:10.1200/JCO.2005.09.004
Paik S, Shak S, Tang G et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826. doi:10.1056/NEJMoa041588
van de Vijver MJ, He YD, van’t Veer LJ et al (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009. doi:10.1056/NEJMoa021967
Berry DA, Cirrincione C, Henderson IC et al (2006) Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295:1658–1667. doi:10.1001/jama.295.14.1658
Montemurro F, Aglietta M (2009) Hormone receptor-positive early breast cancer: controversies in the use of adjuvant chemotherapy. Endocr Relat Cancer 16:1091–1102. doi:10.1677/ERC-09-0033
Colleoni M, Bagnardi V, Rotmensz N et al (2009) Increasing steroid hormone receptors expression defines breast cancer subtypes non responsive to preoperative chemotherapy. Breast Cancer Res Treat 116:359–369. doi:10.1007/s10549-008-0223-y
Carlson RW, Allred DC, Anderson BO et al (2009) Breast cancer. Clinical practice guidelines in oncology. J Natl Compr Cancer Netw 7:122–192
Goldhirsch A, Ingle JN, Gelber RD et al (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–1329. doi:10.1093/annonc/mdp322
Park S, Koo JS, Kim MS et al (2011) Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol 22:1755–1762. doi:10.1093/annonc/mdq678
Castellano I, Allia E, Accortanzo V et al (2010) Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat 124:607–617. doi:10.1007/s10549-010-0761-y
Agoff SN, Swanson PE, Linden H et al (2003) Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol 120:725–731. doi:10.1309/42F0-0D0D-JD0J-5EDT
Hu R, Dawood S, Holmes MD et al (2011) Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res 17:1867–1874. doi:10.1158/1078-0432.CCR-10-2021
Park S, Koo J, Park HS et al (2010) Expression of androgen receptors in primary breast cancer. Ann Oncol 21:488–492. doi:10.1093/annonc/mdp510
Ingle JN, Twito DI, Schaid DJ et al (1991) Combination hormonal therapy with tamoxifen plus fluoxymesterone versus tamoxifen alone in postmenopausal women with metastatic breast cancer. An updated analysis. Cancer 67:886–891
Macedo LF, Guo Z, Tilghman SL et al (2006) Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res 66:7775–7782. doi:10.1158/0008-5472.CAN-05-3984
De Amicis F, Thirugnansampanthan J, Cui Y et al (2010) Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat 121:1–11. doi:10.1007/s10549-009-0436-8
von Elm E, Altman DG, Egger M et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370:1453–1457. doi:10.1016/S0140-6736(07)61602-X
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, New York
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Hammond ME, Hayes DF, Dowsett M et al (2010) American society of clinical oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795. doi:10.1200/JCO.2009.25.6529
Wolff AC, Hammond ME, Schwartz JN et al (2007) American society of clinical oncology/College of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145. doi:10.1200/JCO.2006.09.2775
Rastelli F, Crispino S (2008) Factors predictive of response to hormone therapy in breast cancer. Tumori 94:370–383
Cleator SJ, Ahamed E, Coombes RC et al (2009) A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer 9:S6–S17. doi:10.3816/CBC.2009.s.001
Moe RE, Anderson BO (2007) Androgens and androgen receptors: a clinically neglected sector in breast cancer biology. J Surg Oncol 95:437–439. doi:10.1002/jso.20722
Yu Q, Niu Y, Liu N et al (2011) Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol 22:1288–1294. doi:10.1093/annonc/mdq586
Loibl S, Muller BM, von Minckwitz G et al (2011) Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 130:477–487. doi:10.1007/s10549-011-1715-8
Ravdin PM, Siminoff LA, Davis GJ et al (2001) Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19:980–991
Chen LL, Nolan ME, Silverstein MJ et al (2009) The impact of primary tumor size, lymph node status, and other prognostic factors on the risk of cancer death. Cancer 115:5071–5083. doi:10.1002/cncr.24565
Koo JS, Jung W, Jeong J (2009) The predictive role of E-cadherin and androgen receptor on in vitro chemosensitivity in triple-negative breast Cancer. Jpn J Clin Oncol 39:560–568. doi:10.1093/jjco/hyp065
Acknowledgments
The author(s) indicate no potential conflicts of interest. A major part of this study was presented at the Sixth International Symposium on Hormonal Oncogenesis, Poster Presentations, September 12–16, 2010 in Tokyo, Japan. This study was supported by the Brain Korea 21 Project for Medical Science, Yonsei University, and in part by a grant-in-aid from Sanofi-Aventis Pharmaceutical Co. and Dong-A Pharmaceutical Co.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, S., Park, H.S., Koo, J.S. et al. Higher expression of androgen receptor is a significant predictor for better endocrine-responsiveness in estrogen receptor-positive breast cancers. Breast Cancer Res Treat 133, 311–320 (2012). https://doi.org/10.1007/s10549-011-1950-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1950-z