Abstract
Objective
Prostate cancer recurrence impacts patient quality of life and risk of prostate-cancer specific death following definitive treatment. We investigate differences in disease-free survival among white, black, Hispanic, and Asian patients in a large, population-based database.
Methods
Merged Surveillance, Epidemiology, and End Results Program (SEER) and Medicare files provided data on 23,353 white patients, 2,814 black patients, 480 Hispanic patients, and 566 Asian patients diagnosed at age 65–84 years with clinically localized prostate cancer between 1986 and 1996 in five SEER sites. Patients were followed through 1998. Racial differences in disease-free survival were assessed using Kaplan–Meier survival curves and Cox regression models.
Results
The 75th percentile disease-free survival time for black patients was 13 months less than that for white patients (95% confidence interval [CI]: 6.2–19.8 months), 29.7 months less than that for Hispanic patients (95% CI: 4.4–55.0 months), and 39.1 months less than that for Asian patients (95% CI: 12.1–66.1 months). In multivariate analysis, black race predicted shorter disease-free survival among surgery patients, but not among radiation patients.
Conclusions
Black patients experienced shorter disease-free survival compared to white, Hispanic, and Asian patients, and the disease-free survival of white, Hispanic, and Asian patients were not statistically different. Earlier recurrence of prostate cancer may help explain black patients’ increased risk of mortality from prostate cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With an estimated 232,090 new cases and 30,350 deaths projected to occur in 2005 [1], prostate cancer is the leading cancer diagnosis and the second most common cause of cancer-related death among American males. Prostate cancer incidence rates for black American men are approximately twice the rates for white American men [2, 3]. Black patients more often present with advanced prostate cancer than whites and, when diagnosed with late stage disease, have a worse prognosis [4, 5].
Our recent study of clinically localized prostate cancer demonstrated worse overall and prostate cancer-specific survival for black patients compared to white patients after controlling for many potential confounders, including treatment and comorbidity [6]. Possible explanations for these survival differences include biological differences in measurable and unmeasurable tumor and host characteristics that affect prognosis or response to therapy and behavioral and environmental factors affecting access to health care and its optimal utilization, including prompt diagnosis and the choice, quality, and effectiveness of health care.
The current study examines racial differences in rates of clinical progression leading to subsequent treatment or death following initial treatment of Medicare patients with clinically localized prostate cancer. We extend the findings of our earlier study by examining recurrence rates as a measure of tumor biology and treatment efficacy, and including Hispanic and Asian patients in the analysis.
Materials and methods
Data sources
The data used for this study were derived from the linked Surveillance, Epidemiology and End-Results (SEER)–Medicare database. SEER–Medicare files are a collaborative effort between the SEER program of the National Cancer Institute (NCI) and the Medicare program, run by the Centers for Medicare & Medicaid Services [7]. The SEER program collects cancer data on a routine basis from designated population-based cancer registries in various areas of the country. Data collected include date of incident cancer diagnosis, patient demographic characteristics, cancer histology, grade, and stage, type of treatment recommended or provided within 4 months of diagnosis, follow-up of vital status, and cause of death where applicable [8]. Medicare provides health insurance coverage to 97% of Americans aged 65 years or older, and data from this source include health services claims for care provided by physicians, inpatient hospital stays, hospital outpatient clinics, home health care agencies, skilled nursing facilities, and hospice programs. Medicare data provide information about other diseases and conditions that may influence cancer treatment and survival. SEER–Medicare data represent a large, population-based source of information with which to study care provided to cancer patients throughout the course of their treatment.
Primary and additional treatment
SEER data and Medicare claims covering the first 6 months after diagnosis were examined to identify initial treatment, as this time frame gives adequate time to initiate treatment, even allowing for the multidisciplinary consultations many prostate cancer patients obtain. We defined surgery as procedures performed with curative intent or in anticipation of a subsequent curative procedure, including radical prostatectomy (RP) and procedures performed on regional lymph nodes. Medicare ICD-9 codes 60.3–60.6x, 40.1x, 40.2x, 40.3, 40.5x and CPT codes 55810, 55812, 55815, 55840, 55842, and 55845 as well as SEER site specific surgery codes 30–90 were used to identify such procedures. Individuals undergoing surgery and radiation therapy (XRT) were also included in the surgery group, as additional XRT may follow incomplete or unsuccessful surgery. XRT was defined as external beam therapy, brachytherapy, or therapeutic isotope radiation therapy as listed in SEER in the absence of concomitant surgery, or by inpatient and outpatient codes indicating XRT. Medicare ICD-9 codes 92.2x, V58.0, V66.1, V67.1, CPT codes 77261–77799 excluding 77600–77620, and revenue center codes 0330–0339 as well as SEER radiation codes 1–6 were used to identify such procedures. SEER and Medicare data agreement on surgery and radiation therapy ascertainment is greater than 90% [9, 10].
