Abstract
Purpose
Gestational diabetes mellitus (GDM), a state of glucose intolerance associated with pregnancy, is increasing in prevalence. Data regarding the cancer risk associated with GDM are sparse and limited to cancers of the breast and pancreas. This study was conducted to examine the risk of incident overall and site-specific malignancies associated with prior GDM in a historical cohort of women in a large health maintenance organization in Israel.
Methods
All pregnant women aged 15–50 years who underwent 50-g glucose challenge tests between 13 March 1995 and 27 May 2009, without history of malignancy, diabetes, and infertility, were included. Clinical and demographic parameters at index date including age, socioeconomic level, BMI, and parity were collected. Diagnosis of gestational diabetes was based on the 100-g oral glucose tolerance test using Carpenter and Coustan criteria. Cancer diagnoses were obtained from the Israel Cancer Register through linkage data.
Results
Among the 185,315 women who had undergone glucose challenge during the study period, 11,264 (6.1%) were diagnosed with GDM. During a total follow-up period of 1.05 million person-years (mean = 5.19 ± 3.9, median = 4.3), 2,034 incident cases of cancer were identified. GDM was associated with a hazard ratio (HR) of 7.06 (95% CI: 1.69–29.45) for pancreatic cancer (nine cases) and a HR of 1.70 (95% CI: 0.97–2.99) for hematological malignancies (177 cases). The association between GDM and hematological malignancies was limited to women with 5 or more years of follow-up (HR = 4.53; 95% CI: 1.81–11.31).
Conclusion
GDM is associated with an increased risk of pancreatic cancer and hematologic malignancies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of carbohydrate intolerance with onset or first recognition during pregnancy [1]. Pregnancy is normally accompanied by progressive insulin resistance due to a combination of increased maternal adiposity and the insulin-desensitizing effects of placental hormones [2]. While most women are able to compensate through increased insulin secretion, women with GDM become hyperglycemic, possibly exposing various chronic metabolic abnormalities. GDM complicates 2–5% of pregnancies in the United States [3] with prevalence rates varying by ethnic group [3, 4]. Recent publications have reported an increase in prevalence [5], which may be attributed to factors such as an increase in maternal age, epidemic obesity, and a decrease in daily physical activity [6].
GDM is associated with adverse pregnancy outcomes such as fetal macrosomia and an increased Cesarean section rate [7]. In addition, women diagnosed with GDM and their offspring have an increased lifetime risk of developing type 2 diabetes mellitus (T2DM) [8]. Recently, we calculated the 10-year risk of diabetes mellitus (DM) among Israeli women with previous GDM to be between 10.1 and 51.5%, depending on the level of glucose intolerance [9].
Previous studies have demonstrated an association between states of glucose intolerance [10–12] and of overt DM [13, 14] and cancer. Positive associations with malignancies of the pancreas [12–15], colon [12–14], and liver [12, 13, 16] are well established. Among women, strong evidence links endometrial cancer [14, 16, 17] with DM, while associations with breast [12, 17–19] and ovarian [14, 20] cancer have not been fully established. In our previous study on patients with DM, we found an increased cancer risk among women, but not among men [14]. It has been hypothesized that similar to their risk of future DM, women with GDM are also susceptible to the associated increased risk of malignancy [21].
Few studies have investigated the relationship between GDM and cancer, indicating associations with breast [22] and pancreatic [23] cancers. However, findings have been inconsistent [24], possibly due to methodological limitations such as the use of self-reported information on GDM status and relatively small study populations. The main goal of the current historical cohort study was to evaluate the risk of malignancy among women diagnosed with GDM in a large health maintenance organization (HMO) in Israel using its automated clinical databases.
Methods
Settings
This historical cohort study was conducted in Maccabi Healthcare Services (MHS), the second largest HMO in Israel, insuring 1.9 million members countrywide. In 2008, MHS covered 24.6% of the total population and 26.1% of the 1.56 million Israeli women aged 15–45 years. The annual number of births in MHS (n = 39,291) comprised 24.1% of all births in Israel [25]. According to the Israeli National Health Insurance Act, MHS may not bar any citizen who wishes to join it, and therefore, every section in the Israeli population is represented in MHS. Nonetheless, MHS coverage in the non-Jewish populations is substantially lower compared with the Jewish one.
