Abstract
Background
There is a well-recognized male excess in childhood cancer incidence; however, it is unclear whether there is etiologic heterogeneity by sex when defined by epidemiologic risk factors.
Methods
Using a 5-state registry-linkage study (cases n = 16,411; controls n = 69,816), we estimated sex-stratified odds ratios (OR) and 95% confidence intervals (95% CI) between birth and demographic characteristics for 16 pediatric cancers. Evidence of statistical interaction (p-interaction < 0.01) by sex was evaluated for each characteristic in each cancer.
Results
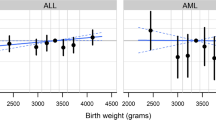
Males comprised > 50% of cases for all cancers, except Wilms tumor (49.6%). Sex interacted with a number of risk factors (all p-interaction < 0.01) including gestational age for ALL (female, 40 vs. 37–39 weeks OR: 0.84, 95% CI 0.73–0.97) and ependymoma (female, 40 vs. 37–39 OR: 1.78, 95% CI 1.14–2.79; female, ≥ 41 OR: 2.01. 95% CI 1.29–3.14), birth order for AML (female, ≥ 3rd vs. 1st OR: 1.39, 95% CI 1.01–1.92), maternal education for Hodgkin lymphoma (male, any college vs. < high school[HS] OR: 1.47, 95% CI 1.03–2.09) and Wilms tumor (female, any college vs. HS OR: 0.74, 95% CI 0.59–0.93), maternal race/ethnicity for neuroblastoma (male, black vs. white OR: 2.21, 95% CI 1.21–4.03; male, Hispanic vs. white OR: 1.86, 95% CI 1.26–2.75; female, Asian/Pacific Islander vs. white OR: 0.28, 95% CI 0.12–0.69), and paternal age (years) for hepatoblastoma in males (< 24 vs. 25–29 OR: 2.17, 95% CI 1.13–4.19; ≥ 35 vs. 25–29 OR: 2.44, 95% CI 1.28–4.64).
Conclusions
These findings suggest etiologic heterogeneity by sex for childhood cancers for gestational age, maternal education, and race/ethnicity and paternal age.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a general male excess in childhood cancer incidence that is particularly pronounced for leukemias, lymphomas, sarcomas, and brain tumors [1,2,3], which comprise over 60% of childhood cancer diagnoses [3]. The increased incidence among males varies by tumor type but may depend on sex differences in risk factor profiles, which have been largely unexplored to date, or biologic sex differences. Concerning biology, sex differences in the immune system may be particularly important as males have lower innate and adaptive immune responses than females as demonstrated by higher rates of infectious diseases in males and greater levels of autoimmune diseases in females [4,5,6,7]. Additionally, the dosage of the X chromosome, which contains many immune-related genes [8], may play an important role in the observed sex disparities in childhood cancer development as the Y chromosome is primarily responsible for sex determination [4]. Sex variation in the hormonal milieu may also impact childhood cancer development in adolescents as estrogen is thought to increase the immune response [9, 10] whereas increasing testosterone may dampen immune responses [11] as observed in adults. These hypotheses, though important, are challenging to investigate in childhood cancer considering the tremendous histologic and anatomic heterogeneity of cancers leading to small sample sizes of biologic specimens to accompany the requisite epidemiologic data.
Therefore, it is important to examine sex differences in risk factor profiles for childhood cancers to identify those that may increase the development of malignancies among males. There are few established, strong non-genetic risk factors for childhood cancer except ionizing radiation [12]. Using record linkages, we have previously demonstrated variation in the modest associations between birthweight [13], parental age [14], parental race/ethnicity [15], child sex [16], birth order [13], plurality [17], socioeconomic status [18] and childhood cancers. However, most of the analyses were not stratified by sex. By doing so, we may identify risk factors that are sex divergent and help us better understand mechanisms for the male excess in cancer development. Using a population-based registry-linkage study from five states, we estimated sex-stratified associations between birth/parental characteristics and 16 of the most common pediatric malignancies diagnosed in children aged 0–14 years at diagnosis.
