Abstract
Objective
Chronic lymphocytic leukemia (CLL) is generally considered to be non-radiogenic and is excluded from several programs that compensate workers for illnesses resulting from occupational exposures. Questions about whether this exclusion is justified prompted a Congressional mandate to the National Institute for Occupational Safety and Health (NIOSH) to, further, examine the radiogenicity of CLL. This study revisits the question of CLL radiogenicity by examining epidemiologic evidence from occupationally and medically-exposed populations.
Methods
A systematic review of radiation-exposed cohorts was conducted to investigate the association between radiation and CLL. Exploratory power calculations for a pooled occupational study were performed to examine the feasibility of assessing CLL radiogenicity epidemiologically.
Results
There is a bias against reporting CLL results, because of the disease’s presumed non-radiogenicity. In medical cohort studies that provide risk estimates for CLL, risk is elevated, though non-significantly, in almost all studies with more than 15 years average follow-up. The results of occupational studies are less consistent.
Conclusions
Studies with adequate follow-up time and power are needed to better understand CLL radiogenicity. Power analyses show that a pooled study might detect risk on the order of radiation induced non-CLL leukemia, but is unlikely to detect smaller risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The etiology of chronic lymphocytic leukemia (CLL) is largely unknown. Studies have demonstrated the involvement of immune system components and genetic abnormalities in the pathogenesis of CLL [1, 2]. Observation of trisomy 12, abnormalities in chromosome 13q14 [3], familial clustering [4], and the phenomenon of anticipation [5] in CLL patients suggest the involvement of multiple genetic pathways.
The role of environmental exposures, such as ionizing radiation and chemicals, in CLL etiology is unclear. Several studies have noted elevations of CLL among farmers [6–8], suggesting increased CLL risk due to exposure to herbicides or pesticides [9]. Exposure to ionizing radiation has also been examined as a potential risk factor for CLL. Since no increases in CLL were observed among the Life Span Study cohort of atomic bomb survivors [10] or among large cohorts treated therapeutically with radiation [11–13], the disease is generally considered non-radiogenic [14].
The US Energy Employees Occupational Illness Compensation Act (EEOICPA) considers CLL non-radiogenic; in fact, CLL is the only cancer assigned a causation probability of zero under EEOICPA [15]. The decision to exclude CLL from compensation stemmed from a lack of evidence for CLL radiogenicity in medically exposed cohorts and in large occupational studies. CLL is also excluded from other compensation programs, including the Radiation Exposure Compensation Act (RECA) [16], which compensates uranium millers and miners and those exposed to fallout from atmospheric nuclear tests. In contrast, other “non-radiogenic” diseases, like prostate cancer and hairy cell leukemia (a malignancy similar to CLL), are compensated under EEOICPA.
It has recently been recognized that the presumption of non-radiogenicity was based, in part, on studies of atomic bomb survivors, a population with a low background rate of the disease [17]. This observation, together with concerns about discrepancies in compensation practices, prompted a Congressional mandate to the National Institute for Occupational Safety and Health (NIOSH) to further investigate the radiogenicity of CLL.
A meta-analysis of the available literature was attempted, but was not feasible due to heterogeneity in study populations and exposures, a lack of explicit reporting of CLL risk estimates, and differences in reporting measures. Instead, a systematic review was conducted to assess the epidemiological evidence pertinent to CLL radiogenicity. In addition, a power analysis was conducted to evaluate the potential of a pooled study of radiation-exposed workers to contribute to the understanding of CLL radiogenicity.
In order to maximize the probability of adequate dosimetry and minimize uncontrolled confounding, this review was restricted to studies of persons exposed to radiation occupationally or as medical patients. The review excluded environmentally exposed populations, such as communities living near nuclear facilities.
Pertinent medically exposed populations include patients treated with therapeutic X-rays or brachytherapy for malignant or benign conditions, given diagnostic X-rays, exposed to Thorotrast, or receiving diagnostic or therapeutic Iodine-131 (131I) for thyroid conditions. Occupational cohorts included in the review comprise nuclear facility workers, radiologists and radiologic technicians, airline crews exposed to cosmic radiation, nuclear test participants, and cleanup workers following nuclear accidents, such as Chernobyl.
Materials and methods
Systematic review
Pubmed and Excerpta Medical (EMBASE) databases were searched through early 2005, with no language restriction, to identify quantitative epidemiological studies of populations exposed to radiation occupationally or during medical treatment or diagnosis. Studies were required to provide risk estimates based on standardized populations or internal comparison groups. Controlling for attained age was required, as CLL is highly correlated with age [18]. Case series, studies of ecologic design, and studies with only proportionate mortality ratio results were excluded.
The initial search used Medical Subject Headings (MeSH) entry terms for “leukemia” and “radiation.” The search was narrowed to target epidemiology studies by requiring a term from the following set: “epidemiology,” “cohort,” “case–control,” “relative risk” and “odds ratio,” “risk ratio,” “standard mortality ratio,” “standard incidence ratio,” “standard rate ratio,” “excess relative risk,” “observed to expected” and “O:E.” The refined search yielded 2,840 unique citations. Abstract scans resulted in the exclusion of many articles. Nearly 900 articles were not epidemiological studies of occupationally or medically-exposed populations. As CLL primarily affects adults, nearly 800 studies focusing on childhood exposures or outcomes were excluded. Smaller numbers of articles were excluded because they reported only on non-CLL leukemia subtypes, studied Asian populations (while CLL is the most prevalent adult leukemia in Western populations [19] it accounts for only 3–5% of leukemia among Asian populations [20]), or followed patients treated for primary lung cancer (because of low survivability) or primary hematopoietic malignancies (due to diagnostic similarity and possible shared etiology with CLL). Populations exposed only to non-ionizing or ultra-violet radiations were excluded, as well.
Following the abstract scan, 293 articles were retained for full-text examination. Additional articles were identified via searches of dissertation abstracts (UMI ProQuest) and of a NIOSH library holding published and unpublished reports from epidemiologic studies of Department of Energy workers. After applying exclusion criteria to the full-text articles, 210 articles remained, where articles did not report CLL-specific results, but suggested that such analyses had been performed, authors were contacted with requests for CLL results or pertinent data files. In most cases these requests were not successful.
A number of medical studies reported CLL results, but failed to differentiate results by chemotherapy or by radiotherapy status. Where these studies are informative (i.e., no CLL deaths or cases observed despite a mixture of therapies) they are discussed. Where updates of specific occupational or medical populations exist, this review presents results from the latest update, except where an earlier study provided CLL risk results and the update did not, or where one study gave mortality results and another, incidence results. The original CLL results from study cohorts later included in pooled analyses are discussed.
Power analysis
Power calculations were completed to examine the feasibility of evaluating the relation between external radiation and CLL through a pooled cohort mortality study of radiation-monitored workers. Egret® Siz software [21] was used to perform the analyses. A Poisson model was assumed where external dose was categorized into four groups: 0–10 millisievert (mSv), 10–50 mSv, 50–100 mSv, and >100 mSv. These cutpoints were chosen to be consistent with studies on which the dose distribution was based [Los Alamos National Laboratory (LANL) and the Portsmouth Naval Shipyard (PNS)]. Age and sex, known risk factors for CLL, were also considered. In specifications for age and sex sampling fractions by dose, basic assumptions were that younger workers have lower cumulative doses than older workers, and that the ratio of males to females increases with dose category.
Expected relative risks (RRs) for CLL for each variable were specified in the model. The RRs for CLL at each level of external dose were derived from the National Academies’ Biological Effects of Ionizing Radiation (BEIR) V model [14] for non-CLL leukemia. Also, a more conservative analysis was run using RRs consistent with lymphoma (excess relative risk (ERR) per Sv = 0.178), obtained from the Centers for Disease Control and Prevention (CDC) and National Cancer Institute (NCI) working group tables [22]. Midpoints of each dose category were used to calculate the RR; the highest category used a dose midpoint of 150 mSv (leukemia: RR at >100 mSv = 1.63, NHL: RR at >100 mSv = 1.02). CLL RRs for age were estimated from CDC Wonder [23] age-specific CLL rates. The RR comparing females to males was assumed to be 0.5, based on the literature [24]. An annual case rate for CLL among the baseline stratum (females, age 25–44) of 2.0735 per 10,000,000 was assumed, again based on CLL mortality rates available from CDC Wonder.
