Abstract
Purpose
Studies of cancer patient survival typically report relative survival or cause-specific survival using data from patients diagnosed many years in the past. From a risk-communication perspective, such measures are suboptimal for several reasons; their interpretation is not transparent for non-specialists, competing causes of death are ignored and the estimates are unsuitable to predict the outcome of newly diagnosed patients. In this paper, we discuss the relative merits of recently developed alternatives to traditionally reported measures of cancer patient survival.
Methods
In a relative survival framework, using a period approach, we estimated probabilities of death in the presence of competing risks. To illustrate the methods, we present estimates of survival among 23,353 initially untreated, or hormonally treated men with intermediate- or high-risk localized prostate cancer using Swedish population-based data.
Results
Among all groups of newly diagnosed patients, the probability of dying from prostate cancer, accounting for competing risks, was lower compared to the corresponding estimates where competing risks were ignored. Accounting for competing deaths was particularly important for patients aged more than 70 years at diagnosis in order to avoid overestimating the risk of dying from prostate cancer.
Conclusions
We argue that period estimates of survival, accounting for competing risks, provide the tools to communicate the actual risk that cancer patients, diagnosed today, face to die from their disease. Such measures should offer a more useful basis for risk communication between patients and clinicians and we advocate their use as means to answer prognostic questions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population-based cancer patient survival is useful for monitoring and evaluating the effectiveness of cancer patient care as it provides estimates of survival that are representative of the entire population. The most commonly reported measures of survival among cancer patients, in such studies, are cause-specific survival and relative survival [1]. Cause-specific survival uses information from death certificates or chart reviews to identify cancer deaths whereas relative survival takes an indirect approach to identifying cancer deaths by contrasting the all-cause survival among the patients to the expected survival for a comparable group in the general population who are assumed to be free of the cancer of interest. Both measures aim to estimate survival in the hypothetical scenario where cancer is the only possible cause of death [2]. In the statistical literature, this hypothetical construct is called net survival; it is useful for purposes such as studying temporal trends in survival or comparing survival between countries where it is desirable to correct for differences in non-cancer mortality. Net survival is also relevant in a randomized clinical trial setting if the primary objective to be addressed concerns demonstrating a putative effect between treatment arms [3].
Thus, the commonly reported 10-year cause-specific survival and 10-year relative survival each provide an estimate of the probability of not dying of cancer within 10 years of diagnosis in the hypothetical situation where one cannot die of other causes than the cancer of interest. From a patient perspective, such information is often of less value since the risk of deaths from other causes is a fact that must be accounted for when discussing the prognosis and the available treatment options. In the statistical literature, probabilities of death estimated in the presence of competing causes of death are called crude (as opposed to net) probabilities of death. The 10-year crude probability of death due to cancer will be lower than the 10-year net probability of death since once we let patients die of other causes than the endpoint of interest, the probability of dying of cancer is lower. The crude probability of death is sometimes referred to as cumulative incidence [4] or the absolute probability of death [5]. Unfortunately, each of these terms (including crude probability) is also used for other statistical measures.
Our aim with this study is to clarify the interpretation of standard and more recent measures of population-based cancer patient survival. In addition to discussing the relative merits of crude and net survival, we also explain how combining period analysis with estimates of crude survival can provide more accurate estimates of the prognosis among newly diagnosed cancer patients compared to the conventionally reported cohort estimates. For illustration, we present estimates of risk category- and treatment-specific prostate cancer survival using population-based data from Sweden.
Methods
Data
The National Prostate Cancer Register of Sweden captures more than 97 % of all men diagnosed with prostate cancer compared with the Swedish National Cancer Register to which reporting is mandatory and regulated by law [6]. In addition to the information available in the Swedish National Cancer Register, the National Prostate Cancer Register contains information about tumor characteristics at the date of diagnosis and primary treatment within 6 months from diagnosis.
We investigated men with intermediate- and high-risk prostate cancer diagnosed between 1996 and 2008. The intermediate-risk category was defined as clinical local stage T1-2, N0, NX-, M0, MX, and serum levels of prostate–specific antigen (PSA) between 10 and 20 ng/ml or Gleason score 7, and the locally advanced high-risk category as T3-4, N0, NX, M0, MX, and/or PSA between 20 and 50 ng/ml, and/or Gleason score 8 or higher according to a modified version of National Comprehensive Cancer Network® classification [7]. Patients who received curatively intended treatment were excluded, leaving 29,647 patients in the investigated cohort.
