Abstract
While checkpoint inhibitors have been approved in patients with newly metastatic PDL1-positive triple negative breast cancer, similar clinical benefit with immunotherapy alone or in combination with chemotherapy has not been observed in patients with hormone receptor-positive, HER2− negative breast cancer in the metastatic setting. However, in the ISPY2 trial, an increase in pathologic response has been observed with the addition of immunotherapy (± PARP inhibition) to chemotherapy compared to chemotherapy alone in patients with high-risk hormone receptor-positive, HER2− breast cancer. We review strategies to enhance the immunotherapeutic activity in this subtype of breast cancer, including combinations of checkpoint inhibition with chemotherapy, endocrine therapy, PARP inhibitors, HDAC inhibitors, CDK4/6 inhibitors, and radiotherapy. Combinations with agents targeting novel immunotherapeutic targets are also discussed. Though there remains an unmet need for immunotherapy approaches in patients with hormone-receptor positive breast cancer, there are a number of approaches that may lead to increased anti-tumor activity with immunotherapy in this tumor subtype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
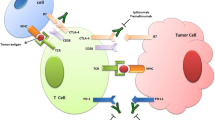
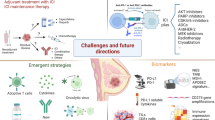
Results from the IMpassion130 [1] and KEYNOTE-355 [2] trials have demonstrated that the combination of chemotherapy and immunotherapy improves progression free survival (PFS) in the front-line treatment of patients with PD-L1+metastatic triple negative breast cancer (TNBC), moving immunotherapy into the treatment algorithms for patients with metastatic breast cancer (mBC). On the other hand, patients with hormone receptor -positive, HER2− negative (HR+, HER2−) cancers have lower levels of tumor infiltrating lymphocytes (TILs) [3] and PD-L1 expression [4] and are traditionally considered immunologically cold tumors. Despite this, a minority of patients will have clinically meaningful responses to immunotherapy and identifying better predictive biomarkers for response and the optimal setting for checkpoint inhibition is imperative. Here, we discuss the current landscape of immunotherapy in HR+, HER2− disease and emerging combination strategies to augment responses to these agents, which are summarized in Fig. 1 and Table 1.
Strategies in the metastatic setting: immunotherapy alone
Anti-PD-1/PD-L1 monotherapy
The phase Ib KEYNOTE-028 trial evaluated pembrolizumab monotherapy among patients with metastatic PD-L1-positive pretreated solid tumors, including a cohort of patients with HR+, HER2− breast cancer [5]. PD-L1 positivity was defined as tumor combined positive score (CPS ≥ 1. Of note, among 261 patients with HR+, HER2− breast cancer who were screened for tumor PD-L1 expression, only 48 (19.5%) had PD-L1 positive tumors. Among 25 enrolled patients with a median number of prior therapies of 9, the ORR was 12% [all partial responses (PR)], and clinical benefit rate (CBR), defined as complete response (CR) plus PR plus stable disease (SD) for at least 6 months, was 20%. The median duration of response was 12.0 months for the three patients with PR, and one patient had sustained response for 69.3 weeks at time of analysis. Of note, all patients with PR had progressed on at least three lines of therapy in the metastatic setting. Median PFS and overall survival (OS) for the entire cohort were 1.8 months and 8.6 months, respectively. The durable responses observed among those who had at least stable disease highlighted both the potential activity of pembrolizumab in a subgroup of this population and the need to identify additional or alternative predictive biomarkers than PD-L1 status for HR+, HER2− patients.
