Abstract
Methods
We searched MEDLINE and EMBASE for epidemiologic studies on occupational exposure to methylene chloride and risk of cancer. Estimates of study-specific odds ratios (ORs) were calculated using inverse-variance-weighted fixed-effects models and random-effects models. Statistical tests for heterogeneity were applied.
Results
We summarized data from five cohort studies and 13 case–control studies. The pooled OR for multiple myeloma was (OR 2.04; 95 % CI 1.31–3.17) in relation to occupational exposure to methylene chloride but not for non-Hodgkin’s lymphoma, leukemia, breast, bronchus, trachea and lung, brain and other CNS, biliary passages and liver, prostate, pancreas, and rectum. Furthermore, we focused on specific outcomes for non-Hodgkin’s lymphoma and multiple myeloma because of exposure misclassification. The pooling OR for non-Hodgkin’s lymphoma and multiple myeloma was 1.42 (95 % CI 1.10–1.83) with moderate degree of heterogeneity among the studies (I 2 = 26.9 %, p = 0.205).
Conclusions
We found an excess risk of multiple myeloma. The non-Hodgkin’s lymphoma and leukemia that have shown weak effects should be investigated further.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylene chloride (dichloromethane) is used as a solvent in paint strippers and removers (30 %), in adhesives (20 %), as a propellant in aerosols (10 %), as a solvent in the manufacture of pharmaceuticals and drugs (10 %), in chemical processing (10 %), as a metal cleaning and finishing solvent (10 %), and in urethane foam blowing (5 %) [1]. Other uses make up the remaining 5 %. Methylene chloride is also widely used in applications such as metal cleaning and degreasing, polyurethane foam manufacturing, triacetate film and fiber manufacturing, food extraction, and aerosol propellants [2]. Current household products that may contain methylene chloride include lubricants, valve cleaners, and degreasers for automobiles, adhesive and varnish removers, paint strippers, and one household herbicide [3]. Methylene chloride is present in these products at percentages ranging from 1 to 90 %.
Methylene chloride is extensively metabolized in mammalian species through two competing pathways: (1) oxidation by the mixed function oxidase enzymes and (2) conjugation with glutathione catalyzed by glutathione-S-transferase(s) (GST). Interest in methylene chloride as a possible human carcinogen was prompted by animal bioassays, reporting an increased incidence of lung and liver tumors in mice inhaling high concentrations of methylene chloride vapor for the majority of their natural lifetime, but not rats, exposed to methylene chloride [4]. Earlier inhalation studies by Burek et al. [5] in rats and hamsters exposed at similar concentrations had shown no evidence of lung or liver tumors. The International Agency for Research on Cancer (IARC) has stated that methylene chloride is possibly carcinogenic to humans (group 2B) [6]. The evaluation was based on a combination of mechanistic data and sufficient evidence in experimental animals, while the evidence for carcinogenicity in humans was assessed as limited.
Several reviews have commented on the association between methylene chloride and risk of cancers in human [7–10]. However, no meta-analysis has been carried out so far. The aim of this paper is to summarize the epidemiological evidence of an association between occupational exposure to methylene chloride and risk of cancer.
Materials and methods
Search strategy
We searched for studies of workers exposed to methylene chloride published in any language using PubMed software to search MEDLINE (US National Library of Medicine, Bethesda, MD) and EMBASE. Combinations of the following keywords were used: “dichloromethane,” “methylene chloride,” “chlorinated solvent,” “solvent,” “cancer,” “carcinogen,” “mortality,” “neoplasm,” “case–control,” “cohort,” “epidemiology.” The search period was 1 January 1990 through 31 October 2012. In addition, we manually reviewed the reference lists from relevant original research and review articles and documents. Unpublished studies were not considered. All searches were carried out independently by two investigators (T.L and Q.E.X), and results were merged.