Statistical methods
Relative survival and excess mortality
Relative survival is the preferred method for estimating net survival in a population-based setting as it captures mortality that is either directly or indirectly related to the cancer without requiring information on cause of death [1]. Relative survival is defined as the ratio of the observed all-cause survival among the patients compared to the expected (all-cause) survival in a disease-free but otherwise comparable population. In practice, patients are matched to the general population on factors which most often include age, sex, and calendar year, although sometimes additional factors, such as socioeconomic status and race, are also feasible. An estimate of the 5-year relative survival of 1.00 suggests that the survival of the patients is just as good (or poor) as that in the general population. It does not mean that all patients are alive 5 years after diagnoses, only that the patients have not experienced any excess mortality associated with the cancer under study during the first 5 years of follow-up. Excess mortality is the mortality analog of relative survival. It is a rate, rather than a proportion, and is expressed as the difference between the observed mortality rate (all-cause) among the patients and the expected mortality rate in a healthy population. Estimates of relative survival below 1.00, or equivalently, an excess mortality rate greater than zero, imply that the survival of the patients is worse than expected and it is assumed that the reason for the difference is entirely due to the cancer in question. One important implication of this is that relative survival captures deaths that can be viewed as indirectly caused by the cancer, for example, excess cardiovascular deaths following cardiotoxic treatments [8–10], or excess deaths from suicides [11]. These are deaths that are difficult to capture using a cause-specific approach since they are typically not classified as death from cancer.
Net versus crude probability of death
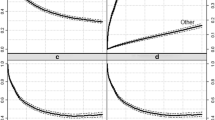
If our primary interest is death due to a specific cancer, then deaths due to other causes (including other forms of cancer) are known as competing risks. Relative survival aims to provide an estimate of net survival, survival in a virtual world where competing causes of death do not exist. This is not a world in which cancer patients live and such estimates are not particularly relevant to individual patients. The solution is provided by the so-called crude survival probabilities, which can be calculated using statistical methods for competing risks. Rather than reporting crude survival probabilities (the probability of not dying), it is common to report the complement, called the crude probability of death. To reinforce the conceptual difference between net and crude probabilities of death, we have estimated both quantities in the cohort of men diagnosed with prostate cancer in Sweden (Fig. 1). The shaded area in Fig. 1a, c represents the net probabilities (i.e., 1—relative survival) of prostate cancer death as a function of years since diagnosis. For a 75-year-old man diagnosed with prostate cancer, the probability of dying of prostate cancer within 10 years is 0.27 in a world where it is not possible to die of other causes (Fig. 1a). When acknowledging the existence of competing causes of death, we see that the crude probability that such a man will die of prostate cancer during the subsequent 10 years is only 0.18 (Fig. 1b). In the same panel, we can also see that the probability of dying of a cause other than prostate cancer within 10 years is 0.43 and the probability of still being alive after 10 years is 0.39. We argue that the right-hand figures are of considerably more interest to clinicians and patients as they not only provide a more accurate prediction of the real-world risk of dying from cancer but also additional information about the overall risk of dying during follow-up. The difference between crude and net probabilities is not as great for the 60-year-old men since the probability of death due to causes other than cancer is not as high (Fig. 1c, d).