Methods
Study population
This case–control study is the result of a previous birth-cancer registry-linkage study from five states: Minnesota (MN), California (CA), New York (NY [excluding New York City]), Texas (TX), and Washington (WA) state [13,14,15, 17, 19, 20]. Children aged 0–14 years (0–4 years of age for CA only) at cancer diagnosis who were born and diagnosed in same state were included. Controls were frequency matched to cases on birth year (MN:1976–2004; NY: 1970–2001; WA: 1980–2004) [14] and by birth year and sex (CA and TX: 1970–2004). Additional MN data arose from a recent linkage (1989–2014) [16, 18]. Study approval was obtained from each state’s department of health and from Institutional Review Boards at all institutions.
Cancer definition
Cancers were classified using the International Classification of Childhood Cancer, Third Edition (ICCC-3) [21]. Cancers with < 300 cases were excluded. Included cancers were acute lymphoid leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma, non-Hodgkin lymphoma, ependymomas, medulloblastoma, primitive neuroectodermal tumors (PNET), other gliomas, neuroblastoma, retinoblastoma, nephroblastoma (Wilms tumor), hepatoblastoma, osteosarcoma, and rhabdomyosarcoma (Supplemental Table 1). Brain tumors were broken into more detailed histologic groupings defined elsewhere [22] and included pilocytic astrocytoma (International Classification of Disease code (ICD): 9421) and other astrocytoma (ICD: 9400, 9380, 9401, 9440, 9441, 9442, 9384, 9432, 9410, 9411, 9420, 9424). Children diagnosed with cancer at < 28 days of life and those with Down syndrome on birth certificates (57 cases and 24 controls) were excluded.
Variables of interest
Data were harmonized across all five studies for maternal and birth characteristics, as previously described [13, 14, 17]. The following variables were included in the fully adjusted models: birthweight (grams: < 2500, 2500–3999, ≥ 4000), gestational age (weeks: < 37, 37–39, 40, ≥ 41), maternal education (< High School [HS]/GED, HS/GED, any college), maternal age (years: < 24, 25–29, ≥ 30), paternal age (years: < 24, 25–29, 30–34, 35 +), maternal and paternal race/ethnicity (non-Hispanic white [NHW], non-Hispanic black [NHB], Hispanic, Asian/Pacific Islander [API]), birth order (first, second, ≥ third), birth year (quartiles: 1970–1985, 1986–1989, 1990–1993, 1994–2014), and state (MN, CA, NY, TX, WA).
Statistical analysis
Logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) as the measure of association between each risk factor and each cancer type in sex-stratified analyses. Models were adjusted for all available risk factors described above. Likelihood Ratio Tests (LRT) were used to obtain p values for statistical interaction between each risk factor and sex. To minimize the rate of false positives, we used a more conservative p value of < 0.01 for statistically significant interactions, determined by 0.05/5 for the five risk factor categories (gestational age, birthweight, birth order, maternal education, and parental race). Additionally, we excluded statistically significant interactions defined by p values that had 95% CIs that included the null. ORs (95% CIs) for strata with fewer than 10 cases have been suppressed.
Results
Sex differences in risk factor distributions
There were 16,411 cases (55.7% male) and 69,816 controls (52.4% male). Overall, male cases and controls had higher birthweight than females (≥ 4000 g: 16% for males and 10% for females in both groups). Male and female cases were similar in their distribution for the other risk factors. For most cancers, there were more males than females (Fig. 1, Supplemental Table 1). The highest percentage of males was observed in lymphomas (approximately 62%), medulloblastoma (65.6%), and hepatoblastoma (60.6%). Conversely, Wilms tumor had a slight female predominance (51.4%).
Among cancer types, a higher percentage of males had high birthweight within the lymphoid and embryonal malignancies, including hepatoblastoma, neuroblastoma, retinoblastoma and Wilms tumor, whereas the sexes were more similar for some brain tumors including ependymoma, medulloblastoma, and other astrocytomas (Supplemental Table 1). For gestational age, the sexes were similar in their distribution among these categories except for ependymoma where 36.4% of females were born at ≥ 41 weeks compared to 29.8% of males and hepatoblastoma where 25.4% of females and 20.3% of males were born at ≥ 41 weeks. The opposite was observed in PNETs (≥ 41 weeks: 34.6% females vs. 39.4% males). For maternal education, there were sex differences in the distribution of children born to mothers with any college education for Hodgkin lymphoma (35.3% females, 48.3% males), medulloblastoma (40.1% female, 48.7% male), and Wilms tumor (44.0% female, 50.5% male). Retinoblastoma and hepatoblastoma had higher percentages of females born to mothers with any college education (45.1% female vs. 40.5% male and 52.4% female vs. 39.3% male, respectively) (Table 1).
