Abstract
Background
Higher birth weight and maternal history of miscarriage has been associated with an increased risk of childhood leukemia. The possibility that this association may be sex-specific has not been explored in detail in previous studies.
Methods
In a retrospective case-control study, 732 childhood (≤14 years) cancer cases from a population-based Registry in Northern England whose hospital birth records could be accessed and 3,723 controls matched for date and hospital of birth to the cases were compared. We examined birth weight for sex-specific associations with childhood cancer. Conditional logistic regression analysis was used for statistical evaluation of associations.
Results
In acute lymphoblastic leukemia (ALL) (225 cases and 1,163 matched controls), birth weight and sex showed a strong interaction (P = 0.003). In boys with ALL, but not in girls, there was a nonlinear association with birth weight (P for trend = 0.008; OR = 3.05 for the highest quintile compared to the second lowest quintile, 95% CI = 1.40–6.64; P = 0.005). When birth weights were adjusted using UK standards for gestational age and sex, the risk associations were similar in statistical significance and magnitude. Maternal history of miscarriage showed an association with all cancers and individually with ALL. The miscarriage association with ALL was statistically significant in boys only (OR = 1.91, 95% CI = 1.07–3.42; P = 0.03). A multivariable model for ALL containing other examined maternal and reproductive variables confirmed the independence of the birth weight and miscarriage associations. There was no birth weight or miscarriage associations in other cancers.
Conclusions
This study confirmed the risk associations with birth weight and miscarriages in childhood ALL. Statistically significant association of size at birth suggested marked differences in etiology between girls and boys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maternal reproductive history (miscarriage, infertility, long intervals between births) and certain birth characteristics (birth weight, birth order) have been previously examined in relation to childhood cancer risk with most studies focusing on leukemia [1]. Increased risk with birth weight, being first-born or with maternal history of miscarriage have been reported. However, these factors have rarely been investigated simultaneously in the same study and mechanisms for these associations are largely unknown although various possibilities have been proposed.
The association with birth weight has been observed in many [2–12] but not all studies; most notably a recent population-based Californian study has reported no association [13]. Several biologic explanations have been proposed for the association with birth weight [3, 14–17] but have not been tested yet. Birth weight is correlated with sex, maternal height and body mass index, gestational age, maternal age, parity and maternal smoking [18, 19], but most studies have been unable to take these additional factors into account. Some previous studies have reported a risk association also with low birth weight in childhood leukemias [10, 20].
Despite the well-established difference in childhood cancer risk between sexes [11, 21–23], the reasons for the higher risk in boys is unclear. Few studies have considered sex-specific associations. However, several genetic associations have been reported exclusively in boys [24–27] including one regarding prenatal loss [28]. Another sex-specific finding is the decreased cancer risk in male twins [29, 30]. This consistent finding is generally attributed to selective early mortality of male twin fetuses or neonates who would otherwise have developed a clinical cancer. The cumulative data suggest that the miscarriage association in childhood cancers should influence boys more than girls. Despite growing recognition of sex differences in disease pathogenesis, response to treatment and prognosis [31], most studies only report their results adjusted for sex rather than doing sex-specific analysis. In light of the generally increased risk for childhood cancers in boys, we hypothesized that the birth weight and miscarriage associations should be stronger in boys. In the present study, we investigated these associations in childhood cancers to test their sex-specificity.
Subjects and methods
Cases
The cases were identified from the ‘Northern Region Young Persons’ Malignant Disease Registry’ (NRYPMDR), a population-based cancer registry [32]. The cases analyzed in this study represent 732 of childhood (≤14 years) cancer cases born and diagnosed in the North of England between 1968 and 1992, and who were born in a hospital. There were a further 701 eligible cases in the registry diagnosed with childhood cancer during the same period but their hospital records were unavailable for this study or they were born at home. The reasons for this were either the closure of the maternity unit or failure to keep earlier birth records in some units. The excluded and included cases were compared for their sex, diagnosis and age-at-diagnosis and no significant differences were found (results not shown). Detailed information on case selection can be found elsewhere [33]. Cases were grouped into leukemias and non-leukemia cancers as shown in Table 1. Within the leukemia group, 225 were acute lymphoblastic leukemia (ALL) and 28 were non-ALL.