Predictions of net probabilities of death (on the left) and crude probabilities of death (on the right) due to prostate cancer among men aged 60 and 75 at diagnosis who were recorded as having intermediate- or high-risk cancer and as treated conservatively or with hormonal treatment in the National Prostate Cancer Register of Sweden between 1996 and 2008
Period analysis
Traditionally reported cohort estimates of cancer patient survival are obtained by following-up cohorts of patients for a number of years after they are diagnosed with cancer. Because cohort estimates of, for example, 10-year survival require following patients diagnosed at least 10 years back in time, they do not fully capture the impact of advances in the diagnosis and treatment on the prognosis of patients diagnosed more recently. Brenner and others [12] suggested an alternative approach to estimation, known as period analysis, which has been shown to be considerably better at predicting the future survival of newly diagnosed patients [13]. In contrast to the cohort approach, where all patients (and their corresponding time at risk) contribute to the analysis, the inclusion of patients and contribution of person-time, in a period analysis, is restricted to a prespecified time window. For example, let us assume that we fix a time window to the years 2005–2009 where the end of 2009 is the last date for which follow-up information is available. For patients who are diagnosed within that window follow-up is calculated in the same manner as in a traditional cohort approach, from the date of diagnosis until the date of death or censoring (administrative or due to loss from follow-up). However, for patients who were diagnosed before the time window only the time at risk that occurs within the window is counted. For example, patients who were diagnosed in 1996, and still alive in 2005, enter their 9th year of follow-up in 2005 and therefore contribute to the estimation of the 9-, 10-, 11-, 12-, and 13-year survival probabilities if they remain alive until the end of 2009. Similarly patients diagnosed in 1997, enter the time window during their 8th year of follow-up and thus contribute to the survival estimates from that year. Time at risk prior to the time window is not included in the survival analysis. Also, patients who die before entering the time window are completely excluded from the analysis. With this approach, estimates of short-term survival are more “up-to-date” than the corresponding estimates in a cohort approach. Because period analysis has proven empirically to be superior to the cohort approach, with respect to its ability to predict future survival, we believe such an approach to estimation provides estimates that are of greater clinical relevance, in particular for risk-communication purposes [12].
Modeling approach
A wide range of statistical models have been proposed for modeling excess mortality [14, 15]. We used flexible parametric survival models [16, 17] which use individual-level data and explicitly estimate a baseline excess mortality rate that may vary nonlinearly with time since diagnosis, by the use of restricted cubic splines [18]. By further allowing delayed entry to the flexible parametric survival model, we adapted a period approach to modeling, using a time window of 2005–2009 inclusive. The choice of period window was based on a desire to capture recent survival experience while, at the same time, providing a sufficient number of cases for the statistical analysis. A window of, for example, 2008–2009 would provide more up-to-date estimates but with larger standard errors. A detailed description of the statistical models that were used is included in the statistical appendix.
Crude probabilities of death were retrieved by re-calculating the survival estimates from the flexible parametric models using competing risk theory adapted for relative survival as described by Lambert et al. [19]. The user-written stpm2 and stpm2cm commands for flexible parametric models in the Stata software (17) (StataCorp. 2009 Stata Statistical Software: Release 11. College Station, TX: StataCorp LP) were used for the statistical analyses.
Results
Table 1 shows the numbers of men and deaths in the full cohort (n = 29,647) by patient and disease characteristics. Among these patients, the distribution of age at diagnosis and selection of conservative or hormonal treatment among men with intermediate-risk cancers was relatively stable throughout the study. In contrast, among men in the high-risk group, the proportion of men older than 79 years at diagnosis increased from 33 % in 1996–1999 to 46 % in 2006–2008, along with the use of hormone therapy. The number of informative subjects in the period analysis (n = 23,353), that is, men that contribute person-time to the chosen period window, is also provided in Table 1 as well as the number of deaths that occurred among these men. Naturally, the number of informative men increases with calendar period of diagnosis as the criteria for entering the period analysis is to be alive at the start of 2005.
Figure 2 shows the predicted crude probabilities of death within the first 10 years after diagnosis for newly diagnosed men managed conservatively, and Fig. 3 shows these probabilities for hormonally treated men. The estimates are shown by risk category, and for a selection of ages at diagnosis. The probability of dying from prostate cancer was similar across the different ages within each risk category of conservatively treated men, whereas for men who received hormone therapy, age at diagnosis was a strong predictor for prostate cancer mortality. For all displayed ages at diagnosis, and both risk groups, the crude probability of death from prostate cancer was higher among hormonally treated men compared to men managed conservatively. Table 2 provides the means to quantify the observed differences in survival at two fixed time points (5 and 10 years) after diagnosis. For example, in the intermediate-risk group of conservatively treated men, the proportion who died from prostate cancer within 10 years after diagnosis was 4.6 % (95 % CI 1.9–7.4 %) for men aged 60 years at diagnosis and 3.9 % (95 % CI 1.9–6.0 %) for men aged 80 years at diagnosis. The corresponding proportions for men treated hormonally, 27.6 % (95 % CI 20.8–34.4 %) for men aged 60 years and 12.2 % (95 % CI 9.0–15.4 %) for men aged 80 years at diagnosis.