Risk factors associated with childhood cancer in both sexes
In adjusted models, there were a number of risk factor-cancer associations that excluded the null value for both sexes (Table 2, Supplemental Table 2). Gestational age < 37 weeks was associated with an increased risk of Wilms tumor (female OR: 1.54, 95% CI 1.06–2.23; male OR: 1.75, 95% CI 1.22–2.52). Low birth weight was associated with an increased risk of hepatoblastoma among males (OR: 3.83, 95% CI 1.97–7.42) and females (OR: 2.23, 95% CI 1.06–4.69). Higher birth order reduced the risk of Wilms tumor (female OR: 0.67, 95% CI 0.51–0.88; male OR: 0.68, 95% CI 0.51–0.89) and rhabdomyosarcoma (female OR: 0.61, 95% CI 0.39–0.95; male OR: 0.63, 95% CI 0.42–0.93). Maternal Hispanic ethnicity was associated with an increased risk of ALL (female OR: 1.67, 95% CI 1.27–2.19; male OR: 1.32, 95% CI 1.03–1.69). Paternal NHB race was inversely associated with ALL (female OR: 0.52, 95% CI 0.29–0.96; male OR: 0.51, 95% CI 0.31–0.82) and paternal Hispanic ethnicity was associated with ALL (female OR: 1.41, 95% CI 1.08–1.85; male OR: 1.51, 95% CI 1.18–1.93).
Statistical interactions between sex and birth characteristics in some childhood cancers
We observed evidence of statistical interaction (p < 0.01) between sex and most risk factors for at least one cancer type (Table 2). There was an interaction between sex and gestational age such that females born at 40 weeks vs. 37–39 had a reduced risk of ALL (OR: 0.84, 95% CI 0.73–0.97; p = 0.0068). Conversely, females born > 37–39 weeks had an increased risk of ependymomas (40 weeks OR: 1.78, 95% CI 1.14–2.79; ≥ 41 weeks OR: 2.01. 95% CI 1.29–3.14; p = 0.0034). For birth order, there was an increased association between ≥ third born and AML (female OR: 1.39, 95% CI 1.01–1.92; p = 0.0036). Maternal education displayed significant interactions with sex for Hodgkin lymphoma (any college, male OR: 1.47, 95% CI 1.03–2.09; p = 0.0048) and Wilms tumor (any college, female OR: 0.74, 95% CI 0.59–0.93; p = 0.0013). Maternal race/ethnicity significantly interacted with sex for neuroblastoma (p < 0.0001) with an increased risk observed among males born to NHB (OR: 2.21, 95% CI 1.21–4.03) and Hispanic mothers (OR: 1.86, 95% CI 1.26–2.75) and an inverse association among females born to API mothers (OR: 0.28, 95% CI 0.12–0.69).
Risk factors associated with childhood cancer in one sex only
A number of risk factors were ostensibly only associated with childhood cancer in one sex or the other, but statistical interaction by sex was not significant (p > 0.01; Table 2, Supplemental Table 2). There was variation in the association between gestational age by sex for other astrocytomas, hepatoblastoma, PNET, and AML. Birthweight varied in association with non-Hodgkin lymphoma, other gliomas, neuroblastoma, ALL, and Wilms tumor by sex. Increasing birth order displayed sex-specific associations for ALL and AML. Level of maternal education varied in association with cancers by sex for ALL, pilocytic astrocytomas, PNET, and Wilms tumor. Younger maternal age was inversely associated with AML, other gliomas, and Wilms tumors in a sex-specific manner, while older maternal age was associated with non-Hodgkin lymphoma in males and rhabdomyosarcoma in females. Similar findings were observed with sex-specific associations for younger paternal age and lymphomas and Wilms tumor. Maternal race/ethnicity varied in association with retinoblastoma by sex as did paternal race/ethnicity and AML, pilocytic, and other astrocytomas.