Person-year (PY) extrapolations were performed for selected cohorts (based on data availability) to complement the power analysis. These cohorts included the US Idaho National Laboratory (INL) cohort [25] and the US Multi-Site Leukemia Case Control Study (LCCS) cohort (Schubauer-Berigan et al., in review), which consists of the contributing cohorts LANL, Hanford, Savannah River Site (SRS), PNS, and Oak Ridge National Laboratory. Required demographic information included age structure of the cohort and age at hire, in order to determine PY begin dates. Datasets were available in-house for the US cohorts. Published data on the age distribution and average date of hire were available for the United Kingdom (UK) National Registry of Radiation Workers (NRRW) [26], Canada’s National Dose Registry [27], and the Russian National Medical Dosimetric Registry of Chernobyl workers [28]; thus these cohorts were included in the analysis. The Life Table Analysis System software [29] and country-specific life tables (and state, age, and race specific, where available) were used to estimate the expected number of deaths, and resulting person years, over several time intervals. Deaths were assumed to occur in the middle of the interval. Persons still anticipated to be alive contributed PY over the entire interval. In addition, a 15 year lagged PY extrapolation was performed on the INL and LCCS cohorts (data were not available for the UK and Canadian cohorts); person-years and deaths which occurred during the first 15 years of follow-up were ignored.
Results
Search results
Only 46 studies (23 medical and 23 occupational) reported risk estimates for CLL, and some of these had overlapping populations (Table 1). In addition, eight medical and eight occupational studies reporting zero CLL cases were considered informative and retained. Studies reporting results both for leukemia and leukemia excluding CLL were retained, although these results are less informative than CLL-specific analyses. The remaining studies provided no CLL risk estimates (report only number of CLL observed).
A number of medical studies had clearly performed pertinent subtype analyses, but did not explicitly report CLL risk estimates, likely because the disease was regarded as non-radiogenic. For example, a study of endometrial cancer patients included CLL as a negative control to detect ascertainment bias [30]. Other authors excluded CLL from the primary or secondary analyses, stating that it is presumed to be non-radiogenic [31], reported only that CLL risk does not appear to be increased without presenting risk estimates [32], or simply mentioned that results were similar for CLL and non-CLL [33]. Other studies did not differentiate by chemotherapy status; those with positive findings [34] are difficult to interpret, as either radiation or chemotherapy could be responsible.
Like the medical studies, some occupational studies performed CLL specific analyses, but did not report CLL risk estimates [35]. Other studies reported a summary of risk estimate for CLL, but excluded CLL from dose–response or time since exposure analyses because the summary estimate was not elevated [36, 37]. However, recent results from a study of radiation workers at PNS demonstrate that a summary risk estimate at or below expectation does not preclude a significant dose–response in internal analyses [38].
Some occupational studies reported risk estimates for all leukemia, but only the number of CLL cases, which is often small. Of occupational studies reporting the number of leukemias found but no subtype data, a few, including analyses of US Department of Energy (DOE) workers at the K-25 site (unpublished report, Dupree et al., 1994) and the X-10 and Y-12 sites [39], French Atomic energy workers [40], and US radiologic technologists [41], reported more than 40 leukemia cases each and might have had sufficient CLL cases to provide useful dose–response or time since exposure results.
In contrast, some studies had valid ascertainment or risk estimation issues that precluded reporting CLL-specific risk estimates. In a study of 131I exposed-patients, Hall et al. report that coding for leukemia did not permit differentiation between acute and chronic leukemia [42]. Darby et al., in their study of women treated by X-rays for metropathia hemorrhagica, reported that risk estimates by subtype were not available for the target population during the study period [43], although they noted that no evidence of an excess of CLL was found in the population. Similarly, in studies of airline workers, Blettner et al. and Zeeb et al. [44, 45] reported only all leukemia, because subtypes could not consistently be determined from death certificates. These legitimate reporting issues may play a role in decisions by other researchers to report risk estimates only for all leukemia combined.
Study results
Medical studies
External radiation and brachytherapy for malignant conditions, non-malignant conditions, and diagnostic purposes
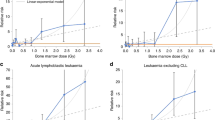
The medical cohort studies tend to exclude CLL from dose–response or time since exposure analyses, but often provide average follow-up time for the cohort. While dose–response or time since exposure analyses would be preferable, average follow-up is also informative, as studies with longer average follow-up are more likely to have a greater percentage of person years at risk with adequate latency. Examination of medical cohort studies [46–61] shows a pattern of risk estimates for irradiated patients below 1.00 with average follow-up of less than 15 years (Fig. 1). With longer follow-up time (≥15 years), all studies have point estimates greater than 1.00 for irradiated patients, with the exception of one low-dose [mean absorbed dose to active bone marrow = 0.09 gray (Gy)] study of tuberculosis patients [60] which found no CLL deaths among the 6,285 exposed cohort members. However, confidence intervals for the majority of these positive results span the null, due in part to the scarcity of CLL cases.
Medical cohort studies and clinical trials: results by follow-up time. Outcome is CLL mortality except where indicated. Point estimate (▲); a incidence studies; b RT + O/E = 1.44, RT− O/E = 2.0; n = number of irradiated study subjects; # of CLL in irradiated pts. Results from Brenner et al. [47] were supplemented by personal communication from Brenner to Silver received 1/14/05
The RRs comparing patients treated with radiotherapy (RT+) to non-irradiated patients (RT−) are not presented in all of these studies, so Fig. 1 displays risk estimates for the irradiated patients. For several studies, the overall risk estimates for irradiated patients were lower than those for their non-irradiated counterparts. These include studies of cervical cancer patients by Boice et al. [51] and by Kleinerman et al. [55], as well as a study of ankylosing spondylitics by Weiss et al. [57]. Follow-up was less than 11 years for the first two studies, but the study of ankylosing spondylitis patients [57] had average follow-up greater than 15 years. Interestingly, the Kleinerman study provided O:E data by time since treatment as well; the results for time periods >20 years since treatment showed a greater O:E ratio for irradiated patients than for their non-irradiated counterparts. This result demonstrates that summary estimates can be misleading, particularly when over influenced by patients with short follow-up (and thus by large numbers of person years in cells with little latency and thus, if latency is long, little risk).
While the studies of testicular cancer presented in Fig. 1 had no CLL cases with fewer than 15 years average follow-up, two other studies did report CLL cases following treatment for this illness, although they failed to provide risk estimates [62, 63]. In addition, Hay et al. studied 517 Scottish testicular cancer patients followed an average of 15.4 years [64] and found two leukemia cases, both CLL, for an all-leukemia O:E ratio of 3.91. No CLL-specific risk estimate is provided, but the CLL risk estimate would also be positive for this study, which had longer follow-up than other testicular cancer research.
Some medical cohort studies did not report mean follow-up time and are, therefore, excluded from Fig. 1. In a study of benign lesions of the locomotor system (ankylosing spondylitis, arthritis, and other conditions) among Swedish patients diagnosed between 1950 and 1964 and followed through 1988, Damber et al. [65] reported a standardized incidence ratio (SIR) for CLL of 1.07 (95% CI = 0.80, 1.41; n = 50). Follow-up time was at least 24 years for surviving patients. In contrast, in an incidence study of Danish patients with in situ and invasive cervical cancer [66], the RR for CLL in patients treated by radiotherapy (n = 7) versus non-irradiated patients remained constant at 0.9 for the follow-up period of over 20 years (average follow-up is not given). A clinical trial for ovarian cancer [67] found no incident lymphatic leukemia cases in the radiation-treated group. Average follow-up was not given by treatment type; median follow-up for the entire cohort was 13.5 years.
Results of six cohort studies that give CLL results but fail to separate risk estimates by chemotherapy status are generally consistent with those shown in Fig. 1. Most have mean follow-up ranging from 3.2 years to 10.2 years; four [68–71] reported either no CLL cases or CLL risk below expectation. While Curtis et al. [72] found a non-significant excess of CLL with average follow-up of 4.6 years, both CLL cases occurred at >10 years after initial ovarian cancer diagnosis. An expansion of this cohort, with average follow-up of 4.1 years, found an overall deficit of CLL in ovarian cancer patients regardless of chemotherapy status [69]. One exception is a study of testicular cancer [34]; with an average follow-up of eight years an elevated O:E of 3.5 was observed, based on only one case.