Controls
Controls were selected from the same date and place of birth of cases by taking the 4th, 8th and 12th birth before and after the index birth by using the birth or admission registers. Out of 3,742 population-based controls randomly selected from the same birthplace and around the same date as the index cases, 19 were excluded because of the ambiguity in the information obtained (mainly the lack of sex information). Thus, the number of controls was 3,723 representing an average control-to-case ratio of 5.1 to 1. Further details of control selection are given elsewhere [33]. For the 225 cases with ALL, there were 1,163 matched controls.
Data
The data included variables other than birth weight and they were included in the analysis as covariates. Estimated duration of gestation was based on both the date of the last menstrual period reported by the mother or the assessment of an obstetrician as extracted from maternity unit records. Gestational age data were missing in 87 cases (12%) and 522 controls (14.0%). Gestational age was categorized as term (37 week or more) and preterm (less than 37 weeks). Maternal age data were missing in an even greater percentage of cases and controls (26.5% of cases and 22.8% of controls). Maternal age association was assessed using five quintiles corresponding to 16–21 years; 22–24 years; 25 and 26 years; 27–30 years; and 31–45 years. Birth weight was recorded in either pounds and ounces or grams (g) but all data were converted into g. Birth weight-for-gestational-age z-scores (standard deviations) were calculated by standardizing the raw birth weights for gestational age and sex, according to sex-specific British reference standards [34]. Because of missing gestational age data, z-scores could not be calculated in all cases and controls. Birth weight associations with childhood cancer risk were sought using both weight in g and the standardized (relative) birth weights (z-scores) to distinguish associations with size at birth and size for gestational age. We used categorized birth weight variable to test the linearity of the birth weight association as well as fractional polynomial regression (see below). Birth weight quintiles were used to estimate the strength of the association. The quintiles were assigned using the birth weight distribution in the controls only. A second set of sex-specific quintiles was defined using the birth weight distribution for each sex (controls only). All reported sex-specific results were obtained using sex-specific birth weight quintiles. The age-specificity of the birth weight association was explored for age groups 0–5 years and 5–15 years.
The association with birth-order was examined as an association with the number of previous live-births (0, 1, 2 or more). This variable was derived from the data on the number of pregnancies and previous pregnancy outcomes obtained from the obstetric records of the mothers. The number of previous pregnancies variable was categorized the same way as the live-births variable (0, 1, 2 or more). Miscarriage and stillbirth data were obtained from obstetric records. These two variables were recorded as presence or absence of miscarriage or stillbirth in the past history because of the small numbers of more than one event.
Statistical analysis
Correlations between variables were assessed using Spearman’s rank correlation. We used conditional logistic regression to evaluate the association between the variables of interest and risk of childhood cancer. Univariate odds ratios (OR) and their corresponding 95% confidence intervals (95% CI) were calculated initially for each of the following variables: sex, gestational age, birth weight of the index child, maternal age, presence of previous miscarriage or stillbirth in maternal history, prior pregnancy outcome, and the total number of previous pregnancies and live-births. Multivariable models were constructed to obtain standardized risk estimates. To examine trend for nonlinear association with birth weight, we used fractional polynomial regression modeling which is based on transformation and fractional polynomials and retains the continuous scale of the data without using arbitrary categories [35]. All statistical tests were two-sided.
Analyses were performed for all cancers and also leukemias (ALL and non-ALL) separately. The number of cases for non-ALL leukemias was too small (n = 28) and the results for this group were not presented separately. As the sex-specificity of any association with childhood cancer was of interest, analyses stratified by sex were done to determine sex-specificity of associations. The interaction between sex and variables of interest was also tested using conditional logistic regression. Positive associations were also checked by using the whole control group in non-conditional logistic regression analysis. All statistical analyses were performed using the statistical software package Stata v9.0 (StataCorp, Texas, USA).