The PD-L1 inhibitor avelumab has similarly been evaluated as monotherapy in pretreated patients with HR+, HER2− mBC, with mixed success. In the phase Ib JAVELIN Solid Tumor trial, 168 patients with pretreated mBC of all subtypes received avelumab monotherapy, including 72 women with HR+, HER2− cancers [6]. Unlike KEYNOTE-028, JAVELIN did not selectively enroll patients with PD-L1 positive tumors. The ORR for the entire cohort was only 3.0% (five patients), of whom three had TNBC and two had HR+, HER2− disease. The one patient with a CR had TNBC. Disease control rate (DCR), defined as those with response or SD, was 28.0% for the entire cohort, and median PFS and OS were 6.0 weeks and 9.2 months, respectively. While PD-L1 expression on tumor cells was not associated with a statistically significant difference in clinical efficacy, PD-L1 expression of ≥ 10% tumor-associated immune cells at any intensity was associated with improved ORR (16.7% vs. 1.6%, p = 0.039).
In 2020, the United States Food and Drug Administration granted accelerated approval to pembrolizumab monotherapy in previously treated, unresectable/metastatic solid tumors with high tumor mutational burden (TMB), defined as ≥ 10 mutations/Mb, based upon the results of KEYNOTE-158, which showed an ORR of 34.3% among 233 patients with 27 tumor types [7]. Analysis of nearly 4000 tumor samples from women with primary or metastatic breast cancer revealed that HR-positive breast cancers have significantly lower mean TMB compared to TNBC and HER2-positive cancers, but among those with metastatic breast cancer, the frequency of breast cancers with high TMB was similar among tumor subtypes (3.7–3.9%) [8]. While less than 5% of mBCs have high TMB, analysis of TMB can identify a minority of patients who might derive benefit from pembrolizumab, independent of PD-L1 expression.
Immunotherapeutic combinations
Based upon the success of combinations of anti-PD-(L)1 therapies in other solid tumors [9, 10], the combination of nivolumab plus ipilimumab is currently being investigated in patients with pretreated HER2-negative mBC who have high TMB (≥ 10 mutations/Mb) in the NIMBUS trial (NCT03789110) [11]. The combination of durvalumab, an anti-PD-1 monoclonal antibody, with tremelimumab, an anti-CTLA4 monoclonal antibody, was evaluated in a pilot study among 18 women with mBC, and did not show activity in patients with HR+, HER2− tumors [12]. While the ORR was 17% in the overall population, no patient with HR-positive mBC responded.
Maintenance anti-PD-1 after chemotherapy
Immunotherapy has also been evaluated as potential maintenance therapy among patients with HER2-negative mBC who have response to first-line chemotherapy, with the goals of reducing the burden of chemotherapy-related toxicity, as well as potentiating an immune response after chemotherapy. In a substudy of the SAFIR02-IMMUNO trial, 199 patients with metastatic HER2-negative mBC without a targetable mutation and who had SD, PR, or CR after six to eight cycles of chemotherapy were randomized 2:1 to receive maintenance durvalumab at 10 mg/kg every 14 days or maintenance chemotherapy [13]. Overall, maintenance durvalumab did not improve PFS compared with chemotherapy, and in the HR-positive subgroup, chemotherapy provided greater PFS benefit than durvalumab [hazard ratio (HR) = 2.08, 95% confidence interval (CI) = 1.28–3.40; p = 0.0025]. While exploratory analyses suggested that patients with PD-L1 positive tumors had improved PFS and OS with durvalumab, the majority of patients with PD-L1-positive tumors had TNBC, with only 14.9% of patients with HR-positive disease having a PD-L1-positive tumor.
Combination strategies in the metastatic setting with chemotherapy, targeted therapies, and/or radiation therapy
Chemotherapy and radiotherapy have the potential to augment responses to immunotherapy, though the degree as to which this happens clinically remains unclear. Chemotherapy and radiotherapy induce immunogenic cell death, resulting in the release of tumor related neoantigens and damage associated molecular patterns which ultimately stimulate cytotoxic T-cells and add to the anti-tumoral potential of immune checkpoint blockade [14]. Additionally, chemotherapy has been shown to have various favorable immunogenic effects on the tumor microenvironment, including reduction in myeloid derived suppressor cells, reduction in regulatory T-cells (Tregs), and promotion of dendritic cell maturation [15, 16].