We identified additional therapy for disease progression as treatment occurring at least 6 months after the end of initial treatment. Additional treatment included surgery and radiation identified using the same codes as those used to identify surgery and radiation as initial treatment. In addition, Medicare CPT codes 79200–79999 identified cancer patients receiving additional XRT. Bilateral orchiectomy was identified using Medicare ICD-9 codes 62.3–62.42 and CPT codes 54520, 54522, 54530, 54535, and 54690. Hormonal therapy was identified using Medicare HCPCS codes J1050, J1051, J9165, J9202, J9217, J9218, J9219, J1950, J3315, and S0175, while chemotherapy was identified from Medicare ICD-9 codes 99.25, V58.1, V66.2, V67.2, CPT codes 96400–96549 and HCPCS codes J9000–J9999, excluding those listed for hormonal therapy. Hormonal therapy was classified as an additional therapy for disease progression if claims were found ≥6 months after diagnosis and no claims were found in a window from 30 days before diagnosis to 180 days after diagnosis, or if there was an interval greater than 1 year between two hormonal therapy claims if the first claim occurred within the window around diagnosis. This second condition distinguishes between neo-adjuvant or adjuvant hormonal therapy and hormonal therapy initiated for disease progression. SEER–Medicare cannot identify patients taking self-administered, oral prescription hormonal drugs, such as flutamide or oral diethylstilbestrol, as these drugs were not covered by Medicare during the period of the study.
Tumor characteristics
Clinically localized prostate cancer refers to prostate cancer thought to be confined within the prostatic capsule based on results from clinical exam and imaging studies. SEER reports pathological staging results from RP and lymphadenectomy when available [11]. Our study focuses on clinically localized prostate cancer. Since clinically advanced cancer precludes attempted surgical cure, we include all surgery patients, assuming that advanced stage arises from surgical findings, to keep the staging consistent with XRT patients, for whom surgical information is unavailable. American Joint Commission on Cancer (AJCC) stage 1 or 2 or, when AJCC staging was missing, SEER historic stage code 1 was used to identify patients with clinically localized cancer [12]. Cancer grade was coded using the International Classification of Diseases for Oncology [13].
Patient characteristics
Race was classified from both SEER and Medicare sources. Individuals were considered Hispanic if they were Hispanic in either source, regardless of other racial/ethnic designations listed. Individuals were considered Asian if they were coded as such in Medicare or as any of the following racial classifications in SEER (without a co-listing of black or Hispanic): Chinese, Japanese, Filipino, Hawaiian, Korean, Asian Indian/Pakistani, Vietnamese, Laotian, Hmong, Kampuchean, Thai, Micronesian not otherwise specified (NOS), Chamorran, Guamanian NOS, Polynesian NOS, Tahitian, Samoan, Tongan, Melanesian NOS, Fiji Islander, New Guinean, Other Asian NOS, or Pacific Islander NOS. Individuals were considered black if they were classified as black in either data file without an additional classification of Hispanic or Asian. Finally, individuals were considered white if they were white in either data file without a classification of black, Hispanic, or Asian. Individuals coded as American Indian, Aleutian, or Eskimo or as both black and Asian was classified as “other” and was not considered for analysis (n=17).
Data on the presence of other health conditions that may affect initial and additional treatment as well as survival were obtained using Medicare inpatient claims for the year prior to diagnosis. A comorbidity score using hospital discharge diagnoses was calculated using the approach outlined by Deyo et al. [14] and Klabunde et al. [15]. We controlled for race and age specific median household income in the census tract and included a binary variable indicating whether the race-specific percentage of those in the tract with less than a high school education was below 25%.
Date of death was available in both SEER and Medicare files. SEER date of death was used for individuals dying of prostate cancer since only SEER data provide cause of death information. Medicare date of death was used for all other individuals because Medicare data provided the longest follow-up (through 31 December 1998).
Endpoints
There were two types of events signifying disease progression—the occurrence of additional treatment or death from prostate cancer as listed in SEER’s cause of death variable. Disease-free survival was measured in months from date of cancer diagnosis to the first progression event or, if neither event occurred, to the earlier of the date of death from a cause other than prostate cancer or 31 December 1998.
Study population
With the SEER–Medicare linkage from 1999, prostate cancer patients diagnosed from 1 January 1986 to 31 December 1996 from five geographic areas were eligible for analyses. The five SEER regions used for this study, Atlanta, Connecticut, Detroit, San Francisco, and Seattle were chosen for comparability with our earlier analysis of the black–white disparity in prostate cancer survival and include nearly all black patients with SEER data from the start of the PSA era. The selected areas include data collected since 1986 and Medicare claims through 31 December 1998. Patients were eligible for the study if they were enrolled in Medicare Part A and Part B from diagnosis to death or the end of the study. HMO enrollees were excluded, as services rendered by an HMO will not be detected in SEER–Medicare data. There were 104,537 patients for potential inclusion. Figure 1 presents a flow chart of the selection of the analysis dataset of 27,213 patients aged 65–84 from five SEER regions with clinically localized invasive prostate cancer covered by Medicare who underwent definitive treatment with surgery or radiation.
Statistical methods
Disease-free survival by race for patients with clinically localized prostate cancer cases in the five SEER sites was assessed using Kaplan–Meier survival curves [16] and Cox regression models. Disease-free survival estimates by race at 12, 60, and 120 months were taken directly from the SAS™ output. Survival curves were used to describe the overall racial comparisons, and log-rank tests were used to analyze the differences between the survival curves. The 75th percentile disease-free survival was used as an outcome measure because no racial groups reached their median survival time. Interactions were tested simultaneously in a single Cox regression model containing race, age, prostate-specific antigen (PSA) testing era (i.e., 1986–1988, the pre-PSA period; 1989–1991, the early PSA period; and 1992–1996, the recent PSA period), marital status, SEER site, primary therapy, tumor stage and grade, and census tract income and poverty level, and all 2×2 interactions between race and the covariates. In this model, race*primary treatment was the only significant interaction using a P-value of 0.1 as a cutoff. The overall, net effect of race on disease-free survival was estimated by fitting a model with only the race variable, coded as an indicator variable with white as the referent. Stage was included as a covariate only for sub-analyses on surgery patients. The final, statistically independent effect of race on disease-free survival was obtained by including race in a Cox regression model with all covariates. The proportional hazards assumption was examined by visually inspecting log(−log[survival])*log(time) plots for departures from parallelism between the groups being compared. There was no interaction between race and time using a P-value of 0.1 as a cutoff, indicating that the proportional hazards assumption was not violated for the effect we were trying to estimate. Statistical tests for main effects were two-sided, with α=0.05.
Results
Table 1 presents demographic and tumor characteristics of the study population. Our study population was comprised of 23,353 white patients (85.8%), 2,814 black patients (10.3%), 480 Hispanic patients (1.8%), and 566 Asian patients (2.1%). All minority groups lived in census tracts with lower education levels than white patients, and more black patients lived in census tracts with greater than 20% of families below the poverty line than all other groups. 48.9% of black patients underwent surgery as their initial treatment, compared with 54.5% of white patients, 57.7% of Hispanic patients, and 62.7% of Asian patients. Black and Asian patients presented with higher grade disease than both white and Hispanic patients
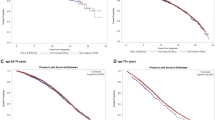
Figure 2 shows Kaplan–Meier curves for disease-free survival by race. Black patients had poorer disease-free survival when compared with all other racial groups. Hispanic and Asian patients trended towards better disease-free survival compared to white patients, but estimates were insufficiently precise to be confident that the difference was real (log-rank P>0.05). Disease-free survival differences were most notable at 120 months from initial treatment, when 58.0% of black patients (95% CI: 54.2–61.7%) were alive without disease recurrence, compared with 65.5% of white patients (95% CI: 64.5–66.5%), 68.0% of Hispanic patients (95% CI: 58.6–77.1%), and 72.1% of Asian patients (95% CI: 64.9–79.2%) (Table 2). The 75th percentile disease-free survival time for black patients was 13 months less than that for white patients (95% CI: 6.2–19.8 months), 29.7 months less than that for Hispanic patients (95% CI: 4.4–55.0 months), and 39.1 months less than that for Asian patients (95% CI: 12.1–66.1 months) (Table 3).