Discussion
We observed a general male excess in all cancers except Wilms tumor, matching our previous report using Surveillance, Epidemiology, and End Results data [1]. In our sex-stratified models, we recapitulated previous associations between risk factors and some cancers for both sexes including low birthweight and hepatoblastoma [19], pre-term birth and Wilms tumor [23], parental Hispanic ethnicity and NHB race in ALL [15, 24], and birth order in Wilms tumor [13, 25]. We observed a reduced risk of rhabdomyosarcoma with increasing birth order for both sexes contradicting a previous null report [26]. In our study, we observed a number of single-sex-only associations that varied by cancer, risk factor, and sex that may have reached statistical significance if sample size was greater. Approximately half of the observed sex-specific associations occurred in each sex. There was an overall U-shaped pattern in the risk of cancers and birthweight, and the increased risk was most often observed in males, particularly for high birthweight in ALL, neuroblastoma, and Wilms tumor. In contrast, a report from the United Kingdom Childhood Cancer Study found high birthweight to be a risk factor among females only [27], but in our US-based study, we had almost 5% more male cases classified as high birthweight suggesting regional variation in factors contributing to high birthweight may underlie these study differences. Using US data, we have previously shown that the association of male sex and most childhood cancers is not mediated by birthweight, suggesting sex itself is a biologic risk factor for some malignancies [16]. Taken together, our previous findings with those here suggest that birthweight is an important risk factor for males in some cancers, and that male sex and birthweight may have independent and important biologic roles in cancer development. Concerning maternal age, there was an overall pattern of reduced risk of some cancers among males born to younger mothers and an increased risk of non-Hodgkin lymphoma in males and rhabdomyosarcoma in females born to older mothers, as has been reported in non-sex-stratified studies [14, 28]. Parental race/ethnicity produced varying results by cancer and sex. The previously reported reduced risk of ALL among children born to NHB parents [15] was driven by paternal NHB race in our study for both sexes.
We also observed a number of statistical interactions between sex and birth characteristics for a few cancers. Statistical interaction was present for neuroblastoma and maternal race/ethnicity such that maternal NHB race and Hispanic ethnicity were associated with increased risk among males, and there was an inverse association between maternal API race in females with neuroblastoma. In our previous report, we found evidence of statistical interaction between parental race/ethnicity and sex and a reduced risk of neuroblastoma among girls with NHB ancestry [15]. Our findings here suggest that the association may be more strongly driven by paternal race/ethnicity than maternal, though the effect estimates for neuroblastoma in females born to NHB fathers included the null in our analyses. We also observed statistical interactions between longer gestational age and ependymoma in females, higher birth order and AML in females, higher maternal education and Hodgkin lymphoma among males, and a U-shaped risk pattern was observed for younger and older paternal age in hepatoblastoma in males. Collectively, our study highlights the potential etiologic heterogeneity by sex for previously reported risk factors of childhood cancers. These findings suggest that biologic sex differences that impact disease risk are present and should be in further investigated in future epidemiologic studies.
The notion that sex itself is a risk factor for cancer development has begun emerging more in the adult literature [29], and we have reported on this phenomenon previously using registry data of various types for pediatric malignancies [1, 2, 16]. There are a number of biologic hypotheses, reviewed extensively elsewhere, [29, 30] that are thought to underlie the male excess in cancer diagnoses including sex differences in immune response [4,5,6,7], X chromosome dosage [4], adolescent hormonal fluctuation [9, 10], epigenetics during fetal development [30], and metabolism over the life course [30]. In our study, we observed a number of sex differences in risk factors that may impact cancer development through a combination of these mechanisms that should be evaluated in the future.
Sex differences for the included risk factors may depend on biologically dimorphic development in utero for males and females such that high birth weight, for example, has a greater impact on males as they are often heavier than females by 150 g on average [31] and have different methylation patterns resulting from the presence of the Y chromosome [30]. These biologic differences may interact with the maternal environment during fetal development thereby creating a permissive environment for some cancers among males as we observed in our study for ALL, neuroblastoma, and Wilms tumor. We observed an increased risk of ependymoma for longer gestational age among females and PNET in males. The increased risk could be due to mode of delivery, which has been shown to increase cancer risk [32, 33] and impact brain development [34]. For maternal age, we observed a consistent inverse association between younger maternal age and AML, other gliomas, and Wilms tumor among males while we found increased risks of non-Hodgkin lymphoma among males, as reported elsewhere in both sexes [28], and rhabdomyosarcoma among females born to mothers aged 30 years and older. The sex differences in these patterns may be due to risk factors present in the gametes of older vs. younger mothers, such as germline de novo mutations or chromosomal abnormalities, and act synergistically with sex for some cancers. Our findings concerning maternal education and parental race/ethnicity highlight the importance of these factors, which are biologically and environmentally influenced, and impact childhood cancer development [18]. We observed that some variation in risk factors by parental sex, such as NHB paternal race in ALL, NHB maternal race and Hispanic ethnicity in males with neuroblastoma, and NHB parental race in females with AML. There are a myriad of social and biologic factors that may influence these relationships that warrant further investigation in epidemiologic and genomic studies in the future.