Clinically, IMpassion130 [1] and KEYNOTE-355 [2] demonstrated that combination chemo-immunotherapy improves PFS in the frontline setting in patients with metastatic, PD-L1 positive TNBC when compared to chemotherapy alone. Success has been seen in other solid tumor types, with combination chemo-immunotherapy approved frontline in the metastatic setting in non-small cell lung cancer [17, 18], small cell lung cancer [19] and PD-L1 positive squamous cell carcinoma of the head and neck [20]. Radiotherapy has also been shown to induce responses at non-irritated sites though the abscopal effect [21] and a variety of trials, predominantly in melanoma and non-small cell lung cancer, have evaluated combinations of immune checkpoint blockade and radiotherapy alone with mixed results [22]. Maintenance immunotherapy is approved after concurrent chemoradiotherapy in both non-small cell lung cancer [23] and esophageal cancer [24]. Given these successful combinations, trials have evaluated the efficacy of these combinations in attempts to improve the response to checkpoint inhibition in HR+, HER2− breast cancer.
Chemotherapy
The combination of pembrolizumab with chemotherapy has been evaluated in patients with HER2-negative breast cancers in a similar approach to IMpassion130 and KEYNOTE-355 without demonstration of a similar survival benefit among patients with HR-positive disease. A single-arm phase II trial evaluated the efficacy of the combination of pembrolizumab and capecitabine in 30 patients with pretreated HER2-negative breast cancer, including 14 patients with endocrine-refractory metastatic HR+, HER2− mBC [25]. In a 21-day cycle, patients received pembrolizumab 200 mg every two weeks and capecitabine 1000 mg/m2 oral (p.o.) twice daily (BID) on days 1–14. Median PFS for the entire cohort was 4.0 months and for the HR-positive subgroup was 5.1 months, which was not statistically better than a historical control of 3.0 months and therefore did not meet the prespecified endpoint. ORR was 14%, with two PRs observed in the HR-positive subgroup, and 21% of HR-positive patients had disease control for greater than one year. One patient with HR-positive disease died of immune related hepatitis.
A second phase II trial randomized patients with HR+, HER2− breast cancer to eribulin with or without pembrolizumab [26]. Eligible patients must have progressed on at least two prior lines of endocrine therapy, and 0–2 lines of prior chemotherapy in the metastatic setting. Patients were treated with 1.4 mg/m2 of eribulin on days 1 and 8 and 200 mg of pembrolizumab on day 1 of a 21-day cycle, and the primary endpoint was PFS. In total, 88 patients were enrolled and 37% were PD-L1 positive, defined as a modified proportion score of at least 1%. The addition of pembrolizumab did not improve PFS compared to eribulin alone in the entire cohort (4.1 months vs 4.2 months) or in the PD-L1 positive subgroup (4.2 vs 4.3 months). OS data was immature at the time of publication. Exploratory analysis of biomarkers for response included PD-L1 status, tumor infiltrating lymphocytes, and TMB, all of which could not identify a population that statistically benefited from the addition of pembrolizumab. Two patients died of immune-related adverse events (irAEs) in the pembrolizumab arm.
Endocrine therapy
While endocrine therapy is the backbone of standard first-line therapies with cyclin-dependent kinases (CDK) 4/6 inhibitors for HR+, HER2− mBC, it has not been frequently investigated in combination with immunotherapy in this population. In theory, endocrine therapy is an attractive combination therapy because it is not immunosuppressive and therefore not likely to blunt anti-tumor T-cell responses to tumor antigens. A phase I trial investigating the combination of tremelimumab and exemestane enrolled 26 women with metastatic HR+, HER2− breast cancer who had progressed on at least one line of systemic therapy in the metastatic setting [27]. While most adverse events were grade 1 or 2, five patients developed dose-limiting toxicities (diarrhea, transaminitis) and one patient had diarrhea refractory to oral steroids and required treatment with infliximab. The best overall response was stable disease in 11 of 26 patients (42%), which was durable for at least 12 weeks, and no patient had PR or CR. While there was no association between clinical response and total circulating CD4+ or CD8+ T-cells, the investigators noted that most patients with SD demonstrated increased co-expression of inducible costimulator (ICOS) on circulating CD4+ and CD8+ T-cells, likely signally immune activation. This combination did not proceed to a phase II trial, but it provided support for the further investigation of endocrine therapy in combination with immunotherapy.