Kaplan–Meier survival curves for clinically localized prostate cancer by race. Curves (unadjusted) show disease-free survival. Log-rank P<0.0001 for black/white comparison and black/Asian comparison. Log-rank P=0.006 for black/Hispanic comparison. Log-rank P>0.05 for Asian/white, Hispanic/white and Hispanic/Asian comparison
Separate multivariate analyses were conducted for surgery and radiation patients (Table 4). Black race was a significant predictor of disease-free survival among surgery patients even with age, comorbidity score, SEER site, census tract income and education level, marital status, tumor grade and stage, and PSA testing era in the model. However, in the model for radiation patients that included the same covariates, black race was not associated with disease-free survival.
Discussion
This study demonstrates worse disease-free survival for black patients when compared to white, Hispanic, and Asian patients. Hispanic and Asian patients appeared to have somewhat better survival than white patients, but confidence intervals overlapped. In multivariate analysis, black race was an independent predictor of disease-free survival overall and among surgery patients.
This study used two indicators of disease recurrence—apparent treatment for prostate cancer recurrence and death from prostate cancer. These indicators constitute accurate and valid estimates of disease recurrence only to the extent that patients with recurrent cancer undergo additional treatment or prostate cancer is documented as their cause of death. Lu-Yao et al. also studied disease progression using the occurrence of an additional treatment 6 months or longer after initial treatment as a proxy for recurrent disease [17]. Their study found that additional treatment occurred in approximately 23% of patients with clinically localized prostate cancer 60 months after treatment with RP, a rate that is consistent with our results.
In order to distinguish treatment for disease recurrence from neo-adjuvant or adjuvant therapy, additional treatment was considered an event only if it occurred 6 months after primary therapy. To assess the impact of misclassifying additional treatment on our conclusions, we repeated our analysis using both 3- and 12-month windows as cutoffs. Black patients experienced reduced disease-free survival compared to all other races regardless of the time interval used. In addition, excluding death from prostate cancer as a failure event also did not materially change the results. Thus, our results are unlikely to be an artifact of misclassifying additional treatment or an unreliable determination of cause of death.
Our measures of cancer recurrence likely underestimate recurrence rates from biochemical (PSA) measures. Additional therapy may be inappropriate for slowly progressing recurrent disease or when advanced patient age or comorbidity limit life expectancy or tolerance of treatment. If these patients recur without additional treatment or documented death from prostate cancer, their recurrences will be undetected using our methods. In addition, Medicare data do not detect self-administered, oral prescription drugs such as single-agent flutamide or diethylstilbestrol during the time period of the study. Furthermore, in our dataset, cause of death information was not available during the last 2 years of the study, preventing detection of late prostate cancer deaths in patients receiving no further prostate cancer treatment.
In addition to medical factors, physician practice patterns and patient preferences influence the relationship between disease recurrence and additional treatment [18, 19]. Increased frequency among black patients of post-treatment assessments of black patients or a greater propensity to institute therapy in black patients once biochemical progression is noted could partly account for the results we found. However, published data indicating that black patients receive poorer follow-up surveillance for disease recurrence and are less likely to receive aggressive therapy for their initial treatment make these explanations unlikely and suggest that our results underestimate the true disease-free survival difference between black patients and other racial groups [20, 21].
It is possible that unmeasured non-medical variables relevant to our measure of disease recurrence may confound our results. SEER–Medicare data provide little information on other environmental, dietary, and lifestyle factors that may affect responses to specific treatments. In addition, important medical predictors of recurrence rates, such as preoperative serum PSA value and tumor size, were not available in the dataset. Residual confounding in measured variables may also bias our results. For example, education and SES information in SEER–Medicare are available only at the census-tract level, which only approximates individual level information [22, 23]. Compared to valid, patient-specific data for these demographic variables any surrogate indicator is inaccurate, but census-tract data provide useful information that distinguishes patient groups and its use is widely accepted. Furthermore, comorbidity measures are limited by the number of comorbid conditions available from claims data.
A major strength of this study is that the use of SEER–Medicare data provide extended follow-up of a large, population-based cohort of prostate cancer patients in diverse locations in the United States. Although the five SEER areas we studied are not statistically representative of the entire US population, they are broadly representative of the urban US [24]. However, the restriction of our analysis to Medicare patient aged 65–84 may limit its relevance to the younger patients currently being diagnosed with prostate cancer.