Figures 2, 3 and Table 2 also provide estimates of the crude probabilities of death from other causes than prostate cancer, and thereby also the probability of still being alive as a function of elapsed time since diagnosis. When these crude probabilities are reported together with the crude probabilities of death, such as in Table 2, the results can easily be converted and communicated in terms of natural frequencies to aid the interpretation of the results further. For illustration, among 100 newly diagnosed 60 year-old men with intermediate-risk prostate cancer and hormonal treatment, we would expect 11 to have died from their cancer, 4 to have died from other causes than cancer, and 85 to still be alive 5 years from today. In the 10-year perspective, we would expect 28 men to have died from prostate cancer, 10 from other causes, and 62 to still be alive.
Compared to net probabilities of death from cancer, the crude probabilities of death from cancer were lower, particularly for older men. Figure 4 summarizes the net and crude 10-year probabilities of dying from prostate cancer by risk and treatment group as a function of age at diagnosis. While the long-term crude prostate cancer mortality among men managed conservatively remained stable within risk category and across different ages at diagnosis, estimates of net mortality began to diverge from the corresponding crude estimates for men older than 70 years at diagnosis. A similar feature was observed among men older than 75 years at diagnosis and treated with hormone therapy although, in general, both the net and the crude mortality due to cancer were higher for all ages and both risk groups compared to conservatively managed men.
Discussion
The great majority of studies of cancer patient survival report estimates of net survival that are only interpretable in the hypothetical situation where patients are assumed immune to death from causes other than the cancer under study. Particularly for elderly patients, such statistics will overestimate the real-world probability of death due to cancer as illustrated in our case of men with intermediate- and high-risk localized prostate cancer. As such, estimates of net survival do not provide an ideal basis for medical decision making where it is necessary to consider the trade-off between the (real-world) probabilities of treatment-related side effects and probabilities of death due to the underlying disease. Estimates of net survival are useful for many other purposes and we are certainly not suggesting they no longer should be presented. For example, to achieve valid international comparisons of cancer survival, it is vital to control for the fact that non-cancer mortality differs across countries in order to enable like-with-like comparisons. Moreover, in studies that evaluate research questions related to disease etiology, net survival should also be the method of choice since, ideally, the results should reflect differences in survival associated with the exposure under study as opposed to differences in the non-cancer mortality.
It is important that both the producers and the consumers of cancer survival statistics become more aware that different end-users are best served by different types of statistics. For example, period estimates of crude survival will in general be more useful in a clinical setting than cohort estimates of net survival. Period estimates have been shown to provide good predictions of long-term net survival [12, 20], in particular, if there have been improvements in survival, either true (e.g., following improvements in treatment) or artificial (e.g., following increased screening activities) over time. However, studies that combine period estimation with competing risk theory in order to predict the outcome of newly diagnosed patients using crude probabilities of death are still rare [21].
The statistical methodology used to estimate the crude probabilities of death relies on the fact that relative survival can be estimated accurately. The key assumptions for estimating relative survival are that survival from prostate cancer is independent from survival from other causes, and that the expected survival in the general population correctly represents the survival that the cancer patients would have experienced in the absence of cancer. Stattin and others have previously demonstrated that the all-cause mortality in patients with low- or intermediate-risk prostate cancer in Sweden was lower than expected, indicating a selection of healthy men for PSA testing and diagnostic work-up leading to a diagnosis of localized prostate cancer [22]. To overcome the strongest selection mechanisms to the cohort and to treatment, we did not include men diagnosed in the low-risk category or men who received curative treatment. Even so, the estimates of crude survival in this study must be interpreted within the context of the limitation of observational data. In particular, the reported estimates should not be interpreted as a basis for assigning treatment to patients.
