Abstract
A meta-analysis of case–control studies on coffee consumption and colorectal cancer risk was conducted. Twenty-four eligible studies published before May 2010 were identified, including a total of 14,846 cases of colorectal, colon or rectal cancer. Compared to non/occasional drinkers, the odds ratios (OR) for drinkers were 0.83 (95% CI 0.73–0.95) for colorectal, 0.93 (95% CI 0.81–1.07) for colon and 0.98 (95% CI 0.85–1.13) for rectal cancer, with significant heterogeneity among studies; the corresponding ORs for the increment of 1 cup/day were 0.94 (95% CI 0.91–0.98), 0.95 (95% CI 0.92–0.98), and 0.97 (95% CI 0.95–0.99). For the highest coffee drinkers, the ORs were 0.70 (95% CI 0.60–0.81) for colorectal cancer, 0.75 (95% CI 0.64–0.88) for colon cancer and 0.87 (95% CI 0.75–1.00) for rectal cancer, when compared to non/low drinkers. The results of this meta-analysis of case–control studies suggest a moderate favorable effect of coffee consumption on colorectal cancer risk. The reduced risk was consistent across study design (hospital vs. population based), geographic area, and various confounding factors considered. It may reflect a real protection but also partly or largely be due to reverse causation, i.e. decreased coffee consumption among cases following the onset of bowel symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After tea, coffee is the most widespread beverage in the world [1]. Colorectal cancer is the fourth most common cancer in the world in men and the third in women [2]. Given the widespread consumption of coffee and the high incidence of colorectal cancer, any relation between them would have appreciable public health relevance.
In 1991, an IARC Working Group Monograph concluded that, in humans, “there is some evidence of an inverse relationship between coffee drinking and cancer of the large bowel” [1] and the inverse relation has further been confirmed [1, 3]. A meta-analysis based on data published up to June 1997 found an overall relative risk (RR) of 0.76 for high versus low coffee drinkers (95% confidence interval CI 0.66–0.89) [4]. This finding, however, came mostly from case–control studies (RR = 0.72, 95% CI 0.61–0.84), including 5,261 cases, whereas no material association was observed in cohort studies (RR = 0.97, 95% CI 0.73–1.29), at that time including only 931 cases. A subsequent meta-analysis of cohort studies only, published in 2009 and based on 12 studies and 5,403 cases, found a RR of 0.91 for high versus low coffee drinkers, of borderline significance (95% CI 0.81–1.02), with lower RR in studies that controlled for tobacco and alcohol consumption [5]. A pooled analysis of 13 prospective studies found a RR of 1.07 (95% CI: 0.89–1.30) for more than 6 cups of coffee per day [6]. A Chinese prospective study, published after the meta- and pooled analysis, found a protective effect of coffee only in ever smokers [7]. The apparent stronger inverse association between coffee intake and colorectal cancer risk found in case–control than in cohort studies might depend on some bias in case–control studies. However, the consistent results in different populations and settings are against any such bias. Likewise, major publication bias is unlikely, as negative results have also attracted interest over the last few years [3].
We combined all available data from case–control studies on coffee drinking and colorectal cancer risk in a systematic meta-analysis [8–31], as the recent meta-analysis included only the results of prospective studies [5], and several case–control studies on coffee drinking and colorectal cancer have been published since the 1997 meta-analysis [4].