Case–control studies of second malignancies following radiotherapy rarely report CLL results. A study of uterine cancer [73], with average follow-up of 8.2 years and control for alkylating agents, found a matched RR for CLL incidence of 0.90 (95% CI = 0.4, 1.9, n = 57). A breast cancer study [31] with an average interval between breast cancer and leukemia diagnoses of 12 years, found a non-significant increased relative risk of subsequent CLL diagnosis of 1.84 (90% CI = 0.5, 6.7, n = 10). CLL was excluded from dose–response analyses; the authors note that the disease is not believed to be radiogenic. The authors restricted the treatment period to 1972 or earlier to reduce the likelihood that chemotherapy was used. Another cervical cancer study, which used CLL as a negative check [11], reported results for this outcome near expectation (incident CLL RR = 1.03, 90% CI = 0.3, 3.9, n = 52). Finally, a study comparing estimated radiation dose from diagnostic X-rays in CLL cases versus controls found a deficit in risk that was statistically significant with a five-year lag; however, no longer lag periods were evaluated [74].
Other case–control studies of second primaries and diagnostic procedures have methodological drawbacks, such a study basing exposure on number of self-and proxy-reported X-rays which found a reporting bias [75]. A study of radiotherapy for various primaries [76] had no dosimetry data, and only evaluated incident CLL risk as a check against study bias.
Thorotrast
The CLL risk estimates for patients exposed to Thorotrast are available from a multi-national cohort study by Travis et al. [77] and a German study by van Kaick et al. [78]. The multinational study saw a sizable but non-significant increase of CLL among Swedish and Danish Thorotrast patients (RR = 9.3, 95% CI = 0.9, 356.6, n = 6). The Danish portion of the cohort was studied previously [79]; an RR of 6.00 (95% CI = 0.77, 121.06, n = 4) was found with follow-up exceeding 20 years. In contrast, van Kaick et al. [78] found a risk ratio of 0.8 (3 CLL cases) when comparing Thorotrast-exposed patients with controls, after 31 years mean follow-up, but this estimate did not adjust for age, sex, or calendar year. Finally, dos Santos Silva’s study of 2,427 Portuguese Thorotrast-exposed patients and 2,558 unexposed patients [80] found no CLL deaths at 15.3 years average follow-up in systemically exposed patients and 38.2 years in locally exposed patients.
131I
Of epidemiologic research involving patients treated with I131, two major studies with follow-up greater than 20 years present CLL risk estimates. Hall et al. studied Swedish patients given 131I between 1950 and 1975, with a mean follow-up of 21 years [42]; results differed by the indication for treatment. While the overall SIR for CLL was 1.08 (n = 65, 95% CI = 0.84, 1.38, n = 65), risk estimates were below expectation for hyperthyroidism but significantly elevated for treatment of thyroid cancer, with a slight, non-significant elevation for diagnostic administration of 131I. SIR results for the entire group of patients were below expectation from two to nine years after exposure, but showed non-significant elevations with longer intervals since exposure. Relative risk estimates generally rose with administered dose category, adjusted for sex, age at exposure, and calendar year, and attained statistical significance for doses at or above 100 mGy for CLL but not for non-CLL.
The other major 131I study, by Ron et al., examined treatment of 35,593 hyperthyroid patients, of whom about 65% were treated with 131I treated 1946–1964 in the US, with a mean follow-up of 21 years [81]. The CLL SMR for patients with Graves’ disease (PY = 368,934) treated by 131I dose was 1.33 (n = 19), while there were no deaths in a smaller group (PY = 13,088) so treated for toxic nodular goiter. Relative risk for CLL decreased with administered activity, with an ERR of −0.008 mCi−1 administered activity. Three smaller studies with average follow-up of 10 years or less report finding no CLL cases [82–84].
Occupational studies
Nuclear facility workers—external exposure
Nuclear facility studies report CLL risk in a variety of ways, complicating comparison between the studies. One of the three occupational studies to present ERR results for CLL explicitly is the International Agency for Research on Cancer (IARC) three-country mortality study of nuclear workers [85], that combined cohorts from the US (Hanford, Oak Ridge National Laboratory, and Rocky Flats), UK (Atomic Weapons Establishment (AWE), Atomic Energy Authority’s Sellafield facility) and Canada (Atomic Energy of Canada Ltd.). The combined cohort included 95,673 workers with average follow-up of 22 years. There were 147 leukemia deaths, with 27 from CLL. For CLL, the ERR per Sv was estimated to be −0.95 (90% CI = <0, 9.4); however, only a two-year lag was employed. Results for leukemia excluding CLL exhibited heterogeneity by facility, but no facility-specific analyses for CLL were presented. An update of the multinational study, analyzing approximately 400,000 monitored workers from 15 countries, has been published recently and reported results for leukemia excluding CLL [86]. The manuscript describing overall and facility-specific CLL analyses for this updated cohort was not available at the time of this analysis.
A mortality study at INL [formerly known as the Idaho National Engineering and Environmental Laboratory (INEEL)], which followed 63,561 workers for an average of 21.2 years [25], also found a negative ERR (−1.45 per Sv, SE = 2.80 × 10−3, n = 21) with a seven-year lag for CLL. Various lag assumptions, up to 20 years, were tested in the analysis of CLL; the maximum risk for non-CLL leukemias was observed with a seven-year lag, but the CLL risk did not vary by lag. No CLL deaths were seen in the highest dose group (≥100 mSv). The CLL ERR was much lower than the ERR for leukemia other than CLL.
The LCCS included cases and controls from a number of nuclear facilities (SRS, Hanford, PNS, ORNL, LANL, and Zia) and had the methodological advantage of controlling for potential chemical confounders, including chemical exposures. The study included 43 CLL deaths; interestingly, none occurred in the highest dose group (workers receiving at least 100 mSv external dose). With a 10-year lag, the ERR was negative (ERR per Sv = −2, 95% CI < 0, 14); however, when workers in the highest dose category were excluded, the estimate was positive, though non-significant (ERR per Sv = 20, 95% CI −3.6, 96) [Schubauer-Berigan et al. in review].
A number of occupational studies report ERR results for leukemia and for leukemia excluding CLL [26, 27, 87–92] (Fig. 2). In theory, if CLL has no relation with radiation exposure, the risk estimates for leukemia excluding CLL would be higher than those for all leukemia. Several studies of the US and Canadian nuclear power cohorts, report ERR results for leukemia and leukemia excluding CLL. The effects of excluding CLL differ depending on whether incidence or mortality is studied and the specific subset of workers studied. For example, in the Canadian studies, while the Sont mortality study [87] of the Canadian National Dose Registry, as mentioned, shows a decreased risk when CLL is excluded, the Ashmore et al. [27] mortality study of this group showed a very slight increase. The Zablotska mortality study [88] of the IARC subset of the Registry, showed a strong increase in risk from 18.9 per Sv (95% CI −2.08, 138) to 52.5 per Sv (95% CI 0.21, 291) when CLL was excluded.
Several mortality studies present O:E ratios or standardized rate ratios (SRR) by external dose category, along with trend tests for CLL (Hanford [36], Mound [93], LANL [94], and an unpublished study by Cragle et al. of Fernald workers). Only the Hanford study [36] had more than four CLL deaths. None of the 6 CLL deaths was observed at or above 100 mSv; overall CLL trend statistics were −0.45 for a two-year lag and −0.34 for a 10-year lag. However, these tests tend were less negative than those reported for leukemia excluding CLL (−0.81 and −0.85 respectively).
Other studies reporting trend tests or risk estimates for leukemia and leukemia excluding CLL include the United Kingdom Atomic Energy Authority (UKAEA) mortality study [95], the Sellafield mortality and morbidity study [96], and an unpublished study by Dupree-Ellis et al. of mortality in Mallinckrodt workers. Only the UKAEA study had more than four CLL cases. The study of 26,395 workers found 24 CLL deaths and a slightly higher SMR for leukemia (SMR = 1.18, 95% CI 0.91, 1.51) than for leukemia excluding CLL (SMR = 1.07, 95% CI 0.76, 1.46) in radiation workers.
A study of the SRS [97] examined CLL as an explicit outcome. However, only SMR analyses were performed and the numbers are quite small. An excess of CLL deaths was observed among white male cohort members compared to the US population, with an SMR of 1.57 (95% CI = 0.67, 2.8, n = 8). The CLL SMR for black males was even higher at 5.01 (95% CI = 0.95, 12.3, n = 3).