Study inclusion criteria and data collection
Studies were included if (1) they used a cohort or case–control study design; (2) the outcome of interest was clearly defined as cancer of an anatomical site; (3) they specifically identified methylene chloride exposure by reference to industrial hygiene records, individual biomarkers, job-exposure matrices, or industrial processes that involved the use of methylene chloride (cohort studies), or included questions regarding methylene chloride exposure (case–control studies); (4) they provided information that can be used to estimate the relation between methylene chloride and cancer risk in terms of odd ratio (OR), relative risk (RR), standardized mortality ratio (SMR), standardized relative risk (SRR), cumulative incidence ratio (CIR), or standardized incidence rate ratio (SIR) and their confidence intervals (CIs) or provided enough data to calculate them (raw data, p value, or variance estimate). Case–control studies that collected non-specific or general exposure information such as “solvents” or “chemicals” or that analyzed findings by a single job title were not included. We excluded case reports and case series. When risks of cancer of different anatomical sites were available in the same publication, we considered each cancer separately.
We developed a questionnaire and recorded study name, year of publication, study design, sample size (cases and controls or cohort size), methylene chloride exposure assessment, study outcomes, duration of follow-up, variables used for adjustment, or matching.
Quality assessment
The quality of the selected studies was assessed independently by two authors (T.L and Q.E.X) using the Newcastle–Ottawa Scale (NOS) [11]. The NOS uses two different tools for case–control and cohort studies and consists of three parameters of quality: selection, comparability, and exposure/outcome assessment. The NOS assigns a maximum of four points for selection, two points for comparability, and three points for exposure or outcome. For stratification purposes, studies with scores of six points or more were considered to be of moderate to good study quality. However, all studied were used for analysis, irrespective of NOS score. Any discrepancies were addressed by a joint reevaluation of the original article.
Statistical analysis
Based on the reported CIs, we estimated the standard errors [seln(effect size)] for the ln(effect size) given by the formula seln(effect size) = [ln(upper limit)−ln(lower limit)] ÷ (2*Z 1−α/2), where for a 95 % CI, Z 1−α/2 equals 1.96 [12]. For the studies for which the 95 % CI was not reported, we calculated them by the Fischer’s exact method using the observed deaths and expected deaths reported in the articles [13].
Overall pooled RRs estimates and their corresponding 95 % CIs were obtained using fixed-effects (Mantel–Haenszel method) and random-effects (DerSimonian and Laird method) methods [14]. Given the significant amount of heterogeneity, only the random-effects estimates are presented. Between-study heterogeneity was assessed using the Q and I 2 statistics, with P Q < 0.10 or I 2 > 25 % indicating significant heterogeneity [15, 16].
The meta-analyses were performed with Stata software (version 10; StataCorp LP, College Station, TX, USA) using a combination of available macros [17]. A p value <0.05 was considered statistically significant for all tests except for the heterogeneity.
Results
Search results
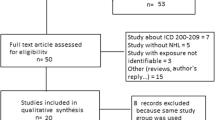
The MEDLINE and EMBASE search identified a total of 3,086 articles. Based on titles and abstracts, 3,004 articles were excluded at first screening because they did not meet the eligibility criteria, such as no original data, human research, methylene chloride data, cancer site, or duplicates of the same study. Full-text copies of the remaining 82 potentially relevant studies were obtained. Sixty-four studies were excluded because they did not meet the inclusion criteria. Five articles have been updated (Lanes et al. [18] updated Lanes et al. [19]. Radican et al. [20] updates Blair et al. [21], Spirtas et al. [22]. Tomenson [23] updates Tomenson et al. [24]. Hearne et al. [25] updates Hearne et al. [26]). Finally, 18 studies (five cohort studies [18, 20, 23, 25, 27] and 13 case–control studies [28–40]) that assessed the association of methylene chloride with risk of cancer met criteria for inclusion in the meta-analysis (Fig. 1).
Study characteristics and study quality
We found six studies on non-Hodgkin’s lymphoma, three studies on multiple myeloma, three studies on leukemia, three studies on breast cancer, five studies on brain and other CNS cancer, five studies on bronchus, trachea, and lung, three studies on rectum cancer, five studies on pancreas cancer, three studies on prostate cancer, and three studies on biliary passages and liver.
Cohort studies were published between 1993 and 2011. Three studies originated from USA, one from UK, and one from Canada. According to the NOS, two studies (40 %) were scored six points or more, indicating a moderate study quality (Table 1). Case–control studies were published between 1994 and 2012. Eight studies originated from America, two from Italy, one from Germany, and two from Canada. According to the NOS, 10 studies (77 %) were scored six points or more, indicating a moderate to good study quality.
The exposure assessment of ten studies was based on a job-exposure matrix. Eight studies used self-reported exposure, occupational histories, and cumulative exposure.