Results
Table 1 presents some characteristics of the participants and descriptive data. In the whole group there were 732 cases and 3,723 controls (average case-to-control ratio: 1/5.1). The male-to-female ratio was 1.19 in cases and 1.09 in controls. In the case group, the most common cancer was leukemia (35%) followed by central nervous system tumors (22%) and neuroblastic tumors (9%).
Birth weight
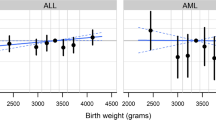
We first examined the linearity of birth weight association using (conditional) fractional polynomial modeling which showed a nonlinear relationship in boys with ALL (P = 0.03). When quintiles based on all controls were used, there was no association in the overall group. In ALL, however, there was an association in boys only (Table 2). Interaction between birth weight and sex was found by both conditional fractional polynomial modeling of continuous birth weight data (P = 0.006) and by conditional logistic regression using the raw birth weight data as a continuous measure and sex being a two-level indicator variable (P = 0.003). Because of the nonlinearity of the association, we present quintile-specific odds ratios using the lowest risk group (second quintile) as reference. In boys with ALL and matched controls, the lowest sex-specific quintile showed a statistically nonsignificant association in the risk direction and the increased risk was statistically significant in each of the higher quintiles with ORs = 2.53, 2.16 and 3.05, respectively (Table 2). The same analysis was repeated using birth weight quintiles that was based on overall birth weight distribution regardless of the sex and a similar result was obtained in boys (not shown). To check whether exclusion of cases due to unavailability of birth records may have introduced any bias, the associations were examined for time periods 1968–1985 and 1986–1992. No difference was found in the results in this split analysis (data not shown).
We examined the age-specificity of the birth weight association as has been reported in some earlier studies [2, 4, 8, 36]. Similar to previous studies, the risk was higher for children who were younger than 5 years of age at diagnosis for boys with ALL (OR = 5.56 for the highest sex-specific birth weight quintile compared with the second quintile, 95% CI = 1.82–17.04; P = 0.003). In older boys the risk was much lower (OR = 1.38 for the highest quintile) and statistically non-significant (P = 0.60). The weak protective effect of higher birth weight in girls was also stronger in younger children (OR = 0.33 for the fourth sex-specific birth weight quintile compared with the third quintile, 95% CI = 0.12–0.03; P = 0.03) and there was no statistically significant association in girls 6–15 years old (ORs = 0.93–1.25).
Most previous studies estimated the risk for high birth weight using a cutoff value of 4,000 g and low birth weight for the cutoff value of 2,500 g. In our group, there were few children with birth weight above 4,000 g (18 boys with ALL and 72 matched controls). We did not confirm the birth weight association using these cutoff values (three birth weight categories) in boys with childhood ALL (OR = 1.36, 95% CI = 0.80–2.33, P = 0.26). We provide the results using five usual birth weight categories in Table 2 for comparisons with other studies.
When adjusted for gestational age in a multivariable conditional logistic regression model (resulting in a slightly smaller sample due to missing gestational ages), the birth weight association in boys with ALL remained unaffected (OR = 3.02 for the highest sex-specific birth weight quintile compared with the second quintile, 95% CI = 1.36–6.67; P = 0.006).
In the smaller maternal age data-positive subgroup, adjustment for maternal age did not change the magnitude of birth weight association (not shown). The birth weight (quintile) association was also adjusted for miscarriage history. Conditional logistic regression analysis confirmed the independence of these associations: the association with the highest quintile remained unchanged (OR = 3.09, 95% CI = 1.40–6.81, P = 0.005) and miscarriage association was also unaffected (OR = 1.97, 95% CI = 1.07–3.63; P = 0.03).