PARP inhibitors
BRCA1 and BRCA2, in conjunction with a variety of other proteins including Chk2, ATM, RAD51 and others, play important roles in the homologous recombination pathway utilized in the repair of DNA double stranded breaks [28]. PARP inhibitors are thus felt to work through “synthetic lethality” in which cells with a deficiency in homologous recombination undergo cell death with PARP inhibition secondary to an inability to maintain genomic stability [29]. Multiple trials have demonstrated the efficacy of PARP inhibition in germline BRCA1/2-mutated, HER2-negative breast cancers [30, 31], with ongoing trials such as TBCRC 048 investigating if deficiencies in other components of the homologous recombination pathway also predict benefit with PARP inhibition [32].
Preclinical data suggest potential synergy with combining of PARP inhibition and immune checkpoint blockade. PARP inhibition and double-stranded DNA breaks have both been shown to increase PD-L1 expression in an ATM/ATR/Chk1 dependent manner [33], and the combination of PARP inhibition with checkpoint inhibition was more effective than either agent alone in an EMT6 breast cancer mouse model [34]. Additionally, PARP inhibition has been shown to result in the accumulation of cytosolic DNA with consequent activation of the cGAS-STING pathway and type I IFN production [35, 36], which can be further augmented when combined with checkpoint inhibition [37].
The phase Ib/II MEDIOLA trial evaluated the safety and efficacy of olaparib with durvalumab in patients with advanced solid malignancies, including a cohort of patients with germline BRCA1/2-mutated, HER2-negative mBC [38]. Patients with HR+, HER2− mBC were eligible if they had a tumor that had progressed on at least one line of endocrine therapy, had previously received an anthracycline or taxane, and received no more than two lines of prior chemotherapy for metastatic disease. Thirty-four patients with mBC were enrolled, of whom 16 (47%) had HR-positive breast cancer. The most common adverse were fatigue, GI effects, and anemia, and the most common grade 3/4 adverse events were anemia (12%), consistent with known toxicities of PARP inhibition. Overall, the median OS was 21.5 months and median PFS was 8.2 months with an 80% DCR at 12 weeks. Median OS and PFS in the HR-positive subgroup were 22.4 and 9.9 months respectively, with 8 PRs and no CR. Though not formally calculated, PD-L1 status did not appear to predict benefit. Intrinsic subtype and TMB also did not correlate with outcomes. Additional studies, such as JAVELIN PARP medley [39], are evaluating the combination of checkpoint and PARP inhibition.
CDK4/6 inhibitors
CDK 4/6 regulate the transition from the G1 to S phases of the cell cycle, and alterations in the CDK4/6 pathway are associated with resistance to endocrine therapy [40]. CDK4/6 inhibition with endocrine therapy is now standard of care among patients with HR+, HER2− breast cancer in the first-line metastatic setting, given improved survival outcomes compared to endocrine therapy alone [41,42,43]. There is preclinical evidence that CDK4/6 inhibitors elicit an anti-tumor immune response through enhanced antigen presentation by tumor cells, reduced proliferation of immunosuppressive Tregs, and stimulation of effector T-cells [44, 45]. In the neoMONARCH study, which randomized post-menopausal women to a two-week run in of neoadjuvant abemaciclib alone, anastrozole alone or the combination of both, post treatment biopsies demonstrated increased gene expression in inflammatory and PD-1 pathways by RNA-seq [46]. In mouse models, the addition of anti-PD-L1 antibody to abemaciclib enhanced tumor regression compared to either agent alone [44].