Statistical analysis and pooling
Non-Hodgkin’s lymphoma
Figure 2 shows the OR and 95 % CI from the individual studies and the pooled OR based on a random-effects model. The average pooled estimate for non-Hodgkin’s lymphoma among methylene chloride exposed workers was 1.28 (95 % CI 0.96–1.70), with a moderate degree of heterogeneity among the studies (I 2 = 29.1 %, p = 0.217). There was no significant association when evaluating cohort studies (OR 1.19; 95 % CI 0.31–4.55) and case–control studies (OR 1.27; 95 % CI 0.93–1.72) separately (Table 2).
Multiple myeloma
The fixed-effects OR for multiple myeloma was elevated at 2.04 (95 % CI 1.31–3.17, I 2 = 0.0 %, p > 0.1, Fig. 3). No association was found when evaluating cohort studies. Furthermore, according to the Glass et al. study [41], we focus on specific outcomes for non-Hodgkin’s lymphoma and multiple myeloma because of exposure misclassification. The pooling OR for non-Hodgkin’s lymphoma and multiple myeloma were 1.42 (95 % CI 1.10–1.83) with moderate degree of heterogeneity among the studies (I 2 = 26.9 %, p = 0.205), 1.38 (95 % CI 1.03–1.85) for case–control studies, 1.69 (95 % CI 0.88–3.26) for cohort studies (data not shown).
Leukemia
The OR for leukemia was 1.19 (95 % CI 0.54–2.65), with a moderate degree of heterogeneity among the studies (I 2 = 44.5 %, p = 0.165). There was no significant association when evaluating cohort studies (OR 1.90; 95 % CI 0.93–3.89) (Fig. 4).
Breast cancer
Figure 5 shows the OR and 95 % CI from the individual studies and the pooled OR based on a random-effects model. The OR for breast cancer was 1.02 (95 % CI 0.48–2.16), with a moderate degree of heterogeneity among the studies (I 2 = 67.4 %, p = 0.046).
Brain and other CNS cancer
The random-effects OR for brain and CNS cancer was 1.11 (95 % CI 0.78–1.58, I 2 = 65.8 %, p < 0.1, Fig. 6). No association was found when evaluating cohort studies and case–control studies separately.
Bronchus, trachea, and lung cancer
The fixed-effects OR for bronchus, trachea, and lung cancer was 0.82 (95 % CI 0.62–1.09, I 2 = 0.0 %, p > 0.1, Fig. 7). No association was found when evaluating cohort studies.
Biliary passages and liver cancer
Figure 8 shows the OR and 95 % CI from the individual studies and the pooled OR based on a random-effects model. The OR for biliary passages and liver cancer was 1.69 (95 % CI 0.30–9.64), with a moderate degree of heterogeneity among the studies (I 2 = 40.9 %, p = 0.193).
Rectum cancer
The fixed-effects OR for rectum cancer was 1.12 (95 % CI 0.71–1.76, I 2 = 0.0 %, p > 0.1, Fig. 9). No association was found when evaluating cohort studies.
Pancreas cancer
The fixed-effects OR for pancreas cancer was 0.97 (95 % CI 0.93–1.01, I 2 = 0.0 %, p > 0.1, Fig. 10) among all the studies. The OR for cohort studies was 0.80 (95 % CI 0.38–1.71).
Prostate cancer
The fixed-effects OR for prostate cancer was 0.76 (95 % CI 0.51–1.14, I 2 = 0.0 %, p > 0.1, Fig. 11).
Discussion
The association between occupational exposure to methylene chloride and risk of cancer has been assessed. Our results demonstrate an increase in the pooled estimate (OR 2.04; 95 % CI 1.31–3.17) for multiple myeloma in relation to occupational exposure to methylene chloride but not for non-Hodgkin’s lymphoma, leukemia, breast, bronchus, trachea and lung, brain and other CNS, biliary passages and liver, prostate, pancreas, and rectum. Furthermore, we focus on specific outcomes for non-Hodgkin’s lymphoma and multiple myeloma because of exposure misclassification, according to the Glass et al. study [41]. The pooling OR for non-Hodgkin’s lymphoma and multiple myeloma were 1.42 (95 % CI 1.10–1.83) with moderate degree of heterogeneity among the studies (I 2 = 26.9 %, p = 0.205), 1.38 (95 % CI 1.03–1.85) for case–control studies, and 1.69 (95 % CI 0.88–3.26) for cohort studies (data not shown).