Materials and methods
Identification, selection, and classification of studies
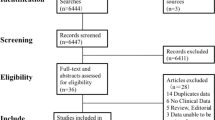
We performed a MEDLINE search in PubMed of articles published from 1966 to May 2010, using the string “(coffee OR caffeine OR diet OR beverages OR drinks) AND (colon OR rectum OR rectal OR colorectal) AND (cancer OR neoplasm) AND risk”, following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [32], and limiting the search to the English language. The PubMed search identified 2,267 articles. From these articles, two of the authors, F.T. (biostatistician) and A.T. (epidemiologist), independently selected articles reporting data on the association of coffee intake with colorectal, colon, and/or rectal cancer risk, from case–control studies only. They also searched in the reference lists of the articles retrieved to obtain other pertinent publications. Abstracts and unpublished results were not included, but no studies were excluded a priori for weakness of design or data quality. No quality score was assigned. We considered 35 articles, and then we applied the following inclusion criteria: (1) a quantitative estimate of the relation [33–37]; (2) at least one of the following: the 95% CI, or standard error, or the distribution of cases and controls in coffee consumption categories, or the p-value for the difference of the OR from unity [38–40]. Studies were also excluded if they reported data for caffeine rather than coffee [41], or if they were based on data updated later, or if part of pooled analyses [42, 43]. Finally, our meta-analysis included 24 case–control studies on coffee intake and risk of colorectal cancer.
Full papers were read and for each paper we registered: first author’s last name, year of publication, country where the study was conducted, number of cases, type of controls (population/hospital), odds ratios (OR) and 95% CIs for the highest level of consumption, the highest versus the lowest amount of coffee to which the OR referred, and the adjustment factors. Discrepancies were discussed and adjudicated.
In most studies, information on coffee intake was collected as cups/day. We converted in cups/day the cups/week reported in three studies [17, 26, 31], the cups/month of one study [14] and grams/day (80 g approximately equal to one cup) in one study [25].
If a study reported more than one OR, we used the multivariate one adjusted for the larger number of available potential confounding factors. For a few studies reporting the adjusted ORs and not the corresponding 95% CIs [8–11, 14], we used the distribution of cases and controls to calculate the standard errors of the corresponding crude ORs, and then the approximate CIs for the reported adjusted ORs [44]. To obtain colorectal cancer risk estimates, we included only studies reporting both colon (or its anatomical subsites) and rectal cancer estimates, together or separately, and excluded studies with information on only one anatomical site.
The 24 identified studies included a total of 14,846 cases of colorectal, or colon, or rectal cancer. Their main characteristics are described in Table 1. Eighteen studies gave information on colorectal or both colon and rectal cancer separately, for a total of 10,952 cases. Fifteen studies gave information on colon cancer, for a total of 8,283 cases, and 13 on rectal cancer, for a total of 5,078 cases. Of the 24 studies, 7 were conducted in North America (6 studies from the USA [8, 13, 16, 21, 27, 31] and 1 from Canada [28], for a total of 7,021 cases), 3 in Northern Europe (1 from Norway [9], 1 from Denmark [18] and 1 from Sweden [19], for a total of 787 cases), 7 in Southern Europe (2 from Italy [20, 22], 1 from Switzerland [26], 2 from France [10, 25], 1 from former Yugoslavia [11] and 1 from Spain [14], for a total of 4,826 cases), 6 studies came from Asia (5 from Japan [15, 17, 23, 29, 30] and 1 from Singapore/China [12], for a total of 2,020 cases), and 1 from South America (Argentina [24], 190 cases). Ten of the 24 studies (5,371 cases) reported data for men [13, 16, 21, 22, 25, 27–31] and 9 (4,372 cases) for women [13, 16, 21, 22, 27–31] separately.
Statistical analysis
To avoid the exclusion of the studies [8, 23, 28] whose reference category included also drinkers of less than one cup/day (occasional drinkers), our reference category included non-drinkers and occasional drinkers. To compute the summary OR for drinkers versus non/occasional drinkers, when one study reported more than one category of coffee consumption, we first calculated the study-specific pooled estimate for drinkers using the fixed-effects model. We also computed the study-specific pooled estimates to compute the overall colorectal, colon and rectal cancer risk in all subjects in studies where risk estimates in men and women were reported only separately.