Logistic regression analysis
In an unconditional analysis, all controls (n = 1,943) were used for comparisons with cases (n = 118), and the birth weight association in boys with ALL remained statistically significant (OR = 2.08 for the highest quintile, 95% CI = 1.11–3.90; P = 0.02). The same approach did not yield a statistically significant (protective) association in girls despite the larger sample size.
Finally, the re-examination of the multivariable model by unconditional analysis confirmed the independence of birth weight (quintile) and miscarriage associations in boys with childhood ALL. In the final model, associations with birth weight quintiles and miscarriage retained statistical significance (data not shown).
Other maternal and reproductive variables
Maternal age
In the most relevant group, boys with ALL and their matched controls had maternal age data in 74.7% and 78.5%, respectively. Despite the smaller numbers, maternal age showed a U-shaped risk association in childhood ALL and again only for boys. When the middle quintile (25 and 26 years) was used as reference, all other quintiles yielded odds ratios greater than 2.0 (2.18–3.50) and all but the lowest quintile (16–21 years) associations were statistically significant (P < 0.05).
Gestational age
Despite the expected strong correlation between birth weight and gestational age (Spearman’s r = 0.37; P < 0.0001), there was no association between gestational age and any groups nor any interaction with sex (Table 3). There were, however, very few preterm babies in the sample.
Number of previous pregnancies and live-births
There was no association with the number of previous pregnancies or live-births either overall or within the ALL group (Table 3). When we investigated specifically the association with being first-born (no previous live-birth), the ORs were greater than unity but were not statistically significant (data not shown).
Previous pregnancy outcome
As a measure of the relationship between reproductive success and ALL risk, the effect of previous live birth was examined. About a third of boys with ALL (43 of 118, 36%) were preceded by a live birth and 287 of 600 matched controls had the same history (48%). Conditional logistic regression analysis showed a significant protective effect of a live birth in the immediately preceding pregnancy in boys (OR = 0.60, 95% CI = 0.38–0.96; P = 0.03). The same analysis in girls yielded a nonsignificant finding (OR = 0.82, 95% CI = 0.51–1.32; P = 0.41).
Miscarriages
The presence of spontaneous miscarriage in maternal history was associated with increased risk for all cancers combined (OR = 1.29; 95% CI = 1.05–1.62, P = 0.02). For the individual cancer groups, this association was significant only for ALL (OR = 1.56; 95% CI = 1.07–2.27, P = 0.02) and osteosarcoma (OR = 4.68; 95% CI = 1.06–20.7, P = 0.04). Stratification by sex showed higher risk in boys (Table 3) although the difference in risk between the sexes did not reach statistical significance (P for interaction for sex and miscarriage = 0.48). This association did not vary between the age groups in either sex. In other cancers, only osteosarcoma showed a risk association with maternal history of miscarriages but this finding was based on a comparison of 12 cases and 54 matched controls. Logistic regression analysis using the whole control group showed an association with miscarriage in the whole ALL group (OR = 1.54, 95% CI = 1.09–2.18; P = 0.01), which was again statistically significant in boys only (OR = 1.67, 95% CI = 1.06–2.65; P = 0.03) with P = 0.21 in girls.
Stillbirths
Maternal history of previous stillbirth was positive for 10 cases (3 in the ALL group) and 48 controls (19 in the ALL group). These numbers were too small for a meaningful analysis.
Discussion
Using data from medical records, in this population-based case–control study of all childhood cancers, we confirmed the birth weight risk association in childhood ALL and also unraveled its nonlinear nature and sex-specificity. The use of standardized birth weight did not alter the results. This approach and the results it brought up will have an impact on future studies of etiologic mechanisms. We also found that the risk of childhood ALL conferred by a history of miscarriage in subsequent offspring was somewhat greater in boys.