Cytochrome P450 2E1 (CYP2E1) and glutathione-S-transferase theta 1 (GSTT1) are two major metabolic pathways in the biotransformation of methylene chloride [36, 42]. Oxidation by P450 pathway via formyl chloride finally leads to the formation of CO, induces cellular toxicity [43]. Barry et al. [36] indicated that CYP2E1 activity was expected to influence the toxicity of methylene chloride. The OR for non-Hodgkin’s lymphoma among women TT for rs2070673 in the CYP2E1 was 4.42 (95 % CI 2.03–9.62). However, Olvera-Bello et al. [44] suggested that methylene chloride was highly cytotoxic in human peripheral blood mononuclear cells, even at doses within the safety range, which could potentially cause an increase in the accumulation of reactive oxygen species via GSTT1 pathway, leading to cell cycle regulation and the mitogenic response.
Exposure misclassification has been a critical limitation among epidemiologic studies. Misclassification can occur with all study designs, and it can concern any of basic elements of an epidemiologic study: the outcome, exposure, or the covariates (confounder, modifiers). From the point of view of exposure assessment, exposure misclassification is of major concern [45]. Most studies assess occupational exposure to specific solvents by means of the job-exposure matrices, self-reported occupational histories, and expert assessment of exposure [46]. Ten of eighteen studies in this meta-analysis have relied on some type of semiquantitative job-exposure matrix (JEM), and sources of data in these studies were based on death certificates or cancer registries. The most recent occupation and type of industry held by the decedent have been reported on death certificates. But no information on most known (or suspected) risk factors, duration of employment, job title, and other occupations were recorded, such as age at menarche, age at first birth, age at menopause, history of benign breast disease, and family history for breast cancer. Thus, the exposure assessment based on usual occupation and type of industry may not accurately reflect the exposures related to the cause of death.
For the occupation data from cancer registries, information collected directly from study participants rather than a proxy respondent is generally a more reliable source of information for job histories. Six studies benefited from an expert’s translation of lifetime occupational history into specific exposures. Nevertheless, retrospective exposure assessment procedure was not based on active measurement, and concentration of exposure could not be estimated in absolute terms. Moreover, the complexity of use of solvents that have been used interchangeably and at times together has made the evaluation of specific exposure difficult. But because of blindly with respect to disease status and controlled nature of the computer-assisted personal interview, the error involved in applying a JEM is non-differential with respect to case/control status and therefore can lead to some attenuation of true odds ratios.
Potential known or unknown confounders may lead to biased results. It has shown that non-Hodgkin’s lymphoma among women exposed to methylene chloride is restricted to the specific genotype/phenotype of CYP2E1, an enzyme involved in the activating/detoxification of methylene chloride [36]. This and other genetic factors are potential effect modifiers that were not addressed in the individual studies. Furthermore, four of the studies explicitly adjusted for smoking [32, 34, 35, 40], although some authors decided not to adjust for it after having ruled out confounding in their data. Thus, lack of control for smoking may have upwardly biased the relative risk estimates in the cohort studies that used external comparisons, but may not have affected case–control studies or cohort studies using internal comparison groups.
Our study identified third implications for the future. First, our meta-analysis provides new information for a carcinogenic effect of methylene chloride in humans, which contributes to the IARC classification of methylene chloride. Second, it is important to establish an increased cancer risk in relation to methylene chloride exposure, particularly non-Hodgkin’s lymphoma and multiple myeloma, as classification differences and independent control groups hinder comparison of consistency across epidemiologic studies of lymphoid cancers and methylene chloride. Third, more than two-thirds of the studies were case–control studies, given semiquantitative exposure assessment, and the cohort studies were of small sizes and uneven exposure information; therefore, the future researches designed with large population-based case–control or cohort studies are needed in order to make reliable inferences on cause–effect mechanisms.
In conclusion, we found an excess risk of multiple myeloma. The non-Hodgkin’s lymphoma and leukemia that have shown weak effects should be investigated further.