Information on all members’ interactions (i.e., visits to outpatient clinics, hospitalizations, laboratory tests, and dispensed medications) is downloaded daily to a central computerized database. In addition, MHS has developed and validated computerized registries of patients suffering from major chronic diseases, including ischemic heart disease, hypertension, and DM [26].
Study population
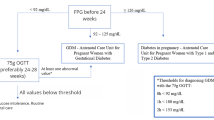
As described in our previous publication [9], we collected data on all pregnant women aged 15–50 years screened for GDM with a 50-g glucose challenge test (GCT) performed in MHS between 13 March 1995 and 27 May 2009. The day of first GCT in the most recent pregnancy was set as the index date. We excluded patients with indication of cancer, DM, and infertility treatments prior to index date.
In accordance with American Diabetes Association guidelines [27], in MHS, all pregnant women are screened for GDM with a 50-g GCT between 24 and 28 weeks of gestation. Women with a serum glucose concentration >140 mg/dL (7.8 mmol/L) 1 h after GCT are referred to a diagnostic 100-g oral glucose tolerance test (OGTT). GDM was defined according to the Carpenter and Coustan modification of the National Diabetes Data Group criteria conversion method [28], based on the presence of two or more of the following values in the 100-g OGTT: fasting serum glucose concentration exceeding 95 mg/dL (5.3 mmol/L), 1-h serum glucose concentration of 180 mg/dL (10 mmol/L) or above, 2-h serum glucose concentration exceeding 155 mg/dL (8.6 mmol/L), and 3-h serum glucose concentration exceeding 140 mg/dL (7.8 mmol/L). All other study subjects were defined as non-GDM.
Data collection and outcomes
Data on cancer occurrence during the study follow-up period were obtained from the Israel National Cancer Register (ICR). Established in 1960, the ICR collects information on invasive and in situ cancer cases diagnosed in medical institutions throughout country with a completeness of above 93.5% for solid tumors and approximately 90% for non-solid tumors [29]. All cancer cases were classified according to the third edition of the International classification of Diseases (ICD-O) and are based on histological reports’ hospital discharge forms, oncology reports, and death certificates. Approximately 92% of registered cases have a valid histology or cytology report. The study population and the ICR were cross-linked by the members’ individual unique identifying number given to all newborns or immigrants to Israel, names, sex, and date of birth. Due to privacy issues, data from the ICR were unavailable for patients who have left MHS (9.5% of the study population).
We examined the MHS database records of women in the study cohort from index date to death, leaving MHS, date of cancer diagnosis, or 1 June 2009, whichever occurred earlier. The MHS database was used to collect a range of clinical and demographic parameters at index date including age, socioeconomic level, body mass index (BMI), parity, and number of general practitioner (GP) visits. BMI was categorized into normal weight (<25 kg/m2), overweight (25–29 kg/m2), and obese (≥30 kg/m2) using the World Health Organization (WHO) classification [30]. Due to the large proportion of women with missing data on BMI, we also included missing BMI as a separate category. Socioeconomic level was categorized into quartiles according to the poverty index of the member’s enumeration area as defined by the 1995 national census based on several parameters including household income, educational qualifications, crowding, material conditions, and car ownership [31]. The study was approved by the MHS institutional review board.
Statistical analyses
All statistical analyses were conducted using a standard statistical package (SPSS 15.0, SPSS, Chicago, IL). Chi-squared test for categorical variables and analysis of variance for continuous variables were performed to determine significant differences in baseline characteristics between study groups. Cox proportional hazards regression model [32] was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) of overall and site-specific cancer incidence. The adequacy of the proportional hazards assumption was tested graphically by the examination of log minus log plots.
Results
During the follow-up period, a total of 185,315 subjects underwent GCT testing of which 11,264 were subsequently diagnosed with GDM. Women with GDM were significantly (p < 0.001) more likely to be older (32.7 vs. 30.6 years), overweight, or obese (36.8% vs. 17.1%) and visit their GP more frequently (Table 1). Through the follow-up period, 125 (0.07%) subjects died and 17,543 left MHS (9.5% of the total population or 5.7% of the total person-years of follow-up).
During a total follow-up period of 1.05 million person-years, 2,034 incident cases of cancer were identified. Follow-up ranged from 0 to 14.2 years, with a 5.19-year average and 4.31-year median. During the first 6 months of follow-up, only eight cancers (three lymphomas, one gastrointestinal, one uterine, one skin, and two other) were diagnosed among GDM cases. Breast cancer was the most common diagnosis comprising 31.3% of all cancers, followed by cancers of the genitalia (25.3%) and thyroid (10.9%).