Miscarriage association in childhood ALL is not new. Three major studies, Oxford Childhood Cancer Study [37], Tristate Study [38] and Childrens Oncology Group Studies [36, 39] have reported associations between maternal history of miscarriage and childhood cancer. In the present study, miscarriage history showed an association which was stronger in boys both in matched analysis and unmatched analysis, which used the whole control group. The sex effect in miscarriage association is biologically plausible. The extreme male predominance at the time of fertilization with estimates ranging between 120 and 160 males per 100 females [40] suggests a disproportionate loss of males during pregnancy to result in the sex ratio of around 1.05 at birth. We interpret these data as suggesting that the excess male frequency in childhood ALL may be due to boys that are destined to be miscarried but survive prenatal selection only to remain at high risk for ALL. Earlier studies identified parental HLA sharing as a risk factor for both recurrent miscarriages and childhood leukemia [reviewed in Ref. 41] and molecular studies revealed a homozygous HLA class II genotype as a male-specific risk marker for childhood ALL [25, 27] while overall HLA class II homozygosity was decreased in newborn boys compared with girls [28]. Thus, increased risks for miscarriage and childhood ALL may be related components of perinatal male disadvantage and may share a similar genetic background. The protective effect of a live birth history in the pregnancy prior to the index case in boys only also supports this hypothesis. The sex effect in the miscarriage association requires independent replication and its possible connection with HLA-mediated risk modification of childhood ALL warrants further investigation.
High birth weight has been associated with a generally increased risk for childhood leukemias, neuroblastoma and Wilms’ tumor in previous studies [2–11]. Despite the sexual dimorphism of birth weight and the sex effect in childhood cancer susceptibility, birth weight association studies generally overlooked sex-specificity of this association. In the sex-matched case–control study of the Nordic countries, sex did not modify the birth weight association [10]. The only other study, which considered sex as an effect modifier in birth weight association in childhood cancer is the UK Childhood Cancer Study Group (UKCCSG) [11]. This multicenter study reported a stronger association (with being heavier than 4,000 g) in girls aged less than 2-years with acute myeloblastic leukemia (AML). We did not have sufficient numbers for AML to examine this association but the mean birth weight in girls with AML was slightly higher (3,467 g, n = 7) than in boys (3,324 g, n = 12). The UKCCSG study, however, did not find the male-specific association that we have noted in ALL. The single-center nature of the present study in an ethnically homogeneous part of England and our demonstration of the nonlinearity of the birth weight association, rather than using cutoff values, may have caused this discrepancy.
Male fetuses grow at a faster rate than female fetuses and subsequently, on average, male infants are heavier than females for the same gestational age [19]. With males being overrepresented in most childhood cancers, a spurious association may arise if sex is not taken into account and if the control group has disproportionate number of males. To control for such differences, in addition to the stratified analysis, we also used birth weights standardized for UK national reference for gestational age. The birth weight associations in ALL did not get stronger when standardized birth weights were used. This observation suggested that size at birth rather than in utero growth trajectory is of etiologic importance in childhood ALL but only in boys. Our dataset had a high proportion of term babies and therefore birth weight was a good indicator of fetal growth.
There may be a genetic component in birth weight determination that also affects susceptibility to leukemia. One of the strongest predictors of birth weight is previous sibling’s birth weight [42]. This finding has been confirmed in leukemic families [10, 11]. One hypothesis proposes that increased growth factor production may increase both birth weight and leukemic cell proliferation [14, 43]. Increasing availability of large biologic sample banks should enable testing of this hypothesis soon. Because body mass and weight also increase adult cancer risk, it has been postulated that cancer risk is proportional to the number of proliferating cells [16, 17]. For all cancers, increased cell division is a risk factor whatever the reason [44]. In childhood leukemia, greater birth weight is thought to be associated with a higher rate of cell proliferation and/or a larger number of precursor cells being at risk of malignant transformation [3]. At present, this is the most parsimonious explanation and may have a genetic as well as an environmental basis. These two hypotheses are not mutually exclusive and a common genetic basis for increased growth factor production and high birth weight is plausible. One other growth factor for both fetal [45, 46] and cancer cell growth [47] is iron. The HFE gene shows a replicated association with childhood ALL in boys only [26], which is likely to be due to the effect of this mutation on body iron content [48]. Iron excess is also linked with gestational diabetes [49] and recently, an HFE association in gestational diabetes has been reported [50]. As gestational diabetes has been proposed one of the possible biological mechanisms of birth weight association with childhood cancer [2], and given the parallels in the epidemiology of diabetes and childhood leukemia, including the sex effect [51], the iron and diabetes connection is also noteworthy. These observations suggest that just like IGF-1, iron may also be connected with both birth weight and leukemia risk.