Some studies of nuclear facility workers report zero CLL cases or deaths arising despite adequate follow-up. Unfortunately, most do not provided the expected number of CLL cases, precluding assessment of whether CLLs are in deficit or close to expectation [98]. While a study by Koshurnikova et al. [99] of mortality in workers at the Mayak facility found no incident CLL cases in workers employed 1948–1972 followed through 1989, an expansion and update of this cohort through 1997 [35] found 11 CLL deaths among 77 deaths from leukemia but no effects of external or internal dose on CLL risk (p > 0.5).
Chernobyl cleanup workers, liquidators, and emergency workers
Three large populations of Chernobyl cleanup workers came from areas that are now Russia, the Ukraine, and Belarus; a smaller group of workers came from Estonia. Many of these workers received substantial dose over a relatively short period of time. Dose varied by year, dropping particularly after 1988. Boice and Holm [100] estimated that for 300,000 workers 1986–1987 working within the 30 km zone the average dose was approximately 100 mGy, with perhaps 4% of the workers receiving >250 mGy.
A study of 162,684 emergency workers from the Russian Federation [92] found 41 incident leukemia cases, of which 28 were CLL (results were provided for leukemia and leukemia excluding CLL). Mean age of entry into the 30 km zone was 34.0, and mean dose was 105 mGy. As shown in Table 2, the ERR for leukemia was lower than that for leukemia excluding CLL; maximum follow-up was less than 10 years.
The smaller group of 4,742 Estonian clean up workers had experienced no incident leukemia cases by end of follow-up in 1993; however, average follow-up of the cohort was only six years [101]. The mean dose to these workers is not known, but appears to have been less than that accrued by other cleanup worker groups [102].
Uranium miners
With respect to occupational radon exposure, one large study of underground miners performed a collaborative analysis of data from 11 international cohorts [103]. The study provided risk estimates for leukemia and leukemia excluding CLL by time since first employment and by cumulative working-level months (WLM). Of 69 leukemia deaths, 33 were from CLL. In early follow-up (<10 years since first employment) the SMR was higher for leukemia (1.93, 95% CI 1.19, 2.95, n = 21) than for leukemia excluding CLL (1.28, 95% CI 0.51, 2.64, n = 7). However, for the time period of 10 years or more since first employment, the SMR for leukemia was 0.99 (95% CI 0.73, 1.31, n = 48), while that for leukemia excluding CLL was 1.08 (95% CI 0.73, 1.55, n = 29). In contrast, an incidence study of Czech uranium miners by Rericha et al. [104] showed positive risk estimates for CLL, with 53 cases. The study used an unusual design involving case-subcohort comparisons with inverse empirical sampling. In internal comparisons of workers at the 80th vs. 20th percentile of cumulative exposure, the CLL RR was significantly elevated at 1.98 (95% CI 1.10, 3.59).
Radiologic technologists and radiologists
A study of CLL mortality among radiologists registered before 1936 found an excess risk of borderline significance (O:E = 2.86, one-sided p = 0.06, with expectation derived from population of the same social class), although only two cases were found [105]. The most recent mortality update of the US radiologic technologists [41] excluded CLL; however, a previous study of this population [106] did analyze CLL mortality. Out of the 103 leukemia deaths observed, 18 were due to CLL. The RRs showed no relation with the number of years certified, but were generally higher those certified before 1940, when doses were presumably higher. No significant increase of CLL was found.
A cancer incidence study [107] in a subset of this population found an SIR of 1.18 (95% CI = 0.72, 1.83, n = 24). The SIR for all leukemia was 1.09 (95% CI = 0.87, 1.32, n = 75). However, these estimates were based on a combination of death records and questionnaire data (weighted for non-response), with not all self-reported diagnoses confirmed by records. In a 2005 study by Linet et al. [108], internal comparisons were performed on a subset of the incidence study cohort. In this study, with 23 incident CLL cases, none of the proxy factors for radiation dose (years worked as a technologist, by calendar time and age; specific work practices) had statistically significant relative risks estimates or showed categorical estimates suggestive of a dose–response.
Airline pilots and cabin crew
Two multinational studies of European pilots have overlapping populations [109, 110]. A cancer incidence study of 10,211 airline pilots by Pukkala et al. [109] found differences in risk by time since first employment. The overall CLL SIR was 1.03 (95% CI = 0.28, 2.64; n = 4), but all CLL cases occurred at >20 years since first employment, where four CLL cases were observed versus 3.2 expected for an SIR of 1.31 (95% CI = 0.36, 3.36). In dose analyses, elevations were restricted to two intermediate dose categories, with SIRs of 2.46 (95% CI = 0.06, 13.7; n = 1) in the 3–9.99 mSv and 2.64 (95% CI = 0.32, 9.52; n = 2) in the 10–19.9 mSv dose categories. The other multinational study [110] used mortality as an endpoint, and reported SMRs for leukemia and leukemia excluding CLL by dose category. Findings were similar to those of Pukkala et al. (risk estimates increased when CLL was excluded except in the intermediate dose category of 15–25 mSv).
Nuclear weapons test participants
Studies conducted by Thaul et al. [37] and Muirhead et al. [111, 112] followed participants in nuclear test series conducted in the 1950s and 1960s. Both compared mortality and cancer incidence among test participants to the same outcomes in groups of non-participant veterans (Table 3). While Thaul et al. reported SMRs below expectation, both studies found non-significantly higher relative risks for test participants than for their non-participant counterparts. Thaul et al. reported better ascertainment among the exposed, potentially biasing the results. In the Muirhead studies, all CLL deaths occurred at least 10 years after first test participation, but most were in the lowest dose categories.
Power analysis
Preliminary power analyses, based on dose and confounder (age, sex) distributions characteristic of US nuclear weapons facilities, show that approximately 11 million PY would be required to detect an RR of 1.63 for CLL comparing >100 mSv to a referent category of 0–10 mSv (Fig. 2), with 80% power (alpha = 0.05). Such an RR is expected for non-CLL leukemia following radiation exposure greater than 100 mSv, per the BEIR V model. However, CLL risks associated with radiation may be smaller those associated with other leukemia subtypes [113]. Assuming risks similar to lymphoma, (RR = 1.02 at >100 mSv, NCI–CDC working group tables [22]), more than half a billion person years would be needed to detect an association with the specifications listed above.
In order to determine when an updated pooled analysis of occupational cohorts would potentially be informative for CLL, given the above power calculations, a PY extrapolation analysis (Table 4) was performed for selected cohorts). A pooled cohort mortality study of the US, UK, and Canadian cohorts, with vital status follow-up until 2002, would yield approximately 13.4 million PY (unlagged estimate) and detect with 80% power a minimum RR at >100 mSv of 1.56 and 1.52, where alpha (significance level) is 5% and 10%, respectively Table 5). Extending follow-up would increase the cumulative PY count for these cohorts, and allow detection of lower relative risks at the same alphas.
However, if a lag period of 15 years is assumed, the PYs decrease greatly, since the first 15 years of follow-up for each worker are ignored. In order to assess the effect of lag on the calculations, the PY extrapolations for the INL and LCCS cohorts (data were readily available) were lagged by 15 years. For these cohorts combined, the PY estimate for 2002 decreased by 42%, while the estimates for follow-up through 2007 and 2012 decreased by 39% and 38%, respectively. Projecting similar PY losses to the combined US, UK, and Canadian cohorts if a15-year lag is imposed give the following PY estimates: approximately 7.8 million by 2002, 9.2 million by 2007, and 10.5 million by 2012. Thus, with such a lag assumption, which is likely more reasonable than a zero lag, the estimated 11 million PYs required to detect risks similar to radiation-induced non-CLL leukemia (RR = 1.63 at >100 mSv) would not be reached until after 2012.
The Chernobyl cohort could potentially be added to this pool, although there is some dispute over the validity of diagnoses [100, 114]. Using an unlagged PY extrapolation, the Russian Federation Chernobyl workers would be expected to accumulate two million PY by 2002. From 2003–2007 and 2008–2012, another 591,183 and 530, 658 PY would be expected, respectively. However, with a 15-year lag, this cohort would not even begin to accrue PY until 2002. The Ukraine and Belarus Chernobyl cohorts combined are of similar size and would be expected to contribute PY of the same order of magnitude as the Russian cohort. Study results are pending on a group of over 100,000 Ukrainian cleanup workers followed in an NCI International collaborative study, which includes a case–control study of 110 leukemias (number of CLL cases not given). Mean and median doses of 170,000 workers 1986–1989 are 126 and 112 mGy. Belarus supplied 91,000 cleanup workers 1986–1989. Doses are known for only 9% of workers in this group; from this limited data, doses appear to be relatively low, with mean and median doses for the entire time period of 46 and 25 mGy. Mean and median doses for 1986 were 60 and 53 mGy.