If the patients represent a selected group with better survival than that of the general population, relative survival methods will underestimate the probabilities of prostate cancer death. The requirement that patients are exchangeable to the general population is thus an assumption that needs to be carefully evaluated from study to study. In applications where the assumption of exchangeability is in doubt, cause-specific survival, using external information from death certificates, should be considered as a viable alternative to relative survival. Cause-specific survival does, however, require high-quality information from death certificates to accurately determine whether a death should be considered attributable to the cancer in question or not [23]. Recently, systematic re-classification algorithms for cause of death data have been developed in order to improve the quality of cause-specific survival analyses [24]. In practice, the choice between relative survival and cause-specific survival must be based on subject matter knowledge of which of the two assumptions (exchangeability/accurate cause of death classification) is less likely to be violated.
Studies aimed at producing and presenting contemporary information, targeted toward patients and their treating clinicians that are useful to anticipate the outcome after a diagnosis of cancer should be of greater interest. This has, for example, been recognized by the Finnish Cancer Registry, which publishes not only estimates of relative survival but also of the predicted 5-year crude probabilities of death due to cancer and other causes in their bi-annual report [25]. However, Koller and others assessed the frequency of studies published in the scientific literature on the subject of competing risks between the years 2000 and 2010 in 119 core clinical journals. The authors concluded that, even in general clinical journals with the highest impact factors, competing risks were often ignored, that the application of inappropriate statistical methods was a frequent problem and that a better recognition of competing risks in the clinical community is needed [26].
Producing statistics for risk communication requires prediction models that not only include prognostic factors that influence the risk of dying from the disease under study, but also on factors that affects the non-cancer mortality. In the current application, the model was predominantly based on factors associated with the disease (risk group and treatment). While further adjustments for, for example, the general health status of the patients would improve the predictions, such information was unfortunately not available on an individual-level basis in our data. One possibility to overcome this weakness in the future studies would be to utilize Swedish nationwide health registers, such as the Hospital Discharge Register to incorporate general disease burden into both the expected mortality rate data for Sweden and the patient data. Alternatively, a “health status-adjusted” age could be derived and incorporated in the analysis as described previously by Feuer and others in a cause-specific setting within the surveillance, epidemiology, and end results program (SEER) [27].
Presenting data for risk communication in a manner that is understandable for the end-user is crucial to increase the usefulness of cancer patient survival statistics. While, for example, age-standardized estimates of net survival serve as a useful summary measure of patient survival in many situations (e.g., international comparisons of survival, studies of disease etiology, etc.), we argue that the same is not true for estimates of crude survival [28, 29]. In particular, age-standardized estimates of crude survival would be of limited use in studies where an individual-level prediction of prognosis is the goal. To this end, factors that have strong impact on non-cancer mortality, such as age, cannot longer be viewed as a nuisance that merely need to be averaged over (by direct or indirect standardization). In contrast, crude survival typically needs to be estimated for a broad range of covariate patterns and a structured presentation of such results is important. Risk-communication strategies that have shown strong or preliminary evidence for improving patient understanding and decision making include presenting absolute risks rather than relative risks as well as natural frequencies in place of percentages [30]. Crude probabilities of death provide a good example of where the predicted prognosis can be conveyed in terms of natural frequencies. For example, statements like “For 100 patients similar to you, with respect to age and tumor characteristics, 66 patients are expected to be alive 10 years after diagnosis, whereas 5 patients are expected to die from the cancer and 29 from other causes,” should be more informative to patients and clinicians than statistics that assume that cancer is the only possible cause of death. However, communication of cancer patient survival is complicated by fact that it changes with elapsed time since diagnosis. While natural frequencies can easily be reported at a fixed point in time after diagnosis, say within 5 and 10 years, some form of visual representation of how the risk accumulates over time is recommended [31]. We present stacked line graphs representing the cumulative crude probabilities of death, in addition to the 5- and 10-year probabilities quantified in terms of natural frequencies. While the graphs provide a more complete picture, the prognosis after prostate cancer, the numerical summary of the natural frequencies at fixed intervals after diagnosis also serves as an example of how the graphs should be interpreted.
In conclusion, with this article, we are hoping to convey the message that producers and consumers of cancer survival statistics should be aware that different audiences are best served by different types of statistics. In particular, we advocate the use of crude, rather than net, survival probabilities to communicate health risks between clinicians and patients as they provide estimates of probabilities of death in the real world in which the patients live. Even in situations where estimates of crude survival are not available, clinicians should be aware that estimates of net survival are based on a hypothetical world and are thus of limited use to answer prognostic questions.