A multicohort phase Ib study is currently evaluating the efficacy and safety of the combination of pembrolizumab and abemaciclib, including two patient cohorts with HR+, HER2− mBC [47]. Eligible patients must be naïve to CDK4/6 inhibition and have received at least one but not more than two lines of systemic therapy. The first cohort is evaluating the combination of pembrolizumab and abemaciclib alone, and the second cohort is evaluating this combination with anastrozole. Early data from 28 patients in the pembrolizumab and abemaciclib arm, all with tumors which had progressed on endocrine therapy, demonstrated an ORR of 29%, with PR in 8 patients. Median PFS and OS were 8.9 months and 26.3 months, respectively. Adverse events were consistent with known toxicities of immune checkpoint inhibition and CDK4/6 inhibition, and the most common grade 3/4 adverse was neutropenia.
Recently, a non-randomized phase II trial evaluated the combination of nivolumab, abemaciclib, and either fulvestrant or letrozole in the first- or second-line setting among women with HR+, HER2− mBC [48]. In contrast to the combination of pembrolizumab and abemaciclib ± anastrozole, this combination resulted in significant toxicity, with trial closure for safety concerns. Among 17 women who were enrolled, over half had grade 3 or higher immune-related adverse events, and one died of treatment-related toxicity (interstitial lung disease). While the ORR were 54.5% and 20% among patients who received fulvestrant and letrozole, respectively, the significant toxicity associated with this combination raises concerns.
Histone deacetylase (HDAC) inhibitors
Histone deacetylases (HDAC) are epigenetic modifiers responsible for acetylation modulation which subsequently opens chromatin and allows for transcription of genes and results in cell cycle arrest, differentiation and/or cell death [49]. There are currently four HDAC inhibitors approved for various malignancies, with ongoing investigations evaluating the anti-tumor potential of these agents in breast cancer.
In HR-positive disease, preclinical data have demonstrated that HDAC inhibition in MCF-7 breast cancer models induces estrogen receptor alpha degradation, downregulates estrogen receptor transcription and reduces tumor growth [50]. The combination of checkpoint inhibition with the HDAC inhibitor entinostat in 4T1 bearing mice resulted in greater anti-tumor efficacy than immune checkpoint alone, and while the combination did not increase TILs, the addition of entinostat reduced myeloid-derived stem cells and tumor associated Tregs.
A phase II trial investigated the combination of tamoxifen, vorinostat, and pembrolizumab among 34 women with heavily-pretreated HR+, HER2− mBC [51]. While the ORR was only 4% and CBR only 19%, the combination was well-tolerated without excess toxicity beyond what was expected from endocrine therapy and checkpoint inhibitors. Among the five patients with clinical benefit, increased markers of CD8+ T-cell exhaustion and treatment-induced depletion of Tregs correlated with improved response and prolonged PFS. Of note, only two patients had PD-L1 positive tumors, and both were non-responders to therapy, indicating the need to identify alternative biomarkers of response to these therapies. Ultimately, this study was terminated early due to low efficacy in unselected patients.
Radiation
A phase II study evaluated the efficacy of pembrolizumab with palliative radiation therapy to non-visceral metastases in patients with metastatic HR+, HER2− mBC [52]. Patients received pembrolizumab 200 mg 2–7 days prior to 20 Gy of radiation over five fractions. Eight patients were enrolled at the time of interim analysis, but the trial was closed due to futility after no responses. Five of eight patients had progressive disease as best response, and there were no abscopal responses. Paired biopsies were attempted but only collected in two patients, in which there appeared to be an increase in stromal TILs. It was hypothesized that fractionation schedule, radiation to bone lesions as opposed to alternative sites, and the overall treatment-refractory population may have contributed to lack of response.
Novel immunomodulatory therapies in the metastatic setting
As the complex interplay between the immune system, stroma and tumor becomes better understood, multiple co-stimulatory and co-inhibitory receptors have been described with the potential to increase responses to immunotherapy in traditionally immunologically “cold” tumor like HR-positive breast cancers. There are preclinical data supporting the anti-tumoral potential of OX40 and GITR agonist antibodies or LAG3, TIM3, and adenosine receptor antagonism in breast cancer, typically in combination with anti-PD1/PD-L1 blockade.