Our results should be considered in light of the study strengths and limitations. While it has several major strengths such as its population-based nature, unbiased source of information, inclusion of all childhood cancers, sample size and ethnically homogeneous study base, the limitations include our inability to include all of the cases identified for the study period, insufficient power for analysis by type for some cancers, and lack of environmental exposure data. We were also unable to examine the recently reported effect modification of the birth weight association by maternal pregnancy weight [12]. The main reason for not including a case was inability to access the birth records. Cases diagnosed earlier in the study period would be more likely to be excluded because of the lack of birth records and temporal trend in birth weight could distort the results. Our use of controls matched for birthplace and birth date, however, was a safeguard against this. Another possibility is that a bias may have been introduced because of the exclusion of births at home. This is deemed to be an infrequent occurrence and the same exclusion criteria also applied to controls. We are confident that the inclusion of 732 of eligible cases diagnosed in the defined time period and region did not distort the results obtained. In any case, however, the findings of this study, namely the sex-specificity of the birth weight association and the differences in the strength of the miscarriage association in childhood ALL need to be replicated in an independent study.
In conclusion, our findings have provided further insight to the previously known birth weight association in childhood ALL by showing that it concerns boys more strongly. This aspect of these associations has not been widely explored before. When sex is taken into account, genetic association studies also reveal stronger results for boys [24–27]. These results should encourage further investigations on sex specificity of risk associations in childhood ALL. The findings are useful in that mechanisms of these associations may be easier to study in the light of the sex effect.
References
Linet MS, Wacholder S, Zahm SH (2003) Interpreting epidemiologic research: lessons from studies of childhood cancer. Pediatrics 112(1 Pt 2):218–232
Daling JR, Starzyk P, Olshan AF, Weiss NS (1984) Birth weight and the incidence of childhood cancer. J Natl Cancer Inst 72(5):1039–1041
Westergaard T, Andersen PK, Pedersen JB, et al. (1997) Birth characteristics, sibling patterns, and acute leukemia risk in childhood: a population-based cohort study. J Natl Cancer Inst 89(13):939–947
Yeazel MW, Ross JA, Buckley JD, Woods WG, Ruccione K, Robison LL (1997) High birth weight and risk of specific childhood cancers: a report from the Children’s Cancer Group. J Pediatr 131(5):671–677
Schuz J, Kaletsch U, Meinert R, Kaatsch P, Spix C, Michaelis J (2001) Risk factors for neuroblastoma at different stages of disease. Results from a population-based case–control study in Germany. J Clin Epidemiol 54(7):702–709
Schuz J, Kaletsch U, Meinert R, Kaatsch P, Michaelis J (2001) High-birth weight and other risk factors for Wilms tumour: results of a population-based case–control study. Eur J Pediatr 160(6):333–338
Murray L, McCarron P, Bailie K, et al. (2002) Association of early life factors and acute lymphoblastic leukaemia in childhood: historical cohort study. Br J Cancer 86(3):356–361
Okcu MF, Goodman KJ, Carozza SE, et al. (2002) Birth weight, ethnicity, and occurrence of cancer in children: a population-based, incident case–control study in the State of Texas, USA. Cancer Causes Control 13(7):595–602