Statistical appendix
This appendix describes the statistical model used to predict crude probabilities of death from cancer and other causes among patients diagnosed with prostate cancer in Sweden between the years 1996 and 2008.
Period estimation of excess mortality
We fitted flexible parametric survival models with delayed entry, thereby adapting a period approach. The baseline excess mortality rate was modeled using 5 degrees of freedom, df (where the interior knots were placed at the 20, 40, 60, and 80th percentiles of the distribution of the uncensored log survival times whereas the boundary knots were placed at the extremes of the same distribution). The effect of age at diagnosis on excess mortality was modeled continuously using a restricted cubic spline with 4 df (where the knots were placed at the 5, 25, 50, 75, and 95th percentiles of the distribution of the observed ages at diagnosis in our cohort). The number and location of the knots for the splines were chosen subjectively but previous studies have shown how the overall conclusions are insensitive to the configuration of the knots [19, 32]. We also carried out sensitivity analyses that confirmed our results were insensitive to the knot positioning.
In addition to including the main effects of age at diagnosis, risk category, and treatment in the model, we considered possible interaction effects. The effect of treatment was modified by age (p = 0.0025) and by risk category (p = 0.031). The proportional excess hazards assumption was tested by evaluating the statistical significance of interaction terms between each covariate and the time scale. There was evidence of all effects being time-dependent (p (age) <0.001, p (risk category) = 0.0128 and p (treatment) <0.001). The final model consisted of all main effects as well as the statistically significant interaction terms.
The model was estimated using the stpm2 module in the Stata software (StataCorp. 2009 Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Estimation of crude probabilities of death from cancer and other causes
The crude probability of death due to cancer, \( P_{{{\text{cr,}}\;{\text{can}}}} \left( t \right) \), was calculating after having fitted a relative survival model by evaluating
Here, R(u) and λ(u) denote the relative survival and excess mortality, respectively, estimated from the flexible parametric model, whereas \( S^{*} \left( u \right) \) represents the expected survival in the general population. Similarly, the crude probability of death due to other causes than cancer,\( P_{{{\text{cr,}}\,{\text{oth}}}} \left( t \right) \), can be calculated using
where the only difference is that λ(u) has been substituted by \( h^{*} (u) \) denoting the expected mortality rate in the general population. Both \( S^{*} \left( u \right) \) and \( h^{*} (u) \) are assumed known and were retrieved from the human mortality database (www.mortality.org). The above integrals were evaluated numerically, and the variance estimates that were used to estimate confidence intervals were obtained using post-estimation commands to the stpm2 module in Stata.
References
Dickman PW, Adami HO (2006) Interpreting trends in cancer patient survival. J Intern Med 260:103–117
Perme MP, Stare J, Esteve J (2012) On estimation in relative survival. Biometrics 68:113–120
Dignam JJ, Kocherginsky MN (2008) Choice and interpretation of statistical tests used when competing risks are present. J Clin Oncol 26:4027–4034
Putter H, Fiocco M, Geskus RB (2007) Tutorial in biostatistics: competing risks and multi-state models. Stat Med 26:2389–2430
Gail MH (2008) Estimation and interpretation of models of absolute risk from epidemiologic data, including family-based studies. Lifetime Data Anal 14:18–36
Adolfsson J, Garmo H, Varenhorst E, Ahlgren G, Ahlstrand C, Andren O, Bill-Axelsson BO, Damber JE, Hellström K, Hellström M, Holmberg E et al (2007) Clinical characteristics and primary treatment of prostate cancer in Sweden between 1996 and 2005. Scand J Urol Nephrol 2007(41):456–477
National Comprehensive Cancer Network (2010) Clinical practice guidelines in oncology, Prostate cancer, Version 1.2010
D’Amico AV, Denham JW, Crook J, Chen MH, Goldhaber SZ, Lamb DS, Joseph D, Tai KH, Malone S, Ludgate C, Steigler A, Kantoff PW (2007) Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol 25:2420–2425