References
Holbrook M (2003) Methylene chloride. Kirk-Othmer Encycl Chem Technol 16:371–380
NTP (1986) Toxicology and carcinogenesis studies of dichloromethane (methylene chloride) (CAS No. 75-09-2) in F344/N rats and B6C3F1 mice (inhalation studies). Natl Toxicol Program Tech Rep Ser 306:1–208
HPD (2009) Household Products Database. National Library of Medicine. http://hpd.nlm.nih.gov/ingredients.htm and search on CAS number
Mennear JH, McConnell EE, Huff JE, Renne RA, Giddens E (1988) Inhalation toxicity and carcinogenesis studies of methylene chloride (dichloromethane) in F344/N rats and B6C3F1 mice. Ann N Y Acad Sci 534:343–351
Burek JD, Nitschke KD, Bell TJ, Wackerle DL, Childs RC, Beyer JE, DA Dittenber RL, McKenna MJ (1984) Methylene chloride: a two-year inhalation toxicity and oncogenicity study in rats and hamsters. Fundam Appl Toxicol 4:30–47
IARC (1999) Dichloromethane. In: Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide. Volume 71, IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. International Agency for Research on Cancer, Lyon, pp 251–315
Lynge E, Anttila A, Hemminki K (1997) Organic solvents and cancer. Cancer Causes Control 8:406–419
Dell LD, Mundt KA, McDonald M, Tritschler JP, Mundt DJ (1999) Critical review of the epidemiology literature on the potential cancer risks of methylene chloride. Int Arch Occup Environ Health 72:429–442
Cooper GS, Scott CS, Bale AS (2011) Insights from epidemiology into dichloromethane and cancer risk. Int J Environ Res Public Health 8:3380–3398
Starr TB, Matanoski G, Anders MW, Andersen ME (2006) Workshop overview: reassessment of the cancer risk of dichloromethane in humans. Toxicol Sci 91:20–28
The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. Available from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 31 Jan 2012
Bradburn M (2004) Updated and new commands for meta-analysis in STATA. Cancer Research UK Medical Statistics Group. Centre for Statistics in Medicine, Oxford
Dean AG, Sullivan KM, Soe MM (2010) OpenEpi Open Source Epidemiologic Statistics for Public Health. Version 2.3.1. Available http://www.OpenEpi.com. Accessed 20 Oct 2010
Harris RJ, Bradburn M, Deeks JJ, Harbord RM, Altman DG, Sterne JAC (2008) Metan: fixed- and random-effects meta-analysis. Stata J 8:3–28
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Takkouche B, Cadarso-Suarez C, Spiegelman D (1999) Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 150:206–215
Sterne J (2009) Meta-analysis in Stata: an updated collection from the Stata journal. Stata Press, College Station, TX
Lanes SF, Rothman KJ, Dreyer NA, Soden KJ (1993) Mortality update of cellulose fiber production workers. Scand J Work Environ Health 19:426–428
Lanes SF, Cohen A, Rothman KJ, Dreyer NA, Soden KJ (1990) Mortality of cellulose fiber production workers. Scand J Work Environ Health 16:247–251
Radican L, Blair A, Stewart P, Wartenberg D (2008) Mortality of aircraft maintenance workers exposed to trichloroethylene and other hydrocarbons and chemicals: extended follow-up. J Occup Environ Med 50:1306–1319
Blair A, Hartge P, Stewart PA, McAdams M, Lubin J (1998) Mortality and cancer incidence of aircraft maintenance workers exposed to trichloroethylene and other organic solvents and chemicals: extended follow up. J Occup Environ Med 55:161–171
Spirtas R, Stewart PA, Lee JS et al (1991) Retrospective cohort mortality study of workers at an aircraft maintenance facility. I Epidemiological results. Br J Ind Med 48:515–530
Tomenson JA (2011) Update of a cohort mortality study of workers exposed to methylene chloride employed at a plant producing cellulose triacetate film base. Int Arch Occup Environ Health 84:889–897
Tomenson JA, Bonner SM, Heijne CG, Farrar DG, Cummings TF (1997) Mortality of workers exposed to methylene chloride employed at a plant producing cellulose triacetate film base. J Occup Environ Med 54:470–476
Hearne FT, Pifer JW (1999) Mortality study of two overlapping cohorts of photographic film base manufacturing employees exposed to methylene chloride. J Occup Environ Med 41:1154–1169