Although we present the first sex-stratified risk factor analysis for a wide range of pediatric malignancies using population-based registry data, our findings should be interpreted in light of the following limitations. While 27% of cases and 29% of controls were missing maternal education, we have adjusted for it in our analysis as it serves as a proxy for socioeconomic status, which is important in pediatric cancer risk [15, 18]. We do not have information on other variables associated with childhood cancer such as mode of delivery [33], breast feeding information, or parental occupation [35], which may produce varying associations by sex for some cancers as we have reported for mode of delivery [36]. While presenting information on a number of childhood cancers, including rarer malignancies like osteosarcoma, our study does not have information on histologic or molecular subtypes for each cancer, which may vary by sex and be useful for further uncovering etiologic heterogeneity. This may be particularly important in cancers such as medulloblastoma where sex differences by the four main subgroups have been identified [37]. In ALL, we have reported on sex differences in the risk of death based on age [38], which corresponds to some established cytogenomic subtypes [39]. However, much population-based research remains to be done in childhood cancer to identify and characterize sex differences in molecular subtypes of disease incidence and risk. With analyses of statistical interaction, it is important to note that the presence of statistical interaction does not imply biologic interaction with the identified risk factors and sex [40]. Further, it has been argued that interaction effects on opposite sides of the null for comparison groups, in our case sex, may be spurious associations, particularly when there are null results for the overall association [41]. While we did observe significant effect estimates in one sex for most identified interactions between sex and the studied risk factors, which have established associations overall with childhood cancers, all confidence intervals for the effect estimates in the opposite sex included the null. Therefore, some of the observed statistical interactions may be due to false negative findings, but to avoid this, we have used a more stringent p value for interaction determination. This lack of precision could be due to sample size limitations and necessitates further investigation in larger studies. Our findings serve as a starting point to investigate the role of biologic interactions of sex and risk factors in future animal models and molecular epidemiology studies. For example, studying immune modulation exposures such as daycare attendance, early life infections or allergies, or breast feeding at hospital discharge, where milk composition has been shown to vary by sex [42], and breast feeding duration may shed light on sex differences in risk profiles as these exposures may interact with sex and alter the immune environment leading to cancer development.
In our large, population-based study of etiologic heterogeneity by sex for 16 childhood cancers, we identified a number of sex-specific associations for previously reported risk factors of childhood cancers. The observed variation may depend on biologic sex differences in fetal and postnatal development and may represent a complex relationship between prenatal, parental, and environmental exposures and cancer development that differs in males and females.
Data availability
The data are proprietary and sharing is limited based on each registry’s rules.