Hjalgrim LL, Westergaard T, Rostgaard K, et al. (2003) Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol 158(8):724–735
Hjalgrim LL, Rostgaard K, Hjalgrim H, et al. (2004) Birth weight and risk for childhood leukemia in Denmark, Sweden, Norway, and Iceland. J Natl Cancer Inst 96(20):1549–1556
Roman E, Simpson J, Ansell P, Lightfoot T, Mitchell C, Eden TO (2005) Perinatal and reproductive factors: a report on haematological malignancies from the UKCCS. Eur J Cancer 41(5):749–759
McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS (2006) Birth weight, maternal weight and childhood leukaemia. Br J Cancer 94(11):1738–1744
Reynolds P, Von Behren J, Elkin EP (2002) Birth characteristics and leukemia in young children. Am J Epidemiol 155(7):603–613
Ross JA, Perentesis JP, Robison LL, Davies SM (1996) Big babies and infant leukemia: a role for insulin-like growth factor-1? Cancer Causes Control 7(5):553–559
Vorwerk P, Wex H, Hohmann B, Mohnike K, Schmidt U, Mittler U (2002) Expression of components of the IGF signalling system in childhood acute lymphoblastic leukaemia. Mol Pathol 55(1):40–45
Albanes D, Winick M (1988) Are cell number and cell proliferation risk factors for cancer? J Natl Cancer Inst 80(10):772–774
Albanes D (1990) Energy balance, body size, and cancer. Crit Rev Oncol Hematol 10(3):283–303
Hindmarsh PC, Geary MP, Rodeck CH, Kingdom JC, Cole TJ (2002) Intrauterine growth and its relationship to size and shape at birth. Pediatr Res 52(2):263–268
Storms MR, Van Howe RS (2004) Birthweight by gestational age and sex at a rural referral center. J Perinatol 24(4):236–240
Schuz J, Kaatsch P, Kaletsch U, Meinert R, Michaelis J (1999) Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol 28(4):631–639
McNally RJ, Rowland D, Roman E, Cartwright RA (1997) Age and sex distributions of hematological malignancies in the U.K. Hematol Oncol 15(4):173–189
Pearce MS, Parker L (2001) Childhood cancer registrations in the developing world: still more boys than girls. Int J Cancer 91(3):402–406
Cartwright RA, Gurney KA, Moorman AV (2002) Sex ratios and the risks of haematological malignancies. Br J Haematol 118(4):1071–1077
Taylor GM, Dearden S, Payne N, et al. (1998) Evidence that an HLA-DQA1-DQB1 haplotype influences susceptibility to childhood common acute lymphoblastic leukaemia in boys provides further support for an infection-related aetiology. Br J Cancer 78(5):561–565
Dorak MT, Lawson T, Machulla HK, Darke C, Mills KI, Burnett AK (1999) Unravelling an HLA-DR association in childhood acute lymphoblastic leukemia. Blood 94(2):694–700
Dorak MT, Sproul AM, Gibson BE, Burnett AK, Worwood M (1999) The C282Y mutation of HFE is another male-specific risk factor for childhood ALL. Blood 94(11):3957–3958
Dorak MT, Oguz FS, Yalman N, et al. (2002) A male-specific increase in the HLA-DRB4 (DR53) frequency in high-risk and relapsed childhood ALL. Leuk Res 26(7):651–656
Dorak MT, Lawson T, Machulla HK, Mills KI, Burnett AK (2002) Increased heterozygosity for MHC class II lineages in newborn males. Genes Immun 3(5):263–269
Hewitt D, Lashof JC, Stewart AM (1966) Childhood cancer in twins. Cancer 19(2):157–161
Inskip PD, Harvey EB, Boice JD Jr, et al. (1991) Incidence of childhood cancer in twins. Cancer Causes Control 2(5):315–324
Pinn VW (2003) Sex and gender factors in medical studies: implications for health and clinical practice. JAMA 289(4):397–400
Cotterill SJ, Parker L, Malcolm AJ, Reid M, More L, Craft AW (2000) Incidence and survival for cancer in children and young adults in the North of England, 1968–1995: a report from the Northern Region Young Persons’ Malignant Disease Registry. Br J Cancer 83(3):397–403