Discussion
Summary of findings
Evaluation of CLL radiogenicity is hampered by the exclusion of CLL from many analyses, as well as the failure to report CLL-specific results. The majority of occupational and medical radiation epidemiology studies do not examine CLL as an endpoint. While some exclude CLL because of legitimate concerns about ascertainment, others exclude CLL because the authors believe, based on evidence from other studies, that the disease is non-radiogenic. This a priori exclusion of CLL precludes comprehensive assessment of its relation with radiation exposure.
In cohort studies of patients treated with external beam radiation or brachytherapy, risk estimates appear to rise with average time since treatment. However, average follow-up time is strongly related to indication for treatment (malignancy versus benign condition, and type of malignancy). Dosimetry is lacking for most of these studies, but average dose to bone marrow differs by indication for treatment as well. Thus, while it appears that studies with adequate latency tend to show increased risk of CLL after radiation exposure, the small number of studies reporting results, as well as the lack of dosimetry data, limit the interpretation of this suggestive finding. Case–control studies reporting CLL results for medical populations are very limited.
Interestingly, with the exception of a cohort of tuberculosis patients (Davis et al. 1989), all the studies with summary risk estimates below 1.00 were of malignant conditions, whereas studies of benign conditions showed elevated CLL risk estimates, although the elevations were not statistically significant. This difference is likely a function of follow-up time, and the relative survivability of malignant versus benign conditions. In addition, patients treated for malignant conditions, with the exception of testicular and cervical cancer, tend to be older than those treated for benign conditions, and are, therefore, more likely to have follow-up attenuated by competing causes of death.
Studies of patients exposed to Thorotrast studies present no compelling evidence of CLL radiogenicity from this type of treatment. The results for 131I treatment are mixed, with crude risk estimates positive for the two studies with >20 years of follow-up and no cases observed in three studies where follow-up was at most 10 years. However, in the studies with positive overall results, differences by indication for treatment and the lack of a clear dose–response suggests the need for more investigation.
Occupational studies tend to have average follow-up of at least 15 years; however, many report mortality, rather than cancer incidence, and the percent deceased among these cohorts is usually less than 25%, limiting study power. Studies of nuclear facility workers do not demonstrate consistent evidence of CLL radiogenicity; studies of two large cohorts, INL [85] and IARC [25], reported non-significant CLL deficits, while a study of a smaller cohort, LANL [94], reported a significant positive trend for CLL, based on very few cases. Studies of radiologists and radiologic technologists tend to show higher CLL risks for those employed in the early years (prior to 1940 or 1950), when doses were presumably higher; however, the absence of actual dosimetry is a limitation of these studies. Non-significant elevations were also seen in some studies of nuclear test participants compared to non-participant veterans. Two large-multinational studies of airline pilots and crew were generally negative; non-significant elevations were found only among workers with >20 years since first employment and in intermediate dose categories. In occupational studies reporting risk for all leukemia and leukemia excluding CLL, the effects of excluding CLL vary, with some risk estimates increasing and others are decreasing. The confidence intervals for the two outcomes are not mutually exclusive, limiting interpretation and highlighting the need for explicit reporting of CLL risk.
Other issues hinder epidemiologic assessment of CLL radiogenicity. Disease misclassification is problematic, as evidenced by the Muirhead et al. study [111] of nuclear test participants, where six diagnostic discrepancies between CLL and NHL were found and the Weiss et al. [57] study of ankylosing spondylitis, in which a death certified as a CLL was noted as an acute myeloid leukemia in the medical records. Historically, CLL has been difficult to distinguish from other B-cell malignancies, such as NHL, leading to misdiagnoses [115]. Case ascertainment for mortality studies is particularly problematic because death certificates often fail to identify leukemia subtype [115]. CLL is an indolent disease and may not manifest for decades. In the 1970s, only 30–40% of cases were diagnosed asymptomatically [18]. With the advent of routine blood counting, 70–80% of CLL patients are diagnosed incidentally [24]. Ascertainment limitations are more severe for earlier deaths, where histology was not often performed and patients may have been first diagnosed when in blast crisis and, therefore, noted as acute or unspecified leukemia. These misdiagnoses may lead to underascertainment of CLL in the early years of study cohorts. Furthermore, mortality studies likely miss CLL cases, particularly where follow-up is short and only underlying cause of death, as recorded on the death certificate, is considered.
The risk status of study subjects can also complicate interpretation and generalization of study results. Prior exposures or genetic susceptibility contributing to an original malignancy may increase the risk of second primaries, potentially including CLL. Compromises in the immune system, whether genetic, resulting from the original malignancy, or resulting from treatment, may increase this risk. Some “benign” conditions may mark increased susceptibility or an underlying malignancy. For example, in a minority of cases, uterine bleeding could be symptomatic of an undiagnosed uterine cancer. Ankylosing spondylitis has an immune system component and may confer differential susceptibility to malignancies, including CLL. However, in general, patients treated for benign conditions are less likely to have such risk factors than patients treated for malignancies.
Uncontrolled confounding, which may lead to biased results, is particularly problematic in medical studies that fail to differentiate between treatment arms. Occupational studies may also suffer from uncontrolled confounding, as nuclear facility workers were exposed not only to external and internal radiation, but also to a number of potentially carcinogenic chemicals, during employment. Controlling for exposures which increase CLL risk is problematic, as these are generally unknown.
The use of short-lag periods is particularly problematic. Richardson et al. [113] recently published a narrative review on the radiogenicity of CLL emphasizing the importance of adequate follow-up time in studies of CLL; studies having a short follow-up time could potentially miss an effect from ionizing radiation if not enough time has elapsed after exposure for CLL to manifest. The authors suggest that latency for CLL may be upwards by 15 years [113]. Results from the medical cohort studies described in this review support this hypothesis. The majority of occupational and medical studies employ a two-year lag in analyses of leukemia; though perhaps suitable in the study of acute leukemia, such a lag assumption is probably not consistent with the disease course of CLL.
Future directions
Currently, therapeutic radiation is rarely used as treatment for non-malignant conditions; thus new patient populations of this type are unlikely to emerge. Updates of existing patient populations may provide more data on CLL risk, but follow-up of these cohorts generally already exceeds 20 years. In many studies of second malignancies, average follow-up time is quite short, especially for those first primaries which tend to be rapidly fatal. Studies of second primaries in patients with first primaries such as lung cancer, with a five-year survival rate below 20%, are particularly inappropriate for estimating the treatment-induced risk of subsequent CLL. Studies of breast cancer and prostate cancer are somewhat better, although the average age at diagnosis, particularly with prostate cancer, limits long-term survival rates. Testicular cancer may be the most suitable candidate for further study once sufficient follow-up accrues; patients are often young and 15-year survival rates exceed 75%. In the future, progress in diagnosis and treatment may improve patient survival, and hence follow-up time, making these populations more informative for CLL risk. However, the growing use of adjuvant chemotherapy as treatment for many conditions, as well as questions of underlying susceptibility, are problematic for evaluating radiation-associated risk of CLL.
Occupational studies are a more likely source of further information on CLL radiogenicity in the near future. The NIOSH-commissioned CLL-specific results of the IARC 15 country study, with 65 cases of CLL (both underlying and contributing causes) should prove informative, especially in the examination of alternate lag assumptions. Adequate power to detect a CLL risk on the order of radiation-induced leukemia requires a large sample size. Power analyses found that an updated pooled analysis of a number of existing occupational cohorts might garner sufficient person years, depending on the level of excess risk. However, occupational epidemiological studies are unlikely to rule out an association between CLL and radiation if the excess risk is very small (i.e., an RR of 1.02 at >100 mSv per the lymphoma risk model).