To assess the relation of colorectal cancer risk for an increment of one cup of coffee per day, when such estimate was not reported in the original study, we estimated the study-specific OR by relating the natural logarithm of the OR to the corresponding mean value of coffee intake across exposure categories, using the method proposed by Greenland and Longnecker [45, 46], taking into account the fact that estimates of risk for subsequent levels of intake are correlated. Since the highest category of consumption was usually open, we considered it of the same amplitude as the previous category. When the number of subjects in each level was not available, we calculated the dose-risk slopes using the variance-weighted least squares regression. Then we pooled the study-specific estimates by a random-effects model, to obtain the summary OR [47]. To compare the results of our meta-analysis with those of a meta-analysis of prospective cohort studies [5], we also considered the ORs for the highest versus the lowest categories of consumption in each study. In these analyses, the reference category was non/low drinkers, which included non-drinkers or the lowest amount of drinking as classified in each study, independently of the absolute number of drinks/day, and excluding studies where the amount of drinking was not split into two or more categories [24, 29, 30]. Summary measures were calculated using random-effects model that considers both within-study and between-study variations [47]. We also explored the differences by type of controls (population or hospital), sex and geographic regions (North America, Northern Europe, Southern Europe or Asia), given the various sources of heterogeneity and the international differences in coffee consumption.
We presented combined estimates using forest plots. In these graphs, a square was plotted for each study whose centre projection on the underlying scale corresponds to the study-specific OR. The area of the square is proportional to the inverse of the variance of the natural logarithm of the OR, thus giving a measure of the amount of information available from each estimate. A diamond was used to plot the summary OR, the centre of which represents the OR and the extremes show the 95% CIs. We assessed the statistical heterogeneity among studies using the χ2 test and quantified the inconsistency using the I2 statistic [48]; results were defined as heterogeneous for p values <0.10 [49]. There was no evidence of publication bias overall as tested using funnel plots [50] and Begg’s and Egger’s tests [51]. All the analyses were performed using the Stata® statistical package (version 10; StataCorp, College Station, TX, USA).
Results
Table 2 shows the summary ORs of colorectal, colon and rectal cancer for coffee drinkers versus non/occasional drinkers. The summary ORs were 0.83 (based on 13 studies and 9,568 cases) for colorectal (p for heterogeneity <0.001; I 2 = 80.0%), 0.93 (based on 11 studies and 7,537 cases) for colon (p for heterogeneity <0.001; I 2 = 81.7%), and 0.98 (based on 10 studies and 4,594 cases) for rectal cancer (p for heterogeneity <0.001; I 2 = 71.2%). Table 2 also shows the summary ORs for an increment of one cup of coffee/day, which were 0.94 (95% CI 0.91–0.98) for colorectal (p for heterogeneity <0.001; I 2 = 69.3%), 0.95 (95% CI 0.92–0.98) for colon (p for heterogeneity = 0.002; I 2 = 60.8%), and 0.97 (95% CI 0.95–0.99) for rectal cancer (p for heterogeneity = 0.347; I 2 = 10.2%).
Figure 1 shows the ORs for colorectal, colon, and rectal cancer for each case–control study and the summary OR for the highest category of coffee consumption compared to the lowest one (including non-drinkers and labeled non/low drinkers) as classified in each case–control study, independently of the number of drinks/day, and including only studies splitting coffee drinkers of ≥1 drink/day at least into two categories. Significant heterogeneity was found between the studies included for the summary estimates of OR for colorectal and colon, but not rectal cancer. The summary ORs were 0.70 (95% CI 0.60–0.81) for colorectal cancer (15 studies, Fig. 1a), 0.75 (95% CI 0.64–0.88) for colon cancer (14 studies, Fig. 1b), and 0.87 (95% CI 0.75–1.00) for rectal cancer (12 studies, Fig. 1c).