IMP321 is a recombinant soluble LAG3-Ig protein that was originally developed as a LAG3 antagonist but was later determined to be a potent stimulator of major histocompatibility complex class II. This agent was evaluated with single agent paclitaxel in a phase I/II trial in patients with HER2-negative mBC [53]. Patients received IMP321 subcutaneously at various doses every two weeks on days 2 and 16 of a 28-day cycle for up to 12 injections with weekly paclitaxel 80 mg/m2 on days 1, 8 and 15. Thirty patients were enrolled, most of whom were HR-positive, and the ORR was 50%, which compared favorably to historical control with weekly paclitaxel alone. IMP321 increased the number of monocytes in a dose dependent manner and additionally increased dendritic cells, activated CD8+ cells and natural killer cells. These results prompted the randomized phase IIb AIPAC trial of paclitaxel alone compared to IMP321 plus paclitaxel in 227 patients with HR+, HER2− mBC [54]. PFS and OS was not improved with the addition of IMP321 with a median PFS of 7.29 months in both arms, though the combination did improve OS in patients younger than 65 years of age with a median OS of 21.9 months vs. 14.8 months in with paclitaxel alone (p = 0.012).
Multiple ongoing phase I/II trials are evaluating novel immuno-oncology combinations in advanced refractory solid malignancies including breast cancer. While TNBC remains the focus of many of these trials, investigations with combinatorial immunotherapeutic approaches are ongoing in HR-positive disease.
Combination with chemotherapy in the neoadjuvant setting
In early-stage breast cancer, the benefits of neoadjuvant systemic therapy include less morbid breast surgery [55], the ability to assess tumor response to therapy, and evidence that pathologic complete response (pCR) is associated with improved long-term clinical outcomes [56]. However, higher rates of pCR are observed in patients with HER2-positive and triple-negative cancers, with a pCR rate of less than 20% in HR+, HER2− breast cancer. Combinations of chemotherapy with immunotherapy have been increasingly investigated in the neoadjuvant setting.
I-SPY2 is an ongoing multicenter, multicohort trial developed as a platform to evaluate the efficacy of novel agents in conjunction with neoadjuvant chemotherapy for high-risk operable breast cancers. These novel agents are “graduated” if they meet a predefined, subtype specific efficacy threshold. Arms in I-SPY2 are open for patients with high-risk HR+, HER2− tumors, which are defined as tumors at least 2.5 cm with a high risk MammaPrint.
The addition of pembrolizumab to paclitaxel demonstrated efficacy and was the first novel agent graduated in the I-SPY2 trial [57]. Patients with HER2-negative breast cancer were treated with pembrolizumab 200 mg every three weeks for 4 cycles in combination with weekly paclitaxel (T) 80 mg/m2 followed by standard dose dense doxorubicin plus cyclophosphamide (AC) alone. Sixty-nine patients were randomized to pembrolizumab and 181 were randomized to control. Forty patients randomized to pembrolizumab group had HR+, HER2− breast cancer. In the HR-positive subgroup, the estimated pCR rate was 30% as compared to 13% in the control group. Adverse events were mostly grade 1–2 and consistent with known adverse events of immunotherapy, though notably six patients developed adrenal insufficiency. KEYNOTE-756 [58] and CheckMate 7FL [59] are ongoing phase III trials incorporating immune checkpoint inhibition with standard neoadjuvant chemotherapy with AC-T followed by continued immune checkpoint inhibition with endocrine therapy.
The combination of olaparib, durvalumab and paclitaxel followed by standard dose dense AC was evaluated in an arm of the I-SPY2 trial and graduated after 13 months [60]. Patients were treated with three cycles of durvalumab 1.5 g every 4 weeks along with olaparib 100 mg p.o. BID for weeks 1–11 and weekly T, followed by dose dense AC. Seventy-three patients were enrolled, 52 of whom had HR+, HER2− breast cancer. The estimated pCR rate was 28% and this combination was generally well tolerated. Future investigations with this combination are ongoing.