References
Williams LA, Richardson M, Marcotte EL et al (2019) Sex ratio among childhood cancers by single year of age. Pediatr Blood Cancer. https://doi.org/10.1002/pbc.27620
Williams LA, Hubbard AK, Scheurer ME et al (2020) Trends in pediatric central nervous system tumor incidence by global region from 1988 to 2012. Int J Epidemiol dyaa. https://doi.org/10.1093/ije/dyaa176
Ward E, Desantis C, Robbins A et al (2014) Childhood and adolescent cancer statistics, 2014. CA: Cancer J Clin 64:83–103. https://doi.org/10.3322/caac.21219
Libert C, Dejager L, Pinheiro I (2010) The X chromosome in immune functions: When a chromosome makes the difference. Nat Rev Immunol 10:594–604. https://doi.org/10.1038/nri2815
Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638. https://doi.org/10.1038/nri.2016.90
Washburn T, Medearis D, Child B (1965) Sex differences in susceptibilty to infections. Pediatrics 35:57–64
Piccini P, Montagnani C, De Martino M (2018) Gender disparity in pediatrics: A review of the current literature. Ital J Pediatr 44:4–9. https://doi.org/10.1186/s13052-017-0437-x
Spatz A, Borg C, Feunteun J (2004) X-chromosome genetics and human cancer. Nat Rev Cancer 4:617–629. https://doi.org/10.1038/nrc1413
Khan D, Ahmed SA (2016) The immune system is a natural target for estrogen action: opposing effects of estrogen in two prototypical autoimmune diseases. Front Immunol 6:1–8. https://doi.org/10.3389/fimmu.2015.00635
Khan D, Ahmed A (2012) Estrogen and signaling in the cells of the immune system. Adv Neuroimmune Biol 3:73–93. https://doi.org/10.3233/NIB-2012-012039
Furman D, Hejblum BP, Simon N et al (2014) Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci 111:869–874. https://doi.org/10.1073/pnas.1321060111
Spector LG, Pankratz N, Marcotte EL (2015) Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am 62:11–25. https://doi.org/10.1016/j.pcl.2014.09.013.Genetic
Von Behren J, Spector LG, Mueller BA et al (2011) Birth order and risk of childhood cancer: a pooled analysis from five US States. Int J Cancer 128:2709–2716. https://doi.org/10.1002/ijc.25593
Johnson KJ, Carozza SE, Chow EJ et al (2009) Parental age and risk of childhood cancer: a pooled analysis. Epidemiology 20:475–483. https://doi.org/10.1097/EDE.0b013e3181a5a332
Chow EJ, Puumala SE, Mueller BA et al (2010) Childhood cancer in relation to parental race and ethnicity: a 5-state pooled analysis. Cancer 116:3045–3053. https://doi.org/10.1002/cncr.25099
Williams LA, Richardson M, Kehm RD et al (2018) The association between sex and most childhood cancers is not mediated by birthweight. Cancer Epidemiol 57:7–12
Puumala SE, Carozza SE, Chow EJ et al (2009) Childhood cancer among twins and higher order multiples. Cancer Epidemiol Biomarkers Prev 18:162–168. https://doi.org/10.1158/1055-9965.EPI-08-0660
Kehm RD, Spector LG, Poynter JN et al (2017) Socioeconomic status and childhood cancer incidence: a population-based multilevel analysis. Am J Epidemiol 187:612–626. https://doi.org/10.1093/aje/kwx292/4080179/Cigarette-Smoking-and-Risk-of-Early-Natural
O’Neill KA, Murphy MFG, Bunch KJ et al (2015) Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol 44:153–168. https://doi.org/10.1093/ije/dyu265
Johnson KJ, Carozza SE, Chow EJ et al (2011) Birth characteristics and childhood carcinomas. Br J Cancer 105:1396–1401. https://doi.org/10.1038/bjc.2011.359
Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P (2005) International classification of childhood cancer, third edition. Cancer 103:1457-1467. https://doi.org/10.1002/cncr.20910
Ostrom QT, De Blank PM, Kruchko C et al (2014) Alex’s Lemonade stand foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol 16:x1–x35. https://doi.org/10.1093/neuonc/nou327
Daniels JL, Pan IJ, Olshan AF et al (2008) Obstetric history and birth characteristics and Wilms tumor: a report from the children’s oncology group. Cancer Causes Control 19:1103–1110. https://doi.org/10.1007/s10552-008-9174-1
Oksuzyan S, Crespi C, Cockburn M et al (2015) Race/ethnicity and the risk childhood leukemia: a case-control study in California. J Epidemiol Community Heal 69:795–802. https://doi.org/10.1136/jech-2014-204975.Race/ethnicity
Schuz J, Kaatsch P, Kaletsch U et al (1999) Association of childhood cancer with factors related to pregnancy and birth. Int J Epidemiol 28:631–639. https://doi.org/10.1038/sj.bjc.6690395