Hearne FT, Pifer JW, Grose F (1992) Mortality study of 1964–1970 cohort of employees exposed to methylene chloride: an update. OSHA methylene chloride docket H-71. OHSA, Washington, DC
Gibbs GW, Amsel J, Soden K (1996) A cohort mortality study of cellulose triacetate-fiber workers exposed to methylene chloride. J Occup Environ Med 38:693–697
Heineman EF, Cocco P, Gomez MR et al (1994) Occupational exposure to chlorinated aliphatic hydrocarbons and risk of astrocytic brain cancer. Am J Ind Med 26:155–169
Cantor KP, Stewart PA, Brinton LA, Dosemeci M (1995) Occupational exposures and female breast cancer mortality in the United States. J Occup Environ Med 37:336–348
Cocco P, Heineman EF, Dosemeci M (1999) Occupational risk factors for cancer of the central nervous system (CNS) among US women. Am J Ind Med 36:70–74
Kernan GJ, Ji BT, Dosemeci M, Silverman DT, Balbus J, Zahm SH (1999) Occupational risk factors for pancreatic cancer: a case-control study based on death certificates from 24 US States. Am J Ind Med 36:260–270
Dumas S, Parent ME, Siemiatycki J, Brisson J (2000) Rectal cancer and occupational risk factors: a hypothesis-generating, exposure-based case-control study. Int J Cancer 87:874–879
Miligi L, Costantini AS, Benvenuti A et al (2006) Occupational exposure to solvents and the risk of lymphomas. Epidemiology 17:552–561
Seidler A, Mohner M, Berger J et al (2007) Solvent exposure and malignant lymphoma: a population-based case-control study in Germany. J Occup Med Toxicol 2:2
Wang R, Zhang Y, Lan Q et al (2008) Occupational exposure to solvents and risk of non-Hodgkin lymphoma in Connecticut women. Am J Epidemiol 169:176–185
Barry KH, Zhang Y, Lan Q et al (2011) Genetic variation in metabolic genes, occupational solvent exposure, and risk of non-hodgkin lymphoma. Am J Epidemiol 173:404–413
Gold LS, Stewart PA, Milliken K et al (2011) The relationship between multiple myeloma and occupational exposure to six chlorinated solvents. Occup Environ Med 68:391–399
Costantini AS, Benvenuti A, Vineis P et al (2008) Risk of leukemia and multiple myeloma associated with exposure to benzene and other organic solvents: evidence from the Italian multicenter case-control study. Am J Ind Med 51:803–811
Neta G, Stewart PA, Rajaraman P et al (2012) Occupational exposure to chlorinated solvents and risks of glioma and meningioma in adults. Occup Environ Med 69:793–801
Vizcaya D, Christensen KY, Lavoué J, Siemiatycki J (2012) Risk of lung cancer associated with six types of chlorinated solvents: results from two case-control studies in Montreal. Can Occup Environ Med 70:81–85
Glass DC, Gray C, Jolley DJ et al (2003) Leukemia risk associated with low-level benzene exposure. Epidemiology 14:569–577
Bos PM, Zeilmaker MJ, van Eijkeren JC (2006) Application of physiologically based pharmacokinetic modeling in setting acute exposure guideline levels for methylene chloride. Toxicol Sci 91:576–585
Schwer CI, Mutschler M, Stoll P et al (2010) Carbon monoxide releasing molecule-2 inhibits pancreatic stellate cell proliferation by activating p38 mitogen-activated protein kinase/heme oxygenase-1 signaling. Mol Pharmacol 77:660–669
Olvera-Bello AE, Estrada-Muniz E, Elizondo G, Vega L (2010) Susceptibility to the cytogenetic effects of dichloromethane is related to the glutathione S-transferase theta phenotype. Toxicol Lett 199:218–224
Kauppinen TP (1994) Assessment of exposure in occupational epidemiology. Scand J Work Environ Health 20:19–29
Teschke K, Olshan AF, Daniels JL et al (2002) Occupational exposure assessment in case-control studies: opportunities for improvement. J Occup Environ Med 59:575–593 discussion 94
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Xu, Qe., Zhang, Ch. et al. Occupational exposure to methylene chloride and risk of cancer: a meta-analysis. Cancer Causes Control 24, 2037–2049 (2013). https://doi.org/10.1007/s10552-013-0283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-013-0283-0