Another combination under evaluation in the Checkmate 7A8 randomized phase II trial is neoadjuvant palbociclib and anastrozole ± nivolumab [61]. Given the toxicity of a similar combination in the metastatic setting, this study will hopefully provide insight into the tolerability and efficacy of the of anti-PD-1 in combination with CDK4/6 inhibition and endocrine therapy.
Conclusions
While checkpoint inhibition alone or with standard chemotherapy has not shown the same clinical efficacy in patients with HR+, HER2− mBC as has been demonstrated in patients with TNBC, novel combinations with targeted therapies, particularly CDK4/6 inhibitors and PARP inhibitors, show promise. Preclinical data suggest synergy with immune checkpoint inhibition and these targeted agents in patients with HR+, HER2− breast cancer, potentially because of alternative mechanisms of enhancing anti-tumor immune response.
There are several challenges to utilizing immunotherapy in HR+, HER2− breast cancer. It is becoming clear that immune checkpoint inhibition is most efficacious when used in early line settings, and with numerous effective agents in HR+, HER2− mBC, early investigations with immunotherapy have been limited to patients often refractory to multiple agents. Additionally, finding a biomarker predictive of response to immunotherapy remains a challenge. The results of immunotherapy-based clinical trials in the HR-positive population call into question the reliability of PD-L1 expression as a biomarker of response and highlight the need to identify other immune signatures that can predict response and identify candidates for novel treatment strategies. Investigations of novel biomarkers in tumor tissue, liquid biopsies and the tumor microenvironment are ongoing.
In the early-stage setting, the innovative I-SPY2 clinical trial design offers an opportunity for the investigation of novel immunotherapy combinations in the neoadjuvant setting, with the potential to lead to further drug development in the advanced/metastatic setting. However, the risk of long-term toxicities with immunotherapy must be weighed against potential benefit in localized disease, particularly in a patient population with better outcomes when compared to their triple negative counterparts. The emerging safety concerns when combining immunotherapy with endocrine therapy reflects the potential for toxicity with immunotherapy combinations despite well described toxicity profiles as monotherapy. Despite these and challenges further advances in the field of immunotherapy in HR+, HER2− disease can be expected in the coming years.
References
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2(10):1354–1360
Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q (2017) Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 8(19):31347–31354
Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Tourneau CL, van Brummelen EMJ, Varga A et al (2018) Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res 24(12):2804–2811
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M et al (2018) Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat 167(3):671–686
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr et al (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21(10):1353–1365
Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, Lin N, Tolaney SM, Wagle N (2020) Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol 31(3):387–394
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora JE et al (2019) Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381(21):2020–2031
Barroso-Sousa R, Trippa L, Lange P, Andrews C, McArthur HL, Haley BB, Rugo HS, Emens LA, Winer EP, Mittendorf EA et al (2019) Nimbus: a phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1115
Santa-Maria CA, Kato T, Park JH, Kiyotani K, Rademaker A, Shah AN, Gross L, Blanco LZ, Jain S, Flaum L et al (2018) A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget 9(27):18985–18996
Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, Coussy F, Sablin M-P, Debled M, Lefeuvre-Plesse C et al (2021) Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med 27(2):250–255
Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV (2013) Many faces of DAMPs in cancer therapy. Cell Death Dis 4:e631
Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3(5):436–443
Murciano-Goroff YR, Warner AB, Wolchok JD (2020) The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 30(6):507–519
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., Psyrri A, Baste N, Neupane P, Bratland A, et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D (2015) The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 41(6):503–510
Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J (2018) Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 11(1):104
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377(20):1919–1929
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lievre A et al (2021) Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384(13):1191–1203