The same categories of coffee consumption were used in the analyses in strata of geographic area, type of controls, and sex (Table 3). The ORs were 0.76 (95% CI 0.65–0.89) for colorectal, 0.88 (95% CI 0.67–1.16) for colon and 0.96 (95% CI 0.81–1.13) for rectal cancer in North American studies, 0.66 (95% CI 0.41–1.10) for colorectal, 0.55 (95% CI 0.39–0.79) for colon and 0.86 (95% CI 0.43–1.73) for rectal cancer in Northern European studies, 0.72 (95% CI 0.50–1.05) for colorectal, 0.82 (95% CI 0.62–1.10) for colon and 0.96 (95% CI 0.75–1.22) for rectal cancer in Southern European studies, and 0.61 (95% CI 0.50–0.74) for colorectal, 0.60 (95% CI 0.42–0.85; 95% CI 0.45–0.81, respectively) for colon and rectal cancer in Asian studies. Analysis in strata of type of controls gave similar results, with summary ORs of 0.71 (95% CI 0.52–0.88) and 0.73 (95% CI 0.64–0.83), respectively, for population and hospital controls for colorectal cancer; the corresponding ORs were 0.80 (95% CI 0.59–1.07) and 0.70 (95% CI 0.62–0.80) for colon, and 0.88 (95% CI 0.72–1.07) and 0.87 (95% CI 0.69–1.10) for rectal cancer. The summary ORs for colorectal cancer were 0.76 (95% CI 0.61–0.94) in women (based on 2 studies and 1,433 cases) and 1.17 (95% CI 0.66–2.05) in men (based on 3 studies and 1,757 cases). The corresponding values were, for colon cancer, 0.79 (95% CI 0.63–0.99) in women (based on 6 studies and 3,034 cases) and 0.90 (95% CI 0.66–1.21) in men (based on 6 studies and 3,466 cases), and, for rectal cancer, 0.90 (95% CI 0.71–1.13) in women (based on 3 studies and 991 cases) and 1.05 (95% CI 0.83–1.33) in men (based on 3 studies and 1,356 cases). Although the inverse association was apparently stronger or restricted to women, the test for heterogeneity between the ORs in women and men was not significant for colorectal cancer or for each site separately.
The pooled estimate of the effect of the highest level of consumption versus non/low drinkers on the risk of colorectal cancer by calendar year of publication is shown in Fig. 2. The cumulative OR was about 0.6 for studies published up to 1994 and between 0.67 and 0.69 for more recent studies, all statistically significant.
Discussion
This meta-analysis of case–control studies found that the risk of colorectal cancer for regular coffee drinkers was approximately 17% lower than for non/occasional drinkers. The protection was about 30% for the highest coffee drinkers and 6% for an increase in consumption of one cup of coffee/day. The inverse association was stronger for colon than for rectal cancer. The apparently lower pooled OR for colorectal than that of colon or rectal cancer reflects differences in the composition of studies used to estimate the relation of coffee drinking with cancer at various anatomical sites. The same was true for the separate estimates in men and women and that including both sexes.
A meta-analysis of data collected before June 1997 [4] found a cumulative RR of colorectal cancer of 0.97 (95% CI: 0.73–1.29) for cohort studies and 0.72 (95% CI: 0.61–0.84) for case–control studies for high versus low categories of coffee consumption. Thus, our findings confirm the previous ones of case–control studies. In the present analysis, the inverse relation was also consistently observed in populations with different baseline colorectal cancer incidences and different patterns of coffee consumption. A recent meta-analysis of cohort studies based on 12 studies and including 5,403 cases of colorectal cancer found a RR of 0.91 (95% CI: 0.81–1.02) of borderline significance, comparing high to low categories of coffee consumption [5], and a pooled analysis of 13 prospective studies found no association overall and in strata of anatomical site of cancer, sex, smoking, alcohol, body mass index, and physical activity [6]. However, a Chinese prospective study published after the meta- and pooled analysis found an overall RR of 0.89 (95% CI 0.66–1.19) in drinkers of two or more coffee per day and of 0.56 (95% CI 0.35–0.90) in ever smokers [7].
Therefore, there are still differences between results of cohort and case–control studies of coffee and colorectal cancer risk. The discrepancy may partly by be related to the different time-exposure considered, closer to cancer incidence in case–control studies. This is supported by the finding that cohort studies with a follow-up shorter than 10 years are more likely to show an inverse association between coffee intake and colorectal cancer risk than studies with longer follow-up [5].
The consistency of results from case–control studies over calendar years indicates that the inverse association between coffee drinking and colorectal cancer risk is unlikely to be a false positive finding due to selective reporting of earlier studies [52], as the cumulative OR for recent studies, in general larger and more accurately adjusted for potential confounding factors, was only 5–9% higher and, given the interest in the issue, also null results have been published, thus limiting the scope for publication bias. In addition, the finding of a dose-risk relation further supports the real inverse association. However, the categories of low and the high drinkers varied across the studies included in this meta-analysis, as coffee drinking is more frequent in North America and Northern Europe than in Southern Europe and even less common in Asia. Consequently, the overall estimates for high drinkers were based on study-specific definitions and can only be considered indicative of an inverse dose-risk relation.
We cannot exclude some misclassification in colorectal cancer diagnosis. However, most studies included in the meta-analysis reported that cases of colorectal cancer were histologically confirmed, and diagnosis and certification of all intestinal sites, including colon and rectum, have long been sufficiently reliable, and have not substantially changed over the last decades. The distinction between colon and rectum cancer poses some problems (since a large proportion of cancers arise in the recto-sigmoid junction) [53], which should not, however, have affected our estimates based on all intestinal cancers combined and might have weakened the cumulative estimate for colon cancer risk, if the association is stronger for colon than for rectum. Thus, it is unlikely that problems in diagnosis and certification practices may have meaningfully affected the overall risk estimates.
Subjects with digestive tract disease, such as inflammatory bowel disease (a risk factor for colorectal cancer), may avoid coffee because of side effects of coffee or following non-specific medical advice (reverse causation) [54]. However, several possible mechanisms support a real inverse association of coffee intake with colorectal (mainly colon) cancer risk [55]. Coffee contains several antioxidant and antimutagenic compounds with potential anticarcinogenic effects in animal models and cell culture systems [56], whose concentrations in the beverage vary depending on the type of raw coffee (Arabica or robusta), roasting, and preparation [57]. They include phenolic compounds (such as chlorogenic, caffeic, ferulic, and cumaric acids) [58], melanoidins and diterpenes (such as cafestol and kahweol). In particular, cafestol and kahweol reduce the oxidant effect of polycyclic aromatic hydrocarbons and several other carcinogens. Specific possible mechanisms in the colon include the reduction in bile acid secretion (a promoter of colon cancer) [59], reduction in synthesis by down-regulation of the expression of bile acid homeostatic genes [60], an increase in colonic motility (mainly at the rectosigmoid junction and in women) [61]. Patients with type-2 diabetes are at increased risk of colon cancer [62–64]. Coffee has antidiabetic properties [65], and the chlorogenic acid contained in coffee reduces glucose absorption in the intestine [66]. A lower concentration of C-peptide (a marker of insulin secretion) [67] was found in women drinking more than 4 cups/day [68], and a prospective study from New York State observed a threefold higher risk of colorectal and colon cancer in women in the top quartile of C-peptide [69].
We observed an apparently stronger inverse relation in women, although the difference was not significant. If real, this can be partly related to sex hormones that have also been associated to reduced colorectal cancer risk [70, 71], and caffeine has been related to sex-hormone-binding globulins [72]. However, the issue remains open to discussion.
Observational studies included in this meta-analysis may have various sources of bias and confounding. Selection and report bias might have operated in case–control studies; however, the consistency of results between type of controls (population and hospital), geographic regions and participation rate, that was satisfactory in most studies, argues against it. Furthermore, the different coffee consumption measurements and the consequent arbitrary classification of consumption may explain the heterogeneity among studies. Moreover, most studies included did not mention type of coffee power, brewing methods, preparation, and cup size, which may modify the relation, since for example boiled (unfiltered) coffee contains smaller amounts of the lipid components of coffee (diterpenes, such as cafestol and kahweol) with anticarcinogenic activities [73]. An important difficulty concerned the assessment of coffee intake based on patients’ self-reporting. However, recall of coffee drinking has been shown to be satisfactorily reproducible and valid [74, 75] and should not be different on the basis of the disease status or among various types of controls, as coffee is not commonly known to affect colorectal cancer risk. A few early studies did not adjust for relevant confounding factors, such as smoking, body mass index, or physical activity. This may be partly responsible for heterogeneity among studies.
However, the exclusion of each single study did not change the summary estimate, and although we did not search for unpublished data or abstracts, given the difficulties in their interpretation, no significant asymmetry was seen in the funnel plot, indicating that publication bias is unlikely to have materially influenced the results.
We were unable to consider the relation between caffeine and the risk of colorectal cancer because most published studies reported only the number of cups of coffee consumed, with no information on caffeine intake. Although caffeine intake depends on the variety of raw coffee and the preparation method, and it is also found in cola, energy drinks and several drugs, coffee drinking is its major source and is strongly correlated with total caffeine intake in Europe and North America [1].
In conclusion, this systematic meta-analysis of case–control studies provides quantitative evidence of an inverse relation between coffee drinking and colorectal (mainly colon) cancer risk. Although bias and confounding were unlikely responsible for the consistent results in different countries, settings and time, the interpretation of this association in terms of causality remains open to discussion, also considering the lack of information on the relation with duration and other time-related factors, and the quantitative difference in the estimates from case–control and cohort studies [5].
References
IARC (1991) Coffee, tea, mate, methylxanthines and methylglyoxal. Lyon France. pp 1–513
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
La Vecchia C, Tavani A (2007) Coffee and cancer risk: an update. Eur J Cancer Prev 16:385–389
Giovannucci E (1998) Meta-analysis of coffee consumption and risk of colorectal cancer. Am J Epidemiol 147:1043–1052
Je Y, Liu W, Giovannucci E (2009) Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer 124:1662–1668
Zhang X, Albanes D, Beeson WL et al (2010) Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 102:771–783
Peterson S, Yuan JM, Koh WP et al (2010) Coffee intake and risk of colorectal cancer among Chinese in Singapore: the Singapore Chinese health study. Nutr Cancer 62:21–29
Higginson J (1966) Etiological factors in gastrointestinal cancer in man. J Natl Cancer Inst 37:527–545
Bjelke E (1974) Letter: colon cancer and blood-cholesterol. Lancet 1:1116–1117
Macquart-Moulin G, Riboli E, Cornee J, Charnay B, Berthezene P, Day N (1986) Case-control study on colorectal cancer and diet in Marseilles. Int J Cancer 38:183–191
Jarebinski M, Adanja B, Vlajinac H (1989) Case-control study of relationship of some biosocial correlates to rectal cancer patients in Belgrade, Yugoslavia. Neoplasma 36:369–374
Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE (1989) Colorectal cancer and diet in an Asian population—a case-control study among Singapore Chinese. Int J Cancer 43:1007–1016
Rosenberg L, Werler MM, Palmer JR et al (1989) The risks of cancers of the colon and rectum in relation to coffee consumption. Am J Epidemiol 130:895–903
Benito E, Obrador A, Stiggelbout A et al (1990) A population-based case-control study of colorectal cancer in Majorca. I. Dietary factors. Int J Cancer 45:69–76
Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S (1990) A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res 81:1101–1108
Slattery ML, West DW, Robison LM et al (1990) Tobacco, alcohol, coffee, and caffeine as risk factors for colon cancer in a low-risk population. Epidemiology 1:141–145
Hoshiyama Y, Sekine T, Sasaba T (1993) A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med 171:153–165
Olsen J, Kronborg O (1993) Coffee, tobacco and alcohol as risk factors for cancer and adenoma of the large intestine. Int J Epidemiol 22:398–402
Baron JA, Gerhardsson de Verdier M, Ekbom A (1994) Coffee, tea, tobacco, and cancer of the large bowel. Cancer Epidemiol Biomarkers Prev 3:565–570
Centonze S, Boeing H, Leoci C, Guerra V, Misciagna G (1994) Dietary habits and colorectal cancer in a low-risk area. Results from a population-based case-control study in southern Italy. Nutr Cancer 21:233–246
Shannon J, White E, Shattuck AL, Potter JD (1996) Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol Biomarkers Prev 5:495–502
Tavani A, Pregnolato A, La Vecchia C, Negri E, Talamini R, Franceschi S (1997) Coffee and tea intake and risk of cancers of the colon and rectum: a study of 3,530 cases and 7,057 controls. Int J Cancer 73:193–197
Inoue M, Tajima K, Hirose K et al (1998) Tea and coffee consumption and the risk of digestive tract cancers: data from a comparative case-referent study in Japan. Cancer Causes Control 9:209–216
Munoz SE, Navarro A, Lantieri MJ et al (1998) Alcohol, methylxanthine-containing beverages, and colorectal cancer in Cordoba, Argentina. Eur J Cancer Prev 7:207–213
Boutron-Ruault MC, Senesse P, Faivre J, Chatelain N, Belghiti C, Meance S (1999) Foods as risk factors for colorectal cancer: a case-control study in Burgundy (France). Eur J Cancer Prev 8:229–235
Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S (1999) Food groups and colorectal cancer risk. Br J Cancer 79:1283–1287
Slattery ML, Caan BJ, Anderson KE, Potter JD (1999) Intake of fluids and methylxanthine-containing beverages: association with colon cancer. Int J Cancer 81:199–204
Woolcott CG, King WD, Marrett LD (2002) Coffee and tea consumption and cancers of the bladder, colon and rectum. Eur J Cancer Prev 11:137–145
Zhang B, Li X, Nakama H, Zhang X, Wei N, Zhang L (2002) A case-control study on risk of changing food consumption for colorectal cancer. Cancer Invest 20:458–463
Yeh CC, Hsieh LL, Tang R, Chang-Chieh CR, Sung FC (2003) Risk factors for colorectal cancer in Taiwan: a hospital-based case-control study. J Formos Med Assoc 102:305–312
Murtaugh MA, Ma KN, Caan BJ, Slattery ML (2004) Association of fluids from beverages with risk of rectal cancer. Nutr Cancer 49:25–31
Stroup DF, Berlin JA, Morton SC et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Abu-Zeid HA, Choi NW, Hsu PH (1981) Factors associated with risk of cancer of the colon and rectum. Am J Epidemiol 114:442
Fredrikson M, Hardell L, Bengtsson NO, Axelson O (1995) Colon cancer and dietary habits—a case-control study. Int J Oncol 7:133–142
Dales LG, Friedman GD, Ury HK, Grossman S, Williams SR (1979) A case-control study of relationships of diet and other traits to colorectal cancer in American blacks. Am J Epidemiol 109:132–144
Graham S, Dayal H, Swanson M, Mittelman A, Wilkinson G (1978) Diet in the epidemiology of cancer of the colon and rectum. J Natl Cancer Inst 61:709–714
Young TB, Wolf DA (1988) Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 42:167–175
Tuyns AJ, Kaaks R, Haelterman M (1988) Colorectal cancer and the consumption of foods: a case-control study in Belgium. Nutr Cancer 11:189–204
Haenszel W, Berg JW, Segi M, Kurihara M, Locke FB (1973) Large-bowel cancer in Hawaiian Japanese. J Natl Cancer Inst 51:1765–1779
Tajima K, Tominaga S (1985) Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res 76:705–716
Peters RK, Pike MC, Garabrant D, Mack TM (1992) Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 3:457–473
Bidoli E, Franceschi S, Talamini R, Barra S, La Vecchia C (1992) Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer 50:223–229
La Vecchia C, Ferraroni M, Negri E et al (1989) Coffee consumption and digestive tract cancers. Cancer Res 49:1049–1051
Breslow NE, Day NE (1980) Statistical methods in cancer research. In: The analysis of case-control studies, vol 1. Lyon, France: international agency for research on cancer. IARC scientific publications
Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 6:40–57
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9:1–30
Thornton A, Lee P (2000) Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol 53:207–216
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Boffetta P, McLaughlin JK, La Vecchia C, Tarone RE, Lipworth L, Blot WJ (2008) False-positive results in cancer epidemiology: a plea for epistemological modesty. J Natl Cancer Inst 100:988–995
Fernandez E, La Vecchia C, Gonzalez JR, Lucchini F, Negri E, Levi F (2005) Converging patterns of colorectal cancer mortality in Europe. Eur J Cancer 41:430–437
Cole P, Rodu B, Mathisen A (2003) Alcohol-containing mouthwash and oropharyngeal cancer: a review of the epidemiology. J Am Dent Assoc 134:1079–1087
Thomson CA, Martinez ME (2010) Coffee, tea, what beverage for me? Associations between beverage intake and colorectal neoplasia risk. J Natl Cancer Inst 102:749–751
Cavin C, Holzhaeuser D, Scharf G, Constable A, Huber WW, Schilter B (2002) Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol 40:1155–1163
Viani R (1993) The composition of coffee. In: Garattini S (ed) Caffeine, coffee, and health. Raven Press, New York, pp 17–41
Ferruzzi MG (2010) The influence of beverage composition on delivery of phenolic compounds from coffee and tea. Physiol Behav 100:33–41
Potter JD (1992) Reconciling the epidemiology, physiology, and molecular biology of colon cancer. JAMA 268:1573–1577
Ricketts ML, Boekschoten MV, Kreeft AJ et al (2007) The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol 21:1603–1616
Brown SR, Cann PA, Read NW (1990) Effect of coffee on distal colon function. Gut 31:450–453
La Vecchia C, Negri E, Decarli A, Franceschi S (1997) Diabetes mellitus and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 6:1007–1010
Tavani A, Bravi F, Bosetti C et al (2005) Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev 14:2277
Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86:s836–s842
van Dam RM, Hu FB (2005) Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA 294:97–104
McCarty MF (2005) A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med Hypotheses 64:848–853
Bonser AM, Garcia-Webb P (1984) C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci 19:297–352
Wu T, Willett WC, Hankinson SE, Giovannucci E (2005) Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in US women. Diabetes Care 28:1390–1396
Kaaks R, Toniolo P, Akhmedkhanov A et al (2000) Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 92:1592–1600
La Vecchia C, Gallus S, Fernandez E (2005) Hormone replacement therapy and colorectal cancer: an update. J Br Menopause Soc 11:166–172
Chlebowski RT, Wactawski-Wende J, Ritenbaugh C et al (2004) Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med 350:991–1004
Ferrini RL, Barrett-Connor E (1996) Caffeine intake and endogenous sex steroid levels in postmenopausal women. The rancho bernardo study. Am J Epidemiol 144:642–644
George SE, Ramalakshmi K, Mohan Rao LJ (2008) A perception on health benefits of coffee. Crit Rev Food Sci Nutr 48:464–486
D’Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D (1997) An assessment, and reproducibility of food frequency data provided by hospital controls. Eur J Cancer Prev 6:288–293
Ferraroni M, Tavani A, Decarli A et al (2004) Reproducibility and validity of coffee and tea consumption in Italy. Eur J Clin Nutr 58:674–680
Acknowledgments
We thank Dr Patrizia Lettieri for bibliographic research, Dr. Irene Tramacere for helpful assistance to plot the graphs, Mrs Judy Baggott for style editing and Ms Ivana Garimoldi for editorial assistance. This work was conducted with the contribution of the Italian Association for Cancer Research.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galeone, C., Turati, F., La Vecchia, C. et al. Coffee consumption and risk of colorectal cancer: a meta-analysis of case–control studies. Cancer Causes Control 21, 1949–1959 (2010). https://doi.org/10.1007/s10552-010-9623-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-010-9623-5