The power analysis was performed under several assumptions; deviations from those assumptions would alter the sample size calculations. If the baseline case rate is higher (for instance if both underlying and contributing causes of death were considered in a pooled cohort study, or if incident CLL cases were included), then the power of the study would increase and fewer PYs would be required. Inclusion of more high-dose workers, such as the Chernobyl group, would increase power to detect an association, although the shorter average follow-up and relatively young age of most Chernobyl workers could have the opposite effect. Conversely, inclusion of many workers with lower doses than those of US cohorts (e.g., Canadian National Dose Registry, mean dose = 5.8 mSv) could decrease power.
With further follow-up, more workers will shift into the older age categories, thereby increasing power. If the actual sex distribution by dose level in the potential pooled study differed greatly from that assumed in the power calculations, power would also be affected, as CLL is twice as prevalent in males as females. The Canadian National Dose Registry population [27] is approximately 50% female; however, the percent female per dose category used in the power calculations ranged from 20% to 35%.
Finally, consideration of incident CLL cases could increase statistical power while avoiding some of the ascertainment issues of death-certificate based studies. Indeed, the differences in results between Canadian incidence and mortality study data suggest a need for further investigation. However, as US cancer registries provide limited, though improving, coverage of the population, the contribution of this data source may be limited.
Other issues
Improved understanding of CLL risk conferred by internal, as well as external, radiation exposure is necessary. As populations exposed to internal emitters are smaller and the nature of the exposures diverse, achieving adequate study power while controlling for exposure a variates will be quite difficult. Two occupational populations in the former Soviet Union (Mayak and the Siberian Chemical Industrial Complex at Seversk) may present opportunities for further research on CLL risk following exposure to internal emitters, particularly plutonium. A database including 80,000 bioassays for 8,694 Mayak workers [116] has been developed. A registry of Seversk workers has been created, as well, although most of the plutonium-exposed workers had body burdens below the limit of detection [117]. However, these groups are small and have been already followed at length.
Studies of internally exposed worker populations in the US DOE and the UK may contribute additional information with further follow-up, but these cohorts are often small. Studies to date providing pertinent risk estimates for these workers [94, 118, 119] had 3 CLL deaths at most and are thus of limited value. A case–control study of a specific internal exposure nested within a pooled population of these workers is likely the most efficient approach to this issue.
Future research into the basic mechanisms of CLL is critical for improvements in epidemiologic evaluation of this disease. Determination of the proper organ for assessing radiation dose and understanding the mechanisms of CLL disease induction (particularly investigation of whether radiation potentially acts as an initiator, a promoter, or a complete carcinogen) will assist with determination of the optimal lag periods for future studies.
Summary
Up to date there is no conclusive evidence regarding CLL radiogencity. Occupational studies show inconsistent results for CLL radiogenicity, with some studies reporting decreased CLL risk and others reporting elevations; most results are not statistically significant. The observation of positive point estimates in studies with follow-up times greater than 15 years in the medical studies, and no positive estimates in studies with shorter follow-up, while based on a sample of convenience (availability of follow-up information and CLL results), is suggestive. Specifically, this finding is consistent with a long latency for CLL and highlights the need for studies with adequate follow-up time.
A pooled occupational cohort mortality study of US, UK, and Canadian cohorts with follow-up through 2012 would have approximately 10.5 million person-years (with a 15-year lag). Such a study might be informative for CLL if the risk of this disease is similar to that for other leukemias, but not if the risk more closely resembles that of NHL.
References
Ghia P, Caligaris-Cappio F (2006) The origin of B-cell chronic lymphocytic leukemia. Semin Oncol 33(2):150–156
Caporaso N (2006) Chips, candidate genes, and CLL. Blood 108(2):415–416
Sgambati MT, Linet MS, Devesa SS (2001) Chronic lymphocytic leukemia: epidemiological, familial, and genetic aspects. Marcel Dekker, Cheson BDNew York, pp 33–62
Goldin LR, Pfeiffer RM, Li X, Hemminki K (2004) Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish Family-Cancer Database. Blood 104(6):1850–1854
Wiernik PH, Ashwin M, Hu XP, Paietta E, Brown K (2001) Anticipation in familial chronic lymphocytic leukaemia. Br J Haematol 113(2):407–414
Amadori D, Nanni O, Falcini F et al (1995) Chronic lymphocytic leukaemias and non-Hodgkin’s lymphomas by histological type in farming-animal breeding workers: a population case–control study based on job titles. Occup Environ Med 52(6):374–379
Gonzalez CA, Agudo A (1999) Occupational cancer in Spain. Environ Health Perspect 107(Suppl 2):273–277
Lee E, Burnett CA, Lalich N, Cameron LL, Sestito JP (2002) Proportionate mortality of crop and livestock farmers in the United States, 1984–1993. Am J Ind Med 42(5):410–420
Institute of Medicine of the National Academies of Sciences (2003) Committee to review the health effects in Vietnam Veterans of exposure to herbicides (fourth biennial update). Veterans and agent orange: update 2002. The National Academies Press, Washington, DC, pp 372–377
Preston DL, Kato H, Kopecky K, Fujita S (1987) Studies of the mortality of A-bomb survivors. 8. Cancer mortality, 1950–1982. Radiat Res 111(1):151–178
Boice JD, Blettner M, Kleinerman RA et al (1987) Radiation dose and leukemia risk in patients treated for cancer of the cervix. J Natl Cancer Inst 79(6):1295–1311
Darby SC, Doll R, Gill SK, Smith PG (1987) Long term mortality after a single treatment course with X-rays in patients treated for ankylosing spondylitis. Br J Cancer 55(2):179–190
Boice JD, Engholm G, Kleinerman RA et al (1988) Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiat Res 116(1):3–55
National Research Council Committee on the Biological Effects of Ionizing Radiations (1990) Health effects of exposure to low levels of ionizing radiation: BEIR V. National Academy Press, Washington, DC
Guidelines for Determining the Probability of Causation Under the Energy Employees Occupational Illness Compensation Act of 2000; Final Rule. 42 CFR Part 81 (2202)
Radiation Exposure Compensation Act (1990) Public Law no. 101–426 (104 Stat. 925, 42 U.S.C. 2210)
National Institute for Occupational Safety and Health (2005) Report of public meeting to seek input on gaps in chronic lymphocytic leukemia (CLL) radiogenicity research held July 21, 2004. National Institute for Occupational Safety and Health; (NIOSH) 2006-100, Cincinnati, OH
Rozman C, Montserrat E (1995) Chronic lymphocytic leukemia. N Engl J Med 333(16):1052–1057
Gale RP, Foon KA (1987) Biology of chronic lymphocytic leukemia. Semin Hematol 24(4):209–229
Montserrat E , Rozman C (1995) Chronic lymphocytic leukemia: present status. Ann Oncol 6(3):219–235
Egret Siz: Sample Size, Power for Nonlinear Regression [computer program] (1997) Cytel Statistical Software Services, Version 1. Cytel Inc., Cambridge, MA
Land C, Gilbert E, Smith JM et al (2003). Report of the NCI–CDC working group to revise the 1985 NIH radioepidemiological tables. National Institutes of Health; NIH Publication No. 03-5387, Bethesda MD
Centers for Disease Control and Prevention (CDC) CDC Wonder Compressed Mortality Data. Accessed 09/28/2005. Available from: http://www.wonder.cdc.gov
Oscier D, Fegan C, Hillmen P et al (2004) Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol 125(3):294–317
Schubauer-Berigan MK, Macievic GV, Utterback DF, Tseng C, Flora JT (2005) An epidemiologic study of mortality and radiation-related risk of cancer among workers at the Idaho National Engineering and Environmental Laboratory. National Institute for Occupational Safety and Health; NIOSH publication 2005-131, Cincinnati, OH
Muirhead CR, Goodill AA, Haylock RG et al (1999) Occupational radiation exposure and mortality: second analysis of the National Registry for Radiation Workers. J Radiol Prot 19(1):3–26
Ashmore JP, Krewski D, Zielinski JM, Jiang H, Semenciw R, Band PR (1998) First analysis of mortality and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol 148(6):564–574
Ivanov VK, Tsyb AF, Gorsky AI et al (1997) Leukaemia and thyroid cancer in emergency workers of the Chernobyl accident: estimation of radiation risks (1986–1995). Radiat Environ Biophys 36(1):9–16
National Institute for Occupational Safety and Health (2001) PC-Life Table Analysis System version 1.0d. National Institute for Occupational Safety and Health
Holowaty EJ, Darlington GA, Gajalakshmi CK, Toogood PB, Levin W (1995) Leukemia after irradiation for endometrial cancer in Ontario. Cancer 76(4):644–649
Curtis RE, Boice JD, Stovall M, Flannery JT, Moloney WC (1989) Leukemia risk following radiotherapy for breast cancer. J Clin Oncol 7(1):21–29
Smith PG, Doll R (1982) Mortality among patients with ankylosing spondylitis after a single treatment course with X rays. Br Med J (Clin Res Ed) 284(6314):449–460
Hall P, Holm LE (1995) Cancer in iodine-131 exposed patients. J Endocrinol Invest 18(2):147–149
Kleinerman RA, Liebermann JV, Li FP (1985) Second cancer following cancer of the male genital system in Connecticut, 1935–82. Natl Cancer Inst Monogr 68:139–147
Shilnikova NS, Preston DL, Ron E et al (2003) Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res 159(6):787–798
Gilbert ES, Omohundro E, Buchanan JA, Holter NA (1993) Mortality of workers at the Hanford site: 1945–1986. Health Phys 64(6):577–590
Thaul S, Page WF, Crawford H, O’Maonaigh H (2005). The five series study: mortality of military participants in US nuclear weapons tests. National Academy Press, Washington, DC
Silver SR, Daniels RD, Taulbee TD et al (2004) Differences in mortality by radiation monitoring status in an expanded cohort of Portsmouth Naval Shipyard workers. J Occup Environ Med 46(7):677–690
Frome EL, Cragle DL, Watkins JP et al (1997) A mortality study of employees of the nuclear industry in Oak Ridge, Tennessee. Radiat Res 148(1):64–80
Telle-Lamberton M, Bergot D, Gagneau M et al (2004) Cancer mortality among French Atomic Energy Commission workers. Am J Ind Med 45(1):34–44
Mohan AK, Hauptmann M, Freedman DM et al (2003) Cancer and other causes of mortality among radiologic technologists in the United States. Int J Cancer 103(2):259–267
Hall P, Berg G, Bjelkengren G et al (1992) Cancer mortality after iodine-131 therapy for hyperthyroidism. Int J Cancer 50(6):886–890
Darby SC, Reeves G, Key T, Doll R, Stovall M (1994) Mortality in a cohort of women given X-ray therapy for metropathia haemorrhagica. Int J Cancer 56(6):793–801
Blettner M, Zeeb H, Langner I, Hammer GP, Schafft T (2002) Mortality from cancer and other causes among airline cabin attendants in Germany, 1960–1997. Am J Epidemiol 156(6):556–565
Zeeb H, Blettner M, Hammer GP, Langner I (2002) Cohort mortality study of German cockpit crew, 1960–1997. Epidemiology 13(6):693–699
Movsas B, Hanlon AL, Pinover W, Hanks GE (1998) Is there an increased risk of second primaries following prostate irradiation? Int J Radiat Oncol Biol Phys 41(2):251–255
Brenner DJ, Curtis RE, Hall EJ, Ron E (2000) Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 88(2):398–406
Bokemeyer C, Schmoll HJ (1993) Secondary neoplasms following treatment of malignant germ cell tumors. J Clin Oncol 11(9):1703–1709
Travis LB, Boice JD, Travis WD (2003) Second primary cancers after thymoma. Int J Cancer 107(5):868–870
Lavey RS, Eby NL, Prosnitz LR (1990) Impact of radiation therapy and/or chemotherapy on the risk for a second malignancy after breast cancer. Cancer 66(5):874–881
Boice JD, Day NE, Andersen A et al (1985) Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. J Natl Cancer Inst 74(5):955–975
Osterlind A, Rorth M, Prener A (1985) Second cancer following cancer of the male genital system in Denmark, 1943–80. Natl Cancer Inst Monogr 68:341–347
Redman JR, Vugrin D, Arlin ZA et al (1984) Leukemia following treatment of germ cell tumors in men. J Clin Oncol 2(10):1080–1087
Horwich A, Bell J (1994) Mortality and cancer incidence following radiotherapy for seminoma of the testis. Radiother Oncol 30(3):193–198
Kleinerman RA, Boice JD, Storm HH et al (1995) Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer 76(3):442–452
Fossa SD, Langmark F, Aass N, Andersen A, Lothe R, Borresen AL (1990) Second non-germ cell malignancies after radiotherapy of testicular cancer with or without chemotherapy. Br J Cancer 61(4):639–643
Weiss HA, Darby SC, Fearn T, Doll R (1995) Leukemia mortality after X-ray treatment for ankylosing spondylitis. Radiat Res 142(1):1–11
Wick RR, Nekolla EA, Gossner W, Kellerer AM (1999) Late effects in ankylosing spondylitis patients treated with 224Ra. Radiat Res 152(6 Suppl):S8–S11
Inskip PD, Kleinerman RA, Stovall M et al (1993) Leukemia, lymphoma, and multiple myeloma after pelvic radiotherapy for benign disease. Radiat Res 135(1):108–124
Davis FG, Boice JD, Hrubec Z, Monson RR (1989) Cancer mortality in a radiation-exposed cohort of Massachusetts tuberculosis patients. Cancer Res 49(21):6130–6136
Ryberg M, Lundell M, Nilsson B, Pettersson F (1990) Malignant disease after radiation treatment of benign gynaecological disorders. A study of a cohort of metropathia patients. Acta Oncol 29(5):563–567
Moller H, Mellemgaard A, Jacobsen GK, Pedersen D, Storm HH (1993) Incidence of second primary cancer following testicular cancer. Eur J Cancer 29A(5):672–676
Hellbardt A, Mirimanoff RO, Obradovic M, Mermillod B, Paunier JP (1990) The risk of second cancer (SC) in patients treated for testicular seminoma. Int J Radiat Oncol Biol Phys 18(6):1327–1331
Hay JH, Duncan W, Kerr GR (1984) Subsequent malignancies in patients irradiated for testicular tumours. Br J Radiol 57(679):597–602
Damber L, Larsson LG, Johansson L, Norin T (1995) A cohort study with regard to the risk of haematological malignancies in patients treated with X-rays for benign lesions in the locomotor system. I. Epidemiological analyses. Acta Oncol 34(6):713–719
Storm HH (1988) Second primary cancer after treatment for cervical cancer. Late effects after radiotherapy. Cancer 61(4):679–688
Dent SF, Klaassen D, Pater JL, Zee B, Whitehead M (2000) Second primary malignancies following the treatment of early stage ovarian cancer: update of a study by the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG). Ann Oncol 11(1):65–68
Harvey EB, Brinton LA (1985) Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr 68:99–112
Travis LB, Curtis RE, Boice JD, Platz CE, Hankey BF, Fraumeni JF (1996) Second malignant neoplasms among long-term survivors of ovarian cancer. Cancer Res 56(7):1564–1570
Travis LB, Curtis RE, Storm H et al (1997) Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst 89(19):1429–1439
Volk N, Pompe-Kirn V (1997) Second primary cancers in breast cancer patients in Slovenia. Cancer Causes Control 8(5):764–770
Curtis RE, Hoover RN, Kleinerman RA, Harvey EB (1985) Second cancer following cancer of the female genital system in Connecticut, 1935–82. Natl Cancer Inst Monogr 68:113–137
Curtis RE, Boice JD, Stovall M et al (1994) Relationship of leukemia risk to radiation dose following cancer of the uterine corpus. J Natl Cancer Inst 86(17):1315–1324
Boice JD, Morin MM, Glass AG et al (1991) Diagnostic X-ray procedures and risk of leukemia, lymphoma, and multiple myeloma. JAMA 265(10):1290–1294
Gibson R, Graham S, Lilienfeld A, Schuman L, Dowd JE, Levin ML (1972) Irradiation in the epidemiology of leukemia among adults. J Natl Cancer Inst 48(2):301–311