The overall cancer risks among GDM and non-GDM women were 1.93 and 2.13 per 1,000 person-years, respectively. Table 2 shows the hazard ratios for cancer in women with GDM compared to non-GDM women. Women with GDM had an age-adjusted HR of 2.23 (95% CI: 1.21–4.13) for cancers of digestive organs (p = 0.013). GDM was not associated with breast cancer (HR 0.85, 95% CI: 0.62–1.16). Adjusting for socioeconomic level, smoking status, BMI, and parity did not appreciably alter these observations. Table 3 shows stratification of cancers of the digestive tract by specific site. Three cases of pancreatic cancer were identified among women with GDM compared with 6 cases among non-GDM women affording an age-adjusted HR of 6.03 (95% CI: 1.48–24.64). This risk climbed to 7.06 (95% CI: 1.69–29.45) after full adjustment. Pancreatic cancer was diagnosed an average 11 months (0.9, SD 0.62) after pregnancy complicated with GDM and 4 years (4.1, SD 2.55) after uncomplicated pregnancies. Latency analysis was impractical due to the limited number of cases. Liver cancer occurred in three patients, all of them in the non-GDM population.
GDM was associated with a fully adjusted HR of 1.70 (95% CI: 0.97–2.99) for hematological cancers (Table 2) with a borderline significance (p = 0.07). Three of the 14 patients with GDM (21%) who developed hematologic neoplasms were diagnosed with DM prior to malignancy, compared to only 2 such cases among 163 patients with hematologic neoplasms and no prior GDM (0.01%). In cancer subtype analysis, GDM was associated with a fully adjusted HR of 1.58 (95% CI: 0.75–3.33) and 2.20 (95% CI: 0.46–10.81) for non-Hodgkin’s lymphoma (NHL) and acute myeloid leukemia (AML), respectively. The cumulative risk of hematologic neoplasms among GDM and non-GDM patients diverged approximately after 5 years of follow-up (Fig. 1). In a stratified analysis of subjects with a follow-up time of ≥5 years, patients with GDM had a HR of 4.53 (95% CI: 1.81–11.31) to develop hematologic malignancies compared to non-GDM women (Table 4), specifically NHL and Hodgkin’s lymphoma. Among subjects with a shorter follow-up period, no significant differences were observed between GDM and non-GDM women.
Discussion
The present historical cohort study indicates that history of gestational diabetes may be strongly associated with an increased risk of pancreatic cancer. In addition, we found a 4.5-fold risk of hematologic neoplasms in women with more than 5 years of follow-up after GDM diagnosis.
The relationship between GDM and pancreatic cancer has been rarely investigated. The largest investigation to date was an Israeli cohort, conducted by Perrin and colleagues, with 28–40 years of follow-up that documented five incident cases of pancreatic cancer in women with GDM history [23]. The investigators calculated an adjusted RR of 7.1, which is identical to our risk estimate. This study, similar to the current study, used a population-based historical cohort design and identified a similarly high and statically significant relative risk despite a relatively small number of pancreatic cancer cases. However, while Perrin et al. observed 14–35 years between GDM and pancreatic cancer incidence, we witnessed a much shorter 5–19 month time range. This disparity further fuels the controversy on whether DM is causally associated with pancreatic cancer or is a consequence of tumor growth. Hereof, a large meta-analysis [33] reported a significant 50% increase in risk of pancreatic cancer associated with DM diagnosed 5 or more years earlier refuting the reverse causality bias. Interestingly, in a recent study in a similar population, we found an elevated risk of pancreatic cancer in women with a history of DM (HR 1.89), but not in men [14]. Insulin-like growth factor (IGF)-1 is elevated in patients with pancreatic cancer [34] and upregulated in human pancreatic cancer tissues but is unexpressed in surrounding non-cancerous tissues [35]. Thus, insulin resistance and the subsequent increased levels of insulin and IGF may promote cell proliferation, inhibit apoptosis, and enhance angiogenesis, leading to accelerated tumor development and progression [36].
To the best of our knowledge, this is the first study to demonstrate an association between GDM and hematologic malignancies, particularly NHL and AML. The potential relationship between NHL and diabetes was first suggested in the mid-1960s [37]. Since GDM in our cohort was strongly associated with DM [9], investigating the association between DM and NHL can be informative. In their comprehensive meta-analysis on the association between NHL and DM, Mitri and colleagues [38] summarized ten case–control reports and five prospective cohorts which included more than 160,000 diabetic patients and 337 histologically confirmed incident cases of NHL over a mean follow-up period of 7–24 years. This meta-analysis indicated that women with DM had a pooled RR of 1.38 (95% CI: 1.06–1.80) to develop NHL. Similar results were calculated in a second meta-analysis of five studies in which the pooled RR of NHL in female patients with DM was 1.60 (95% CI: 1.15–2.22) [39]. The results of both meta-analyses are well within the 95% confidence interval limits of the HR calculated in our study for hematologic malignancies in patients with GDM and corroborate our findings. Similar to other researchers who found an increasing risk of NHL with increasing duration of diabetes [40, 41], our observation was limited to women with at least 5 years of follow-up. Notwithstanding, several studies suggested that the risk of NHL is particularly high among women at the earlier stages of diabetes. For example, Cerhan et al. reported a significantly elevated risk of NHL (RR = 3.43) in women who were on oral anti-diabetic medication, but not among women on insulin [42].
Previous studies of the association between GDM and breast cancer have produced inconsistent results. Similar to our study, no association between GDM and breast cancer was found in a US population-based case–control study of 1,239 women diagnosed with breast cancer and 1,166 controls [43]. A Scottish cohort study of 753 pregnant women who underwent glucose testing at 13 weeks of pregnancy calculated a positive association between higher glucose levels and an increased risk of breast cancer diagnosed up to 20 years later [21]. An elevated risk was also observed among Israeli women with a history of GDM diagnosed with breast cancer at age 50 and above [24]. In contrast, a population-based case–control study of women first diagnosed before 35 years of age indicated an inverse association with breast cancer (OR: 0.54, 95% CI: 0.37–0.79) [44]. The conflicting results from previous investigations demonstrate the important effect of age at GDM diagnosis on future breast cancer risk. In our analysis, stratification by age revealed a reduced breast cancer risk among women aged below 35 years (RR = 0.73; 95% CI: 0.44–1.21) and an increased risk among women diagnosed with GDM at older age (RR = 1.31; 95% CI: 0.84-2.04), but neither reached statistical significance. One potential confounder that may explain lower breast cancer risk in younger women with GDM is irregular cycles, which were found to associate both with higher risk of GDM [45] and possibly with a lower risk of breast cancer [46].
The present study is one of the largest undertaken to date on GDM and subsequent cancer occurrence with respect to follow-up period and size of cohort. Other study strengths include the historical cohort design, the use of administrative databases to avoid a differential recall bias, and the systematic and comprehensive collection of personal data, which reduces the possibility of bias due to study outcomes. Additionally, unlike most previous studies where the history of GDM or DM was ascertained by self-report, GDM status in our study was based on actual OGTT results during pregnancy.
BMI was missing for 48.6% and 62.6% of the GDM and non-GDM women, respectively. Among women with available BMI measurements, there was no significant association between BMI level and cancer of digestive organs (p = 0.805) or hematological malignancies (p = 0.305). Similarly, previous studies have shown that among women, unlike in men, there is no [47] or little [48] association between body mass index and the aforementioned cancers. Therefore, it is unlikely that missing BMI measurements significantly biased our results.
Inherent to observational studies, our research is subject to several biases. A differential detection bias is of particular concern regarding indolent lymphomas with little or no clinical symptoms. However, our analysis showed no elevated risk of breast or cervical cancer, which would have been more sensitive for such bias. Nearly 9.5% of the population cohort left MHS during the follow-up period. The mean follow-up time of these subjects was 3.3 years, accumulating to 39,218 lost person-years. The expected number of cases during this period is 88, or 4% of the total number of cancers in the study. Although lost-to-follow-up rates were higher among non-GDM women, the relatively low number of cases is unlikely to introduce a substantial selection bias.
In conclusion, the findings of the present study indicate that even after a relatively short follow-up period, women with a history of GDM are more likely to be diagnosed with pancreatic cancer and hematologic neoplasms, particularly NHL. GDM, as an early and transient biomarker for metabolic dysfunction, offers an opportunity for primary interventions that may modify the risk of future malignancy. Although the annual risk of pancreatic cancer and NHL in women are relatively low (10.2 and 15.7 per 100,000) [49], they are among the leading causes of death from cancer [50] and thus require further attention and research, particularly in light of the increasing prevalence of gestational diabetes [5, 6].
Abbreviations
- GDM:
-
Gestational diabetes mellitus
- DM:
-
Diabetes mellitus
- GCT:
-
Glucose challenge test
- OGTT:
-
Oral glucose tolerance test
- HMO:
-
Health maintenance organization
- MHS:
-
Maccabi healthcare services
- NHL:
-
Non-hodgkin’s lymphoma
- AML:
-
Acute myeloid leukemia
References
Metzger BE, Coustan DR (1998) Summary and recommendations of the fourth international workshop-conference on gestational diabetes mellitus. The organizing committee. Diabetes Care 21(Suppl 2):B161–B167
Buchanan TA, Xiang A, Kjos SL, Watanabe R (2007) What is gestational diabetes? Diabetes Care 30(Suppl 2):S105–S111
Ferrara A, Hedderson MM, Quesenberry CP, Selby JV (2002) Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose thresholds. Diabetes Care 25(9):1625–1630
Dornhorst A, Paterson CM, Nicholls JS, Wadsworth J, Chiu DC, Elkeles RS, Johnston DG, Beard RW (1992) High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med 9(9):820–825
Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS (2005) Increasing prevalence of gestational diabetes (GDM) over time and by birth cohort: Kaiser Permanente of colorado GDM screening program. Diabetes Care 28(3):579–584
Ferrara A (2007) Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30(Suppl 2):S141–S146
Kjos SL, Buchanan TA (1999) Gestational diabetes mellitus. N Engl J Med 341(23):1749–1756
Metzger BE (2007) Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 50(4):972–979
Chodick G, Elchalal U, Sella T, Heymann A, Porath A, Kokia E, Shalev V (2010) The risk of overt diabetes mellitus among women with gestational diabetes: a population-based study. Diabet Med 27(7):779–785
Saydah SH, Loria CM, Eberhardt MS, Brancati FL (2003) Abnormal glucose tolerance and the risk of cancer death in the United States. Am J Epidemiol 157(12):1092–1100
Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293(2):194–202
Zhou XH, Qiao Q, Zethelius B, Pyörälä K, Söderberg S, Pajak A, Stehouwer CD, Heine RJ, Jousilahti P, Ruotolo G, Nilsson PM, Calori G, Tuomilehto J, The DECODE Study Group (2010) Diabetes, prediabetes and cancer mortality. Diabetologia 53(9):1867–1876
Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ (2004) Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 159(12):1160–1167
Chodick G, Heymann A, Rosenmann L, Green M, Flash S, Porath A, Kokia E, Shalev V (2010) Diabetes and risk of incident cancer: a large population-based cohort study in Israel. Cancer Causes Control 21(6):879–887
Everhart J, Wright D (1995) Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273(20):1605–1609
Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, Persson I (1991) Cancer risk in patients with diabetes mellitus. Cancer Causes Control 2(5):307–314
La Vecchia C, Negri E, Franceschi S, D’Avanzo B, Boyle P (1994) A case–control study of diabetes mellitus and cancer risk. Br J Cancer 70(5):950–953
Xue F, Michels KB (2007) Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr 86(3):S823–S835
Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, Ram M, Freudenheim JL, Sieri S, Trevisan M, Berrino F (2002) Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomark Prev 11(11):1361–1368
Adler AI, Weiss NS, Kamb ML, Lyon JL (1996) Is diabetes mellitus a risk factor for ovarian cancer? A case–control study in Utah and Washington (United States). Cancer Causes Control 7(4):475–478
Dawson S (2004) Long-term risk of malignant neoplasm associated with gestational glucose intolerance. Cancer 100(1):149–155
Perrin M, Terry M, Kleinhaus K, Yanetz R, Tiram E, Calderon-Margalit R, Friedlander Y, Paltiel O, Harlap S (2008) Gestational diabetes and the risk of breast cancer among women in the Jerusalem Perinatal Study. Breast Cancer Res Treat 108(1):129–135
Perrin MC, Terry MB, Kleinhaus K, Deutsch L, Yanetz R, Tiram E, Calderon R, Friedlander Y, Paltiel O, Harlap S (2007) Gestational diabetes as a risk factor for pancreatic cancer: a prospective cohort study. BMC Med 5:25. doi:1741-7015-5-25
Nechuta S, Paneth N, Velie E (2010) Pregnancy characteristics and maternal breast cancer risk: a review of the epidemiologic literature. Cancer Causes Control 21(7):967–989
Bendelac J (2009) Membership in sick funds 2008. National Insurance Institute, Jerusalem
Chodick G, Heymann A, Shalev V, Kookia E (2003) The epidemiology of diabetes in a large Israeli HMO. Eur J Epidemiol 18:1143–1146
American Diabetes Association (2003) Gestational diabetes mellitus. Diabetes Care 26(Suppl 1):S103–S105
Carpenter M, Coustan D (1982) Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 144:768–773
Israel Center for Disease Control (2003) Investigation into the completeness for the Israel cancer registry. Israel Center for Disease Control Publication 230 (In Hebrew), Jerusalem
World Health Organization (1998) Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity, 3–5 June 1997. World Health Organization, Geneva, Switzerland
Israel Central Bureau of Statistics (1998) 1995 Census of population and housing, Jerusalem
Cox DR (1972) Regression models and life tables (with discussion). J R Stat Soc B 32:187–220
Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M (2005) Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 92(11):2076–2083
Karna E, Surazynski A, Orlowski K, Laszkiewicz J, Puchalski Z, Nawrat P, Palka J (2002) Serum and tissue level of insulin-like growth factor-I (IGF-I) and IGF-I binding proteins as an index of pancreatitis and pancreatic cancer. Int J Exp Pathol 83(5):239–245
Bergmann U, Funatomi H, Yokoyama M, Beger HG, Korc M (1995) Insulin-like growth factor I overexpression in human pancreatic cancer: evidence for autocrine and paracrine roles. Cancer Res 55(10):2007–2011
Li D, Abbruzzese JL (2010) New strategies in pancreatic cancer: emerging epidemiologic and therapeutic concepts. Clin Cancer Res 16(17):4313–4318
Lisker S, Brody J, Beizer L (1966) Abnormal carbohydrate metabolism in patients with malignant blood dyscrasias. Am J Med Sci 252:282–288
Mitri J, Castillo J, Pittas A (2008) Diabetes and risk of non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care 31:2391–2397
PJ ChaoC (2008) Type 2 diabetes mellitus and risk of non-Hodgkin lymphoma: a systematic review and meta-analysis. Am J Epidemiol 168(5):471–480
Cerhan J, Wallace R, Folsom A, Potter J, Sellers T, Zheng W, Lutz C (1997) Medical history risk factors for non-Hodgkin’s lymphoma in older women. J Natl Cancer Inst 89(4):314–318
Hjalgrim H, Frisch M, Ekbom A, Kyvik KO, Melbye M, Green A (1997) Cancer and diabetes—a follow-up study of two population-based cohorts of diabetic patients. J Intern Med 241(6):471–475
Cerhan JR, Bernstein L, Severson RK, Davis S, Colt JS, Blair A, Hartge P (2005) Anthropometrics, physical activity, related medical conditions, and the risk of non-hodgkin lymphoma. Cancer Causes Control 16(10):1203–1214
Troisi R, Weiss H, Hoover R (1998) Pregnancy characteristics and maternal risk of breast cancer. Epidemiology 9(6):641–647
Rollison DE, Giuliano AR, Sellers TA, Laronga C, Sweeney C, Risendal B, Baumgartner KB, Byers T, Slattery ML (2008) Population-based case–control study of diabetes and breast cancer risk in Hispanic and non-Hispanic White women living in US southwestern states. Am J Epidemiol 167(4):447–456
Haver M, Locksmith G, Emmet E (2003) Irregular menses: an independent risk factor for gestational diabetes mellitus. Am J Obstet Gynecol 188(5):1189–1191
Clavel-Chapelon F, E3N Group (2002) Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control 13(9):831–838
Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, Giles GG (2010) Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomark Prev 19(11):2978–2986
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578
U.S. Cancer Statistics Working Group (2010) United States cancer statistics: 1999–2006 incidence and mortality web-based report. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute, Atlanta, US
American Cancer Society (2010) Cancer facts and figures 2010. American Cancer Society, Atlanta, GA
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tal Sella and Gabriel Chodick equally contributed to the study and manuscript.
Rights and permissions
About this article
Cite this article
Sella, T., Chodick, G., Barchana, M. et al. Gestational diabetes and risk of incident primary cancer: a large historical cohort study in Israel. Cancer Causes Control 22, 1513–1520 (2011). https://doi.org/10.1007/s10552-011-9825-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-011-9825-5