Parker L, Cole M, Craft AW, Hey EN (1998) Neonatal vitamin K administration and childhood cancer in the north of England: retrospective case–control study. BMJ316 7126:189–193
Wilcox M, Gardosi J, Mongelli M, Ray C, Johnson I (1993) Birth weight from pregnancies dated by ultrasonography in a multicultural British population. BMJ307 6904:588–591
Royston P, Ambler G, Sauerbrei W (1999) The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 28(5):964–974
Kaye SA, Robison LL, Smithson WA, Gunderson P, King FL, Neglia JP (1991) Maternal reproductive history and birth characteristics in childhood acute lymphoblastic leukemia. Cancer 68(6):1351–1355
Stewart A, Webb J, Hewitt D (1958) A survey of childhood malignancies. BMJ 1:1495–1508
Gibson RW, Bross IDJ, Graham S, et al. (1968) Leukemia in children exposed to multiple risk factors. New Engl J Med 279(17):906–909
Yeazel MW, Buckley JD, Woods WG, Ruccione K, Robison LL (1995) History of maternal fetal loss and increased risk of childhood acute leukemia at an early age. A report from the Childrens Cancer Group. Cancer 75(7):1718–1727
McMillen MM (1979) Differential mortality by sex in fetal and neonatal deaths. Science 204(4388):89–91
Dorak MT, Burnett AK (1992) Major histocompatibility complex, t-complex, and leukemia. Cancer Causes Control 3(3):273–282
Melve KK, Skjaerven R (2003) Birthweight and perinatal mortality: paradoxes, social class, and sibling dependencies. Int J Epidemiol 32(4):625–632
Petridou E, Skalkidou A, Dessypris N, et al. (2000) Endogenous risk factors for childhood leukemia in relation to the IGF system (Greece). The Childhood Haematologists–Oncologists Group. Cancer Causes Control 11(8):765–771
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE (1990) Increased cell division as a cause of human cancer. Cancer Res 50(23):7415–7421
Srivastava S, Mehrotra PK, Srivastava SP, Siddiqui MK (2002) Some essential elements in maternal and cord blood in relation to birth weight and gestational age of the baby. Biol Trace Elem Res 86(2):97–105
Cogswell ME, Parvanta I, Ickes L, Yip R, Brittenham GM (2003) Iron supplementation during pregnancy, anemia, and birth weight: a randomized controlled trial. Am J Clin Nutr 78(4):773–781
Le NT, Richardson DR (2002) The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta 1603(1):31–46
Dorak MT, Burnett AK, Worwood M (2005) HFE gene mutations in susceptibility to childhood leukemia: HuGE review. Genet Med 7(3):159–168
Lao TT, Chan PL, Tam KF (2001) Gestational diabetes mellitus in the last trimester—a feature of maternal iron excess? Diabet Med 18(3):218–223
Cauza E, Hanusch-Enserer U, Bischof M, et al. (2005) Increased C282Y heterozygosity in gestational diabetes. Fetal Diagn Ther 20(5):349–354
Feltbower RG, McKinney PA, Greaves MF, Parslow RC, Bodansky HJ (2004) International parallels in leukaemia and diabetes epidemiology. Arch Dis Child 89(1):54–56
Acknowledgments
The original study was funded by the Department of Health (UK). MTD is supported by the North of England Children’s Cancer Research Fund. The Northern Region Young Persons’ Malignant Disease Registry is funded by the Newcastle Hospitals NHS Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorak, M.T., Pearce, M.S., Hammal, D.M. et al. Examination of gender effect in birth weight and miscarriage associations with childhood cancer (United Kingdom). Cancer Causes Control 18, 219–228 (2007). https://doi.org/10.1007/s10552-006-0093-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-006-0093-8