Boivin JF, Hutchison GB, Evans FB, Abou-Daoud KT, Junod B (1986) Leukemia after radiotherapy for first primary cancers of various anatomic sites. Am J Epidemiol 123(6):993–1003
Travis LB, Hauptmann M, Gaul LK et al (2003) Site-specific cancer incidence and mortality after cerebral angiography with radioactive Thorotrast. Radiat Res 160(6):691–706
van Kaick G, Dalheimer A, Hornik S et al (1999) The German Thorotrast study: recent results and assessment of risks. Radiat Res 152(6 Suppl):S64–S71
Andersson M, Carstensen B, Storm HH (1995) Mortality and cancer incidence after cerebral arteriography with or without Thorotrast. Radiat Res 142(3):305–320
dos Santos Silva I, Malveiro F, Jones ME, Swerdlow AJ (2003) Mortality after radiological investigation with radioactive Thorotrast: a follow-up study of up to fifty years in Portugal. Radiat Res 159(4):521–534
Ron E, Doody MM, Becker DV et al (1998) Cancer mortality following treatment for adult hyperthyroidism. Cooperative Thyrotoxicosis Therapy Follow-up Study Group. JAMA 280(4):347–355
de Vathaire F, Schlumberger M, Delisle MJ et al (1997) Leukaemias and cancers following iodine-131 administration for thyroid cancer. Br J Cancer 75(5):734–739
Dottorini ME, Lomuscio G, Mazzucchelli L, Vignati A, Colombo L (1995) Assessment of female fertility and carcinogenesis after iodine-131 therapy for differentiated thyroid carcinoma. J Nucl Med 36(1):21–27
Brincker H, Hansen HS, Andersen AP (1973) Induction of leukemia by 131-I treatment of thyroid carcinoma. Br J Cancer 28(3):232–237
Cardis E, Gilbert ES, Carpenter L et al (1995) Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res 142(2):117–132
Cardis E, Vrijheid M, Blettner M et al (2005) Risk of cancer after low doses of ionising radiation: retrospective cohort study in 15 countries. BMJ 331(7508):77
Sont WN, Zielinski JM, Ashmore JP et al (2001) First analysis of cancer incidence and occupational radiation exposure based on the National Dose Registry of Canada. Am J Epidemiol 153(4):309–318
Zablotska LB, Ashmore JP, Howe GR (2004) Analysis of mortality among Canadian nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res 161(6):633–641
Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J (2004) Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res 162(5):517–526
McGeoghegan D, Binks K (2000) The mortality and cancer morbidity experience of workers at the Springfields uranium production facility, 1946–95. J Radiol Prot 20(2):111–137
Kubale TL, Daniels RD, Yiin JH et al (2005) A nested case–control study of leukemia mortality and ionizing radiation at the Portsmouth Naval Shipyard. Radiat Res 164(6):810–819
Konogorov AP , Ivanov VK, Chekin SY, Khait SE (2000) A case–control analysis of leukemia in accident emergency workers of Chernobyl. J Environ Pathol Toxicol Oncol 19(1–2):143–151
Wiggs LD, Cox-DeVore CA, Wilkinson GS, Reyes M (1991) Mortality among workers exposed to external ionizing radiation at a nuclear facility in Ohio. J Occup Med 33(5):632–637
Wiggs LD, Johnson ER, Cox-DeVore CA, Voelz GL (1994) Mortality through 1990 among white male workers at the Los Alamos National Laboratory: considering exposures to plutonium and external ionizing radiation. Health Phys 67(6):577–588
Atkinson WD, Law DV, Bromley KJ, Inskip HM (2004) Mortality of employees of the United Kingdom Atomic Energy Authority, 1946–97. Occup Environ Med 61(7):577–585
Omar RZ, Barber JA, Smith PG (1999) Cancer mortality and morbidity among plutonium workers at the Sellafield plant of British Nuclear Fuels. Br J Cancer 79(7–8):1288–1301
Wartenberg D, Brown S, Mohr S, Cragle D, Friedlander B (2001) Are African-American nuclear workers at lower mortality risk than Caucasians? J Occup Environ Med 43(10):861–871
Hadjimichael OC, Ostfeld AM, D’Atri DA, Brubaker RE (1983) Mortality and cancer incidence experience of employees in a nuclear fuels fabrication plant. J Occup Med 25(1):48–61
Koshurnikova NA, Buldakov LA, Bysogolov GD, Bolotnikova MG, Komleva NS, Peternikova VS (1994) Mortality from malignancies of the hematopoietic and lymphatic tissues among personnel of the first nuclear plant in the USSR. Sci Total Environ 142(1–2):19–23
Boice JD, Holm LE (1997) Radiation risk estimates for leukemia and thyroid cancer among Russian emergency workers at Chernobyl. Radiat Environ Biophys 36(3):213–214
Rahu M, Tekkel M, Veidebaum T et al (1997) The Estonian study of Chernobyl cleanup workers: II. Incidence of cancer and mortality. Radiat Res 147(5):653–657
Hatch M, Ron E, Bouville A, Zablotska L, Howe G (2005) The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Epidemiol Rev 27:56–66
Darby SC, Whitley E, Howe GR et al (1995) Radon and cancers other than lung cancer in underground miners: a collaborative analysis of 11 studies. J Natl Cancer Inst 87(5):378–384
Rericha V, Kulich M, Rericha R, Shore DL, Sandler DP (2006) Incidence of leukemia, lymphoma, and multiple myeloma in Czech uranium miners: a case-cohort study. Environ Health Perspect 114(6):818–822
Smith PG, Doll R (1981) Mortality from cancer and all causes among British radiologists. Br J Radiol 54(639):187–194
Doody MM, Mandel JS, Lubin JH, Boice JD (1998) Mortality among United States radiologic technologists, 1926–90. Cancer Causes Control 9(1):67–75
Sigurdson AJ, Doody MM, Rao RS et al (2003) Cancer incidence in the US radiologic technologists health study, 1983–1998. Cancer 97(12):3080–3089
Linet MS, Freedman DM, Mohan AK et al (2005) Incidence of haematopoietic malignancies in US radiologic technologists. Occup Environ Med 62(12):861–867
Pukkala E, Aspholm R, Auvinen A et al (2003) Cancer incidence among 10,211 airline pilots: a Nordic study. Aviat Space Environ Med 74(7):699–706
Langner I, Blettner M, Gundestrup M et al (2004) Cosmic radiation and cancer mortality among airline pilots: results from a European cohort study (ESCAPE). Radiat Environ Biophys 42(4):247–256
Muirhead CR , Bingham D, Haylock RG et al (2003) Follow up of mortality and incidence of cancer 1952–98 in men from the UK who participated in the UK’s atmospheric nuclear weapon tests and experimental programmes. Occup Environ Med 60(3):165–172
Muirhead CR, Bingham D, Haylock RG et al (2003). Mortality and cancer incidence 1952–1998 in UK participants in the UK atmospheric nuclear weapon tests and experimental programmes. National Radiological Protection Board; NRPB-W27, Didcot Oxfordshire
Richardson DB, Wing S, Schroeder J, Schmitz-Feuerhake I, Hoffmann W (2005) Ionizing radiation and chronic lymphocytic leukemia. Environ Health Perspect 113(1):1–5
Dyagil I, Adam M, Beebe GW et al (2002) Histologic verification of leukemia, myelodysplasia, and multiple myeloma diagnoses in patients in Ukraine, 1987–1998. Int J Hematol 76(1):55–60
Finch SC, Linet MS (1992) Chronic leukaemias. Baillieres Clin Haematol 5(1):27–56
Romanov SA, Vasilenko EK, Khokhryakov VF, Jacob P (2002) Studies on the Mayak nuclear workers: dosimetry. Radiat Environ Biophys 41(1):23–28
Kisselev M, Kellerer AM (2002) The potential for studies in other nuclear installations. On the possibility of creating medico-dosimetry registries of workers at the Siberian Chemical Industrial Complex (SCIC) and the Mountain Chemical Industrial Complex (MCIC) in Tomsk, Krasnoyarsk. Radiat Environ Biophys 41(1):81–83
Carpenter LM, Higgins CD, Douglas AJ et al (1998) Cancer mortality in relation to monitoring for radionuclide exposure in three UK nuclear industry workforces. Br J Cancer 78(9):1224–1232
Wiggs LD, Cox-DeVore CA, Voelz GL (1991) Mortality among a cohort of workers monitored for 210Po exposure: 1944–1972. Health Phys 61(1):71–76
Acknowledgment
Funding for this study was provided through an agreement between the U.S. Department of Energy (DOE) and the U.S. Department of Health and Human Services (DHHS). Required disclaimer - The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silver, S.R., Hiratzka, S.L., Schubauer-Berigan, M.K. et al. Chronic lymphocytic leukemia radiogenicity: a systematic review. Cancer Causes Control 18, 1077–1093 (2007). https://doi.org/10.1007/s10552-007-9048-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-007-9048-y