Shah AN, Flaum L, Helenowski I, Santa-Maria CA, Jain S, Rademaker A, Nelson V, Tsarwhas D, Cristofanilli M, Gradishar W (2020) Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000173
Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, Wulf G, Spring L, Sinclair NF, Andrews C et al (2020) Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol 6(10):1598–1605
Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J, Mariani GL et al (2010) Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 16(13):3485–3494
Hakem R (2008) DNA-damage repair; the good, the bad, and the ugly. EMBO J 27(4):589–605
Ratta R, Guida A, Scotte F, Neuzillet Y, Teillet AB, Lebret T, Beuzeboc P (2020) PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review. Prostate Cancer Prostatic Dis 23(4):549–560
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M et al (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379(8):753–763
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A et al (2017) Olaparib for metastatic breast cancer in patients with a germline brca mutation. N Engl J Med 377(6):523–533
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, Shah PD, Ballinger TJ, Yang ES, Vinayak S et al (2020) TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38(36):4274–4282
Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S et al (2017) DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun 8(1):1751
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH et al (2017) PARP Inhibitor Upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 23(14):3711–3720
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, Xie S, Liu JF, Stover EH et al (2018) PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. https://doi.org/10.1016/j.celrep.2018.11.054
Pantelidou C, Sonzogni O, De Oliveria TM, Mehta AK, Kothari A, Wang D, Visal T, Li MK, Pinto J, Castrillon JA et al (2019) PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov 9(6):722–737
Shen J, Zhao W, Ju Z, Wang L, Peng Y, Labrie M, Yap TA, Mills GB, Peng G (2019) PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res 79(2):311–319
Domchek SM, Postel-Vinay S, Im SA, Park YH, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs MG et al (2020) Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 21(9):1155–1164
Yap TA, Konstantinopoulos P, Telli ML, Saraykar S, Beck JT, Galsky MD, Abraham J, Wise DR, Khasraw M, Rubovszky G et al (2020) Abstract P1–19–03: JAVELIN PARP medley, a phase 1b/2 study of avelumab plus talazoparib: results from advanced breast cancer cohorts. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-P1-19-03
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17
Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im S-A, Petrakova K, Bianchi GV, Esteva FJ, Martín M et al (2019) Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 382(6):514–524
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O et al (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475
Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR et al (2018) CDK4/6 inhibition augments antitumor immunity by enhancing t-cell activation. Cancer Discov 8(2):216–233
Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, Rostorfer R, Guarneri V, Huang CS, Barriga S et al (2020) Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR(+)/HER2(-) breast cancer. Clin Cancer Res 26(3):566–580
Rugo HS, Kabos P, Beck JT, Chisamore MJ, Hossain A, Chen Y, Tolaney SM (2020) A phase Ib study of abemaciclib in combination with pembrolizumab for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) locally advanced or metastatic breast cancer (MBC) (NCT02779751): interim results. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl
Masuda J, Tsurutani J, Masuda N, Futamura M, Matsumoto K, Aogi K, Takahashi M, Iwata H, Iwasa T, Mukohara et al (2020) Phase II study of nivolumab in combination with abemaciclib plus endocrine therapy in patients with hormone receptor-positive human epidermal growth factor receptor-2 negative metastatic breast cancer (WJOG11418B, NEWFLAME trial). Abstract PS12-10. In: Virtual San Antonio Breast Cancer Symposium, Texas, 8-11 December 2020.
Bates SE (2020) Epigenetic therapies for cancer. N Engl J Med 383(7):650–663
Yi X, Wei W, Wang SY, Du ZY, Xu YJ, Yu XD (2008) Histone deacetylase inhibitor SAHA induces ERalpha degradation in breast cancer MCF-7 cells by CHIP-mediated ubiquitin pathway and inhibits survival signaling. Biochem Pharmacol 75(9):1697–1705
Terranova-Barberio M, Pawlowska N, Dhawan M, Moasser M, Chien AJ, Melisko ME, Rugo H, Rahimi R, Deal T, Daud A et al (2020) Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun 11(1):3584
Barroso-Sousa R, Krop IE, Trippa L, Tan-Wasielewski Z, Li T, Osmani W, Andrews C, Dillon D, Richardson ET 3rd, Pastorello RG et al (2020) A phase II study of pembrolizumab in combination with palliative radiotherapy for hormone receptor-positive metastatic breast cancer. Clin Breast Cancer 20(3):238–245
Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C et al (2010) First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med 8:71
Wildiers H, Armstrong A, Cuypere E, Dalