Abstract
Purpose
Racial differences in prostate cancer treatment patterns have motivated concerns about over- and undertreatment. We surveyed black and white patients with localized prostate cancer (LPC) regarding their treatment decision-making processes to gain a better perspective on factors associated with LPC treatment choice.
Methods
We conducted a population-based, cross-sectional survey of 260 men (132 black, 128 white) aged ≤75 years, with newly diagnosed LPC. Our primary outcome was treatment choice (either surgery, radiation, or watchful waiting/active surveillance (WW/AS)), and our primary predictors were race and tumor risk level.
Results
Overall, treatment choice did not differ by race. As cancer risk increased, both black and white patients were more likely to undergo surgery and less likely to receive radiation. However, the pattern of WW/AS was different between white and black men. White men were less likely to select WW/AS as cancer risk increased, while risk level was unrelated to black men undergoing WW/AS. Urologist’s recommendation had the greatest impact on men’s treatment choice, followed by tumor risk level, age, and personal preferences.
Conclusions
Although there were no overall racial differences in treatment choice, when stratified by tumor risk level, the pattern of WW/AS was different between white and black patients, suggesting that over- and undertreatment is a larger concern for black than white men. A risk-stratified approach to understand racial disparities in LPC treatment and better strategies to aid black men in their treatment decision-making are needed to reduce racial disparities in prostate cancer outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer remains the most common malignancy in the USA. Prostate cancer exhibits a striking racial disparity as black men have 1.6 times higher incidence and 2.4 times higher mortality compared to white men [1]. Known genetic susceptibility accounts for only a small proportion of the racial variation [2]. The disproportionate burden of prostate cancer in black men may be related to unequal access to medical care and differences in the receipt of treatment [2]. Black men who receive prostate cancer treatment similar to that of white men experience similar outcomes [3, 4]. Historically, black men undergo less aggressive treatment, more watchful waiting (WW), even after adjustment for socioeconomic status (SES) [5–7].
Although the literature raises questions about racial differences in cancer treatment and outcomes, the studies often have important limitations. Many were conducted before the era of widespread PSA screening, while others use cancer registry data that lack information on the presence of comorbidities and/or SES, which may influence men’s treatment options [7, 8]. In addition, there are few studies that investigate the patient’s perspective about how and why they decide on the treatment for their localized prostate cancer (LPC) [9], and even less is known about whether decision-making influences differ among racial groups. It is unclear whether men’s treatment choices correspond to their tumor risk level, which would affect the likelihood of clinical benefit. The risk stratification of prostate cancer has allowed for improved counseling of patients and provides guidance for treatment selection. Concerns of overtreatment of low-risk cancers and undertreatment of high-risk cancers are hotly debated [10–13]. An improved understanding is needed regarding the treatment decision-making for LPC, particularly in a contemporary screen-detected cohort.
Given the paucity of research on racial disparities in prostate cancer treatment decision-making [14] and increasing concern of overtreatment of low-risk cancer [11], we conducted a population-based study of racially and socioeconomically diverse LPC patients. We hypothesized that race and tumor risk affect treatment choice even after adjusting for men’s preferences, SES, and comorbidities.
Materials and Methods
We conducted a cross-sectional survey of black and white men living in the metropolitan Detroit area aged ≤75 years and newly diagnosed with LPC between 2009 and 2010. Cases were identified by Rapid Case Ascertainment (RCA) in the Metropolitan Detroit Cancer Surveillance System (MDCSS), a founding member of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program. RCA reviews pathology reports of malignancy from all area hospitals and clinical laboratories and usually identifies newly incident cancer cases within 3–4 months of diagnosis. RCA allowed us to identify and contact men while they were in the process of making treatment decisions to minimize the potential recall bias. LPC was defined as T1–T2 tumors based on American Joint Committee on Cancer (AJCC) stage criteria. The study received approval from the institutional review board at Wayne State University.
Survey Instrument
The content and design of the survey were developed based on thorough literature review, and refined by the findings of formal semi-structured, in-depth, in-person interviews of 21 men (14 black, 7 white) with newly diagnosed LPC [15, 16]. The survey asked men to report their treatment choice, reasons for the choice, and what treatment options were offered and recommended by their physicians, including urologist, radiation oncologist, and primary care physician (PCP).
Overall, the survey instrument demonstrated good reliability and validity. Internal consistency of the scales (e.g., treatment efficacy/cure, treatment burden) measured by Cronbach’s alpha ranged from 0.63 to 0.87. To evaluate construct validity, we conducted factor analyses of our scales and found the resulting factors were well defined and conformed to our theoretical expectations. The predictive validity of our scales was demonstrated in that the scales discriminated between groups of individuals expected to differ with regard to treatment choice.
Study Population and Sampling
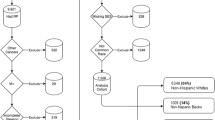
During the study period, a total of 874 potentially eligible LPC cases were identified, and 559 were sampled for inclusion in the study. To achieve similar numbers of white and black men, we sampled white men at a ratio of 1:3. Other racial groups were excluded due to their small numbers in the registry, which reflects the population of the metropolitan Detroit area. After initial physician and patient contact, 168 total patients were excluded as described in Fig. 1. Of the 391 eligible cases, 266 men completed the survey with a response rate of 68 % (white 78 %, black 62 %), 6 of which were excluded from the data analysis. Twenty men were excluded from multinomial logistic regression due to selection of treatment other than the 3 main treatments, including 8 men who were undecided on treatment at the time of survey.
Data Collection
Physicians were notified of our intention to contact his/her patient with the option to exclude participation. If there was no physician objection, we initiated the Dillman method for contacting patients to encourage survey response [17]. This approach consisted of mailing study materials and a small monetary incentive with a multi-method follow-up approach, including postcard reminders and follow-up calls. Participants were encouraged to complete the written survey, and a telephone option was offered for those who did not return a completed survey after reminders (<5 %).
Measures
The primary outcome variable was the patient’s self-reported treatment choice (surgery, radiation, or WW/AS), which was identified from an 8-item list including an open-ended “other” category. There were two primary predictor variables: risk level and race. Cancer risk level was categorized as low, intermediate, or high risk using the American Urological Association endorsed D’Amico criteria [18], which is based on prostate-specific antigen (PSA) level, Gleason score, and stage [19]. Since the focus was on patient perspectives of the treatment decision process, self-reported PSA level and Gleason score were used when available and supplemented by MDCSS. Since patients are unlikely to know the exact stage of their cancer, MDCSS stage data were used. Self-reported demographic variables included age, marital status, education, income, insurance type, employment status, general health status, and the number of comorbidities obtained from a modified Charlson comorbidity index [20]. Patients were asked to report if they saw a urologist, radiation oncologist, and PCP, and what their treatment recommendation was. Since every patient saw a urologist, their specific recommendations were analyzed in detail. About half the patients saw the other specialists, and their recommendations could not be evaluated in detail.
Decision influencing factors were derived using a 12-item scale of Likert questions that asked “how much was your treatment decision influenced by whether the treatment would: get rid of the cancer, be convenient to receive, interfere with sex life, cause leakage of urine,” etc. The Likert response categories ranged from 1 (“Did not influence decision”) to 4 (“Very much influenced decision”). Factor analyses of the scale revealed three well-defined, meaningful factors: desire for “treatment efficacy/cure,” concerns of “treatment burden,” and “worry about side effects” (Cronbach alpha of 0.63, 0.80, and 0.80, respectively). The factor “treatment efficacy/cure” included 2 items (cure and reduce recurrence); “treatment burden” included 4 items (pain, inconvenience, life style, and cost); and “treatment side effects” included 6 items (sexual, urinary, and bowel symptoms).
Statistical Analysis
Five percent of surveys were double entered to confirm data entry accuracy. Self-reported household income was not reported by 10 % of subjects and was imputed using median household income based on census track. All other variables had <5 % missing data and were not differentially missing.
Racial and treatment group differences in demographics, comorbidities, PSA level, Gleason score, general health, perceived cancer seriousness, and worry about cancer were examined using t tests, chi-square analyses, and generalized linear modeling. The effect of race, tumor risk level, desire for treatment efficacy/cure, concern about treatment burden, and worry about treatment side effects on treatment choice were evaluated using multinomial logistic regression. A backward stepwise procedure selected the final predictor variables included in the model. All analyses were completed using SPSS version 20 (SPSS Inc., Chicago, IL), with an alpha <0.05 as significant.
Results
Compared to white men, black men had lower education and income, were less likely to be married/partnered, and less likely to have private insurance (all p ≤ 0.001) (Table 1). There were no racial differences in the percentage of men who reported a family history of prostate cancer, yet black men reported a higher number of relatives with prostate cancer. There were no racial differences in PSA level, Gleason score, stage, risk level, treatment selected, comorbidities, and cancer seriousness perception; however, black men were somewhat more likely to report worse perception of general health (p = 0.06) and a higher degree of cancer worry (p = 0.08). Both races rated treatment efficacy/cure as the factor that most strongly influenced their treatment decisions. Yet, black men reported higher concern regarding treatment burden than white men (p = 0.04) and white men reported higher desire of treatment efficacy/cure than black men (p = 0.04). There were no racial differences in how strongly their decisions were influenced by worry about side effects.
Regardless of race, men who chose surgery were younger than men who chose radiation or WW/AS (p ≤ 0.001). In addition, men who chose surgery reported better perception of general health, lower number of comorbidities, and were more likely to have full-time jobs and private insurance. There were no differences in education, income, marital status, or family history by treatment group. There were significant differences in Gleason score, stage, and risk level based on treatment groups, but not PSA level. Men who chose surgery perceived their cancer as more serious, worried about it more, and had a higher proportion of high-risk disease.
About half of both white and black men reported that their urologist(s) did not recommend any treatment. However, when their urologists gave a recommendation, they were more likely (70 %) to recommend surgery. Almost all men (90 %) who received a surgery recommendation chose surgery. There were no racial differences in the urologist’s treatment recommendation or the congruence between recommendation and treatment selection. Additionally, there were no racial differences in the proportion of men who visited radiation oncologists (50 %) and/or their primary care physician (PCP) (45 %) during the time of treatment decision-making. Over half (59 %) of the men who reported a visit to radiation oncologist selected radiation, while most (65 %) men who reported a visit to PCP selected surgery. Like urologists, approximately half of the radiation oncologists and PCPs did not recommend a specific treatment.
In unadjusted bivariate analyses, all tested variables except race, education, and worry about side effects were significantly associated with treatment choice. In the adjusted analyses, race, age, full-time employment, risk level, worry about cancer, treatment efficacy/cure, treatment burden, and urologist surgery recommendation were significantly associated with treatment choice. There was a significant interaction between race and tumor risk level (p = 0.008) in unadjusted and adjusted analyses (data not shown here but available as a supplement Table for online publication only).
Multinomial logistic regression comparing surgery and WW/AS revealed that younger men (≤65) and men who emphasized treatment efficacy/cure were more likely to receive surgery; while older men and men who emphasized treatment burden were more likely to receive WW/AS (Table 2). More importantly, men who received a surgery recommendation from their urologist were much more likely to receive surgery than those who did not.
When comparing radiation and WW/AS, there was a significant race by risk level interaction. For black men, as tumor risk level increased, they were less likely (OR = 0.31) to receive radiation and more likely to receive WW/AS. For white men, this association was in the opposite direction (OR = 2.4) but it was not statistically significant (p = 0.09, data not shown). In addition, younger men and men who emphasized treatment efficacy/cure were more likely to receive radiation while older men and men who had full-time employment were more likely to choose WW/AS. Furthermore, compared to men who chose radiation, men who chose WW/AS reported greater cancer worry.
When comparing radiation and surgery, as risk level increased both black and white men were less likely to receive radiation over surgery (OR = 0.15, OR = 0.39, respectively). In addition, men concerned about treatment burden were more likely (OR = 1.9) to receive radiation, while men receiving urologist’s surgery recommendation were less likely (OR = 0.16) to receive radiation.
The race by risk level interaction is illustrated in Fig. 2. For both races, as cancer risk increased, the percentage choosing radiation decreased and the percentage choosing surgery increased. For WW/AS, white men with low-risk cancer were most likely to choose this option (29 %) and least likely with high-risk cancer (2 %). Black men chose WW/AS equally for low- and high-risk (10 %) disease, and the proportion was highest for intermediate risk (33 %).
Discussion
Overall, we found no racial differences in the initial treatment choice for LPC with low uptake of WW/AS for both races. Regardless of race, younger patients and patients with higher risk tumors were more likely to select surgery and less likely to select radiation. However, the pattern of undergoing WW/AS differed by race. As cancer risk increased, white men were less likely to select WW/AS while black men did not select WW/AS based on tumor risk level.
There are a few earlier reports that suggest similar interactions between race and tumor aggressiveness [12, 21, 22]. Hoffman et al. [21] reported that among men with aggressive prostate cancers, black men received surgery less often than white men and were more often treated with androgen deprivation or WW. Although our findings support the observation that racial disparities in definitive therapy have decreased significantly over the years [21, 22], the interaction between race and tumor aggressiveness persisted in our contemporary cohort. This is concerning because it could lead to overtreatment of low-risk disease and undertreatment of intermediate/high-risk disease in black men [12]. It also suggests that either a lack of understanding of tumor risk in black men or poor communication between black patients and their physicians may exist. Indeed, Steenland et al. [23] found that poor patient-physician communication was prevalent among black men and that it was associated with not choosing definitive treatment. In our sample, black men had significantly lower SES than whites, which may contribute to lower health literacy and poorer understanding of risks and benefits of treatment options. Racial differences in treatment preferences could also contribute to the differential patterns. For instance, black men were more concerned by the treatment burden associated with surgery than white men, and it played a small but significant role in their treatment choice.
The few previous studies that looked at racial differences in prostate cancer treatment decision-making have shown that differences exist in the type of treatment chosen, with black men more likely to choose nonsurgical options compared to white men [21, 24, 25]. While it is unclear why these differences exist, some hypothesize that physicians may provide black men with different information, either because they think these men would not adhere to medical advice or that they would be less receptive to surgery [21, 24]. In contrast, our findings suggest that physicians provided similar treatment recommendations to both black and white patients, and they were equally receptive to physician recommendation. This is encouraging and supports more recent findings of overall decrease of racial disparity in the use of definitive therapy [21, 22]. Furthermore, we found that both black and white men reported about half of their physicians, regardless of specialty, did not recommend any specific treatment. This is interesting in the context of widespread knowledge that specialists are biased towards the treatment they themselves deliver [26]. Our findings suggest considerable efforts are made by physicians to be impartial as the current evidence warrants. However, we also clearly show that specialists favor their own treatment when providing a specific recommendation.
Compared to a relatively passive WW approach, AS is an active program of surveillance with curative treatment triggered by signs of cancer progression and is considered a reasonable choice for men with low-risk disease [11]. However, only 10 % of men in our sample chose AS, which is consistent with other reports [27, 28]. Given the strong influence of urologist’s recommendation on treatment choice, interventions to decrease overtreatment of low-risk cancers should include urologists. In fact, Davison et al. [29] identified urologist’s recommendation as the most influential factor on the decision to choose AS in a small survey of mostly white, educated men.
Study Strengths and Limitations
This study has important strengths. First, our study is one of few population-based studies that examined the personal, sociocultural, clinical, and physician influences on prostate cancer treatment choices. More importantly, this study is unique in its examination of racial differences in the rationale and forces that motivate treatment choice from the patient’s point of view.
However, there are several limitations. First, although our goal was to survey patients before treatment began, approximately 66 % of men had started treatment. Therefore, recall bias may have affected patient reports. However, men surveyed before and after treatment began were not significantly different in respect to treatment choice, demographics, clinical characteristics, or decision-influencing factors (data not shown). Furthermore, all men were surveyed within 6 months of diagnosis, which should minimize potential recall bias. Second, a small number of patients (n = 31) chose WW/AS in this cohort, and the interaction between race and risk should be interpreted cautiously. Larger studies are needed to confirm this finding. Third, we did not differentiate WW from AS in this study since AS is a relatively new term, and these terms are still used interchangeably by both physicians and patients [11]. In addition, as this survey was done between 2009 and 2010, we expect more patients may choose AS now because of new evidence supporting the safety of AS [31] and the increasing concerns of overdiagnosis and overtreatment of LPC [13]. Fourth, other than the stage at diagnosis, all data were self-reported. However, the high correlations (>0.7, data not shown) between self-reported tumor characteristic (e.g., PSA level and Gleason score) and those reported in the tumor registry give us confidence of the accuracy of the self-reported data. Fifth, our sample has a relatively lower percentage of low-risk disease (18.6 %) compared to most published data [28, 30, 31] This is mainly due to the higher proportion of clinical stage (≥ T2c) recorded in MDCSS, which automatically assigns them to high-risk based on D’Amico criteria. When we recalculated risk level using only PSA level and Gleason score, the proportion of low-risk disease was similar to other published reports, and the race and risk interaction persisted. Finally, our sample was derived from one urban metropolitan area and may not be representative of non-urban locations or different geographic areas.
In conclusion, this racially diverse, population-based study provides an important assessment of personal, sociocultural, and clinical influences of LPC treatment decision-making. Although there were racial differences in some patient preferences, there were no racial differences in overall treatment choices. Regardless of race, men who chose surgery were more influenced by their desire for cure and urologist’s surgery recommendation. Men who chose radiation were more influenced by concerns of treatment burden. White men who chose WW/AS followed an expected pattern of decreasing WW/AS choice as tumor risk increased; black men did not follow this pattern. Over-and undertreatment may be a larger concern for black men compared to white men and deserves further investigation. A risk-stratified approach to understand racial disparities in prostate cancer treatment and better strategies to aid black men in their treatment decision-making are needed to reduce racial disparities in prostate cancer outcomes.
References
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: Cancer J Clin. 2011;61(4):212–36.
Barocas DA, Penson DF. Racial variation in the pattern and quality of care for prostate cancer in the USA: mind the gap. BJU Int. 2010;106(3):322–8.
Optenberg SA, Thompson IM, Friedrichs P, Wojcik B, Stein CR, Kramer B. Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA. 1995;274(20):1599–605.
Underwood 3rd W, Wei J, Rubin MA, Montie JE, Resh J, Sanda MG. Postprostatectomy cancer-free survival of African Americans is similar to non-African Americans after adjustment for baseline cancer severity. Urol Oncol. 2004;22(1):20–4.
Schwartz K, Powell IJ, Underwood 3rd W, George J, Yee C, Banerjee M. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology. 2009;74(6):1296–302.
Shavers VL, Brown ML, Potosky AL, Klabunde CN, Davis WW, Moul JW, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19(2):146–55.
Schapira MM, McAuliffe TL, Nattinger AB. Treatment of localized prostate cancer in African-American compared with Caucasian men. Less use of aggressive therapy for comparable disease. Med Care. 1995;33(11):1079–88.