Tsai HK, D’Amico AV, Sadetsky N, Chen MH, Carroll PR (2007) Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst 99:1516–1524
Van Hemelrijck M, Garmo H, Holmberg L, Ingelsson E, Bratt O, Bill-Axelson A, Lambe M, Stattin P, Adolfsson J (2010) Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol 28:3448–3456
Fang F, Fall K, Mittleman MA, Sparen P, Ye W, Adami HO, Valdimarsdóttir U (2012) Suicide and cardiovascular death after a cancer diagnosis. N Engl J Med 366:1310–1318
Brenner H, Gefeller O, Hakulinen T (2004) Period analysis for ‘up-to-date’ cancer survival data: theory, empirical evaluation, computational realisation and applications. Eur J Cancer 40:326–335
Brenner H, Hakulinen T (2002) Advanced detection of time trends in long-term cancer patient survival: experience from 50 years of cancer registration in Finland. Am J Epidemiol 156:566–577
Dickman PW, Sloggett A, Hills M, Hakulinen T (2004) Regression models for relative survival. Stat Med 23:51–64
Perme MP, Henderson R, Stare J (2009) An approach to estimation in relative survival regression. Biostatistics 10:136–146
Nelson CP, Lambert PC, Squire IB, Jones DR (2007) Flexible parametric models for relative survival, with application in coronary heart disease. Stat Med 26:5486–5498
Lambert PC (2009) Further development of flexible parametric models for survival analysis. Stata J 9:265–290
Durrleman S, Simon R (1989) Flexible regression models with cubic splines. Stat Med 8:551–561
Lambert PC, Dickman PW, Nelson CP, Royston P (2010) Estimating the crude probability of death due to cancer and other causes using relative survival models. Stat Med 29:885–895
Brenner H, Arndt V (2005) Long-term survival rates of patients with prostate cancer in the prostate-specific antigen screening era: population-based estimates for the year 2000 by period analysis. J Clin Oncol 23:441–447
Holmberg L, Robinson D, Sandin F, Bray F, Linklater KM, Klint A, Lambert PC, Adolfsson J, Hamdy FC, Catto J, Møller H (2012) A comparison of prostate cancer survival in England, Norway and Sweden: a population-based study. Cancer Epidemiol 36:e7–e12
Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J (2010) Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst 102:950–958
Hinchliffe SR, Abrams KR, Lambert PC (2012) The impact of under and over-recording of cancer on death certificates in a competing risks analysis: a simulation study. Cancer Epidemiol (Epub ahead of print). doi:10.1016/j.canep.2012.08.012
Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102:1584–1598
Finnish Cancer Registry-Institute for Statistical and Epidemiological Cancer Research (2009) Cancer in Finland 2006 and 2007. Helsinki 2009. Publication No. 76
Koller MT, Raatz H, Steyerberg EW, Wolbers M (2011) Competing risks and the clinical community: irrelevance or ignorance? Stat Med 31:1089–1097
Feuer EJ, Lee M, Mariotto AB, Cronin KA, Scoppa S, Penson DF et al (2012) The Cancer Survival Query System: making survival estimates from the Surveillance, Epidemiology, and End Results program more timely and relevant for recently diagnosed patients. Cancer 118:5652–5662
Brenner H, Hakulinen T (2003) On crude and age-adjusted relative survival rates. J Clin Epidemiol 56:1185–1191
Pokhrel A, Hakulinen T (2008) How to interpret the relative survival ratios of cancer patients. Eur J Cancer 44:2661–2667
Fagerlin A, Zikmund-Fisher BJ, Ubel PA (2011) Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst 103:1436–1443
Lipkus IM (2007) Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Mak 27:696–713
Lambert PC, Royston P (2009) Further development of flexible parametric models for survival analysis. Stata J 9:265–290
Acknowledgments
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden steering group: Pär Stattin chair, Anders Widmark, Stefan Carlsson, Magnus Törnblom, Jan Adolfsson, Anna Bill-Axelson, Ove Andrén, David Robinson, Bill Pettersson, Jonas Hugosson, Jan-Erik Damber, Ola Bratt, Göran Ahlgren, Lars Egevad, and Mats Lambe. This work was supported by the Swedish Cancer Society (grant number CAN 2010/676).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eloranta, S., Adolfsson, J., Lambert, P.C. et al. How can we make cancer survival statistics more useful for patients and clinicians: An illustration using localized prostate cancer in Sweden. Cancer Causes Control 24, 505–515 (2013). https://doi.org/10.1007/s10552-012-0141-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-012-0141-5