Lupo PJ, Danysh HE, Skapek SX et al (2014) Maternal and birth characteristics and childhood rhabdomyosarcoma: a report from the Children’s Oncology Group. Cancer Causes Control 25:905–913. https://doi.org/10.1007/s10552-014-0390-6
Smith A, Lightfoot T, Simpson J, Roman E (2009) Birth weight, sex and childhood cancer: a report from the United Kingdom Childhood Cancer Study. Cancer Epidemiol 33:363–367. https://doi.org/10.1016/j.canep.2009.10.012
Contreras ZA, Hansen J, Ritz B et al (2017) Parental age and childhood cancer risk: a danish population-based registry study. Cancer Epidemiol 49:202–215. https://doi.org/10.1016/j.canep.2017.06.010
Clocchiatti A, Cora E, Zhang Y, Dotto GP (2016) Sexual dimorphism in cancer. Nat Rev Cancer 16:330–339. https://doi.org/10.1038/nrc.2016.30
Rubin JB, Lagas JS, Broestl L et al (2020) Sex differences in cancer mechanisms. Biol Sex Differ 11:1–29. https://doi.org/10.1186/s13293-020-00291-x
Lunde A, Melve KK, Gjessing HK et al (2007) Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol 165:734–741. https://doi.org/10.1093/aje/kwk107
Schüz J, Weihkopf T, Kaatsch P (2007) Medication use during pregnancy and the risk of childhood cancer in the offspring. Eur J Pediatr 166:433–441. https://doi.org/10.1007/s00431-006-0401-z
Marcotte EL, Thomopoulos TP, Infante-Rivard C et al (2016) Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol 3:e176–e185. https://doi.org/10.1016/S2352-3026(16)00002-8
Deoni SC, Adams SH, Li X et al (2019) Cesarean delivery impacts infant brain development. Am J Neuroradiol 40:169–177. https://doi.org/10.3174/ajnr.A5887
Patel DM, Jones RR, Booth BJ et al (2019) Parental occupational exposure to pesticides, animals and organic dust and risk of childhood leukemia and central nervous system tumors: Findings from the International Childhood Cancer Cohort Consortium (I4C). Int J Cancer. https://doi.org/10.1002/ijc.32388
Williams LA, Richardson M, Spector LG, Marcotte EL (2021) Cesarean section is associated with an increased risk of acute lymphoblastic leukemia and hepatoblastoma in children from minnesota. Cancer Epidemiol Biomarkers Prev 30:736–742. https://doi.org/10.1158/1055-9965.EPI-20-1406
Juraschka K, Taylor MD (2019) Medulloblastoma in the age of molecular subgroups: a review: JNSPG 75th Anniversary invited review article. J Neurosurg Pediatr 24:353–363. https://doi.org/10.3171/2019.5.PEDS18381
Williams LA, Spector LG (2019) Survival differences between males and females diagnosed with childhood cancer. JNCI Cancer Spectr 3:1–11
Williams LA, Yang JJ, Hirsch BA et al (2019) Is there etiologic heterogeneity between subtypes of childhood acute lymphoblastic leukemia? A review of variation in risk by subtype. Cancer Epidemiol Biomarkers Prev. https://doi.org/10.1158/1055-9965.EPI-18-0801
Brumback B, Berg A (2008) On effect-measure modification: Relationships among changes in the relative risk, odds ratio, and risk difference. Stat Methods Med Res 27:3453–3465
Weiss NS (2008) Subgroup-specific associations in the face of overall null results: Should we rush in or fear to tread? Cancer Epidemiol Biomarkers Prev 17:1297–1299. https://doi.org/10.1158/1055-9965.EPI-08-0144
Galante L, Milan AM, Reynolds CM et al (2018) Sex-specific human milk composition: The role of infant sex in determining early life nutrition. Nutrients 10:1–11. https://doi.org/10.3390/nu10091194
Funding
The Children’s Cancer Research Fund (LAW), the Washington State Cancer Registry and the Cancer Surveillance System of Western Washington, which provided data, are supported by contract N01-CN-05230 from the National Cancer Institute and the Fred Hutchinson Cancer Research Center. In California and Texas, National Cancer Institute grants R01 CA717450 and R01 CA92670 supported assembly of their respective datasets. In New York, partial support for assembly of the dataset was received from the Centers for Disease Control and Prevention’s National Program of Cancer Registries by cooperative agreement U58DP000783-01 awarded to the New York State Department of Health; contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Williams, L.A., Sample, J., McLaughlin, C.C. et al. Sex differences in associations between birth characteristics and childhood cancers: a five-state registry-linkage study. Cancer Causes Control 32, 1289–1298 (2021). https://doi.org/10.1007/s10552-021-01479-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-021-01479-1