Krupski TL, Kwan L, Afifi AA, Litwin MS. Geographic and socioeconomic variation in the treatment of prostate cancer. J Clin Oncol. 2005;23(31):7881–8.
Zeliadt SB, Moinpour CM, Blough DK, Penson DF, Hall IJ, Smith JL, et al. Preliminary treatment considerations among men with newly diagnosed prostate cancer. Am J Manag Care. 2010;16(5):e121–30.
Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–23.
Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, et al. National Institutes of Health State-of-the-Science Conference: role of active surveillance in the management of men with localized prostate cancer. Ann Intern Med. 2012;156(8):591–5.
Presley CJ, Raldow AC, Cramer LD, Soulos PR, Long JB, Yu JB, et al. A new approach to understanding racial disparities in prostate cancer treatment. J Geriatr Oncol. 2013;4(1):1–8.
The US Preventive Serivice Task Force. Screening for prostate cancer. 2012. http://www.uspreventiveservicestaskforce.org/prostatecancerscreening.htm. Accessed 10/10/2013.
Zeliadt SB, Ramsey SD, Penson DF, Hall IJ, Ekwueme DU, Stroud L, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106(9):1865–74.
Xu J, Dailey RK, Eggly S, Neale AV, Schwartz KL. Men’s perspectives on selecting their prostate cancer treatment. J Natl Med Assoc. 2011;103(June):468–78.
Xu J, Neale AV, Dailey RK, Eggly S, Schwartz KL. Patient perspective on watchful waiting/active surveillance for localized prostate cancer. J Am Board Fam Med : JABFM. 2012;25(6):763–70.
Dillman D. Mail and telephone surveys. New York: John Wiley and Sons Inc.; 1978.
Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31.
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–74.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–83.
Hoffman RM, Harlan LC, Klabunde CN, Gilliland FD, Stephenson RA, Hunt WC, et al. Racial differences in initial treatment for clinically localized prostate cancer. Results from the prostate cancer outcomes study. J Gen Intern Med. 2003;18(10):845–53.
Underwood W, De Monner S, Ubel P, Fagerlin A, Sanda MG, Wei JT. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol. 2004;171(4):1504–7.
Steenland K, Goodman M, Liff J, Diiorio C, Butler S, Roberts P, et al. The effect of race and rural residence on prostate cancer treatment choice among men in Georgia. Urology. 2011;77(3):581–7.
Denberg TD, Beaty BL, Kim FJ, Steiner JF. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. 2005;103(9):1819–25.
Moses KA, Paciorek AT, Penson DF, Carroll PR, Master VA. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2009;28(6):1069–74.
Fowler Jr FJ, McNaughton Collins M, Albertsen PC, Zietman A, Elliott DB, Barry MJ. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–22.
Hamilton AS, Albertsen PC, Johnson TK, Hoffman R, Morrell D, Deapen D, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107(4):576–84.
Barocas DA, Cowan JE, Smith Jr JA, Carroll PR. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180(4):1330–4.
Davison BJ, Goldenberg SL. Patient acceptance of active surveillance as a treatment option for low-risk prostate cancer. BJU Int. 2011;108(11):1787–93.
Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–9.
Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367(3):203–13.
Acknowledgments
This study was supported by a Mentored Research Scholar Grant in Applied and Clinical Research (Grant #MRSGT-06-133-01-CPPB) from American Cancer Society.
Conflict of Interest
All authors declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). The study received approval from the institutional review board at Wayne State University. A waiver of written consent was granted and an information sheet was mailed with each survey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Janisse, J., Ruterbusch, J. et al. Racial Differences in Treatment Decision-Making for Men with Clinically Localized Prostate Cancer: a Population-Based Study. J. Racial and Ethnic Health Disparities 3, 35–45 (2016). https://doi.org/10.1007/s40615-015-0109-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-015-0109-8