During the years during which the patients we studied were diagnosed, PSA testing produced a stage shift in prostate cancer, resulting in lower stage, earlier cancers detected in younger men compared to the cancers we studied. Our results may therefore overstate current recurrence rates, although racial differences would be less affected. The majority of patients in all racial groups in our study were diagnosed during the recent PSA era (1992–1996), but our ability to examine these more recent cancers is sharply limited by having only 2–4 years to follow them. When examined by PSA era, the disease-free survival disparity between white and black patients was least pronounced in the most recent period (1992–1996), but longer follow-up will be required to verify that the disparity has really declined.
In our previous analysis of the same patient population, we found that compared to white patients, black patients with clinically localized prostate cancer had poorer overall and prostate cancer-specific survival for all treatment modalities and especially after surgery, even with control for comorbid medical conditions [6]. We now extend these findings to include higher prostate cancer recurrence rates. The fact that black prostate cancer patients have higher clinical recurrence rates while alive suggests that at least part of the racial difference in prostate cancer mortality is not attributable to delayed initial diagnosis or less effective management of recurrent disease. Poorer disease control implies less technically adequate initial treatment or tumors with more aggressive biological behavior. Such biological differences could reflect racial differences in factors affecting tumor initiation or progression (e.g., nutrition, chemical exposures, chronic stress, genetic variants, etc.).
In conclusion, our study of disease-free survival in prostate cancer patients with clinically localized disease observed worse outcomes for black patients. Among surgery patients, race was a significant predictor of disease-free survival despite adjustments for measurable potential confounders in the model. Longer follow-up is required for accurate estimates of the outcomes of patients diagnosed during the era of PSA screening, and using biochemical recurrence, if feasible, may improve on our measure of disease progression. Earlier recurrence of prostate cancer may help explain black patients’ increased risk of mortality from prostate cancer.
References
InstitutionalAuthorNameAmerican Cancer Society (2005) Cancer facts & figures 2005 American Cancer Society Atlanta, GA
PA Wingo S Bolden T Tong et al. (1996) ArticleTitleCancer statistics for African Americans, 1996 CA Cancer J Clin 46 113–125 Occurrence Handle8624795 Occurrence Handle1:STN:280:BymB3Mzls1Y%3D
Ries LAG, Eisner MP, Kosary CL et al (eds) (2003) SEER Cancer statistics review, 1975–2000. National Cancer Institute, Bethesda, Maryland. http://seer.cancer.gov/csr/1975 2000
I Thompson C Tangen A Tolcher et al. (2001) ArticleTitleAssociation of African–American ethnic background with survival in men with metastatic prostate cancer J Natl Cancer Inst 93 219–225 Occurrence Handle11158191 Occurrence Handle1:STN:280:DC%2BD3M7ot1Kgsg%3D%3D Occurrence Handle10.1093/jnci/93.3.219
A Jemal A Thomas T Murray et al. (2002) ArticleTitleCancer statistics, 2002 CA Cancer J Clin 52 23–47 Occurrence Handle11814064 Occurrence Handle10.3322/canjclin.52.1.23
PA Godley AP Schenck MA Amamoo et al. (2003) ArticleTitleRacial differences in mortality among Medicare recipients after treatment for localized prostate cancer J Natl Cancer Inst 95 IssueID22 1702–1710 Occurrence Handle14625261
AL Potosky GF Riley JD Lubitz et al. (1993) ArticleTitlePotential for cancer related health services research using a linked Medicare-tumor registry database Med Care 31 732–748 Occurrence Handle8336512 Occurrence Handle1:STN:280:ByyA38bpt1Y%3D
JL Warren CN Klabunde D Schrag et al. (2002) ArticleTitleOverview of SEER–Medicare data: content, research applications, and generalizability to the United Stated elderly population Med Care 40 IssueID8S IV3–IV18
GS Cooper B Virnig CN Klabunde et al. (2002) ArticleTitleUse of SEER–Medicare data for measuring cancer surgery Med Care 40 IssueID8S IV43–IV48
BA Virnig JL Warren GS Cooper et al. (2002) ArticleTitleStudying radiation therapy using SEER–Medicare-linked data Med Care 40 IssueID8S IV49–IV54
GL Lu-Yao SL Yao (1997) ArticleTitlePopulation-based study of long-term survival in patients with clinically localized prostate cancer Lancet 349 906–910 Occurrence Handle9093251 Occurrence Handle1:STN:280:ByiB2cjhtFM%3D Occurrence Handle10.1016/S0140-6736(96)09380-4
InstitutionalAuthorNameAmerican Joint Committee on Cancer (AJCC) (2002) Prostate FL Greene DL Pge ID Fleming AG Fritz CM Balch (Eds) et al. AJCC cancer staging manual EditionNumber6th edn Springer New York (NY) 309–316
A Fritz L Ries (1998) The SEER program code manual EditionNumber3rd edn National Cancer Institute Bethesda (MD)
R Deyo DC Cherkin MA Ciol (1992) ArticleTitleAdapting a clinical comorbidity index for use with ICD-9-CM administrative databases J Clin Epidemiol 45 613 Occurrence Handle1607900 Occurrence Handle1:STN:280:By2A3c%2FjvFY%3D Occurrence Handle10.1016/0895-4356(92)90133-8
CN Klabunde AL Potosky JM Legler et al. (2000) ArticleTitleDevelopment of a comorbidity index using physician claims data J Clin Epidemiol 53 1258–1267 Occurrence Handle11146273 Occurrence Handle1:STN:280:DC%2BD3M%2FptlSrtQ%3D%3D Occurrence Handle10.1016/S0895-4356(00)00256-0
EL Kaplan P Meier (1958) ArticleTitleNonparametric estimation from incomplete observations J Am Stat Assoc 45 7–481
GL Lu-Yao AL Potosky PC Albertsen et al. (1996) ArticleTitleFollow-up prostate cancer treatments after radical prostatectomy: a population-based study J Natl Cancer Inst 88 IssueID3–4 166–173 Occurrence Handle8632490 Occurrence Handle1:STN:280:BymB3s7ptlU%3D
D Scherr PW Swindle PT Scardino (2003) ArticleTitleNational comprehensive cancer network guidelines for the management of prostate cancer Urology 61 IssueID2A 14–24 Occurrence Handle12667883 Occurrence Handle10.1016/S0090-4295(02)02395-6
CC Schulman JE Altwein AR Zlotta (2000) ArticleTitleTreatment options after failure of local curative treatments in prostate cancer: a controversial issue Br J Urol 86 1014–1022 Occurrence Handle1:STN:280:DC%2BD3M%2FntFKntg%3D%3D
VL Shavers M Brown CN Klabunde et al. (2004) ArticleTitleRace/ethnicity and the intensity of medical monitoring under ‘watchful waiting’ for prostate cancer Med Care 42 IssueID3 239–250 Occurrence Handle15076823 Occurrence Handle10.1097/01.mlr.0000117361.61444.71
SB Zeliadt DF Penson PC Albertsen et al. (2003) ArticleTitleRD Race independently predicts prostate specific antigen testing frequency following prostate cancer diagnosis Cancer 98 IssueID2 496–503 Occurrence Handle12879465 Occurrence Handle10.1002/cncr.11492
H Greenwald N Polissar E Borgatta et al. (1994) ArticleTitleDetecting survival effects of socioeconomic status: problems in the use of aggregate data J Clin Epidemiol 47 903–909 Occurrence Handle7730894 Occurrence Handle1:STN:280:ByqB2cfjsl0%3D Occurrence Handle10.1016/0895-4356(94)90194-5
RK Kwok BC Yankaskas (2001) ArticleTitleThe use of census data for determining race and education as SES indicators: a validation study Ann Epidemiol 11 171–177 Occurrence Handle11293403 Occurrence Handle1:STN:280:DC%2BD3M3jsFKmuw%3D%3D Occurrence Handle10.1016/S1047-2797(00)00205-2
AB Nattinger TL McAuliffe MM Schapira (1997) ArticleTitleGeneralizability of the surveillance, epidemiology, and end results registry population: factors relevant to epidemiologic and health care research J Clin Epidemiol 50 IssueID8 939–945 Occurrence Handle9291879 Occurrence Handle1:STN:280:ByiH3sjjsVI%3D Occurrence Handle10.1016/S0895-4356(97)00099-1
Acknowledgment
The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database. This research was supported by 1 P60 MD00244-01 National Center on Minority Health and Health Disparities and by 1 U01 CA114629-01 National Cancer Institute and PC040795 Department of Defense (to P. Godley). The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study used the linked SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. All authors contributed equally to the preparation and submission of this manuscript.
Rights and permissions
About this article
Cite this article
Cohen, J.H., Schoenbach, V.J., Kaufman, J.S. et al. Racial Differences in Clinical Progression Among Medicare Recipients After Treatment for Localized Prostate Cancer (United States). Cancer Causes Control 17, 803–811 (2006). https://doi.org/10.1007/s10552-006-0017-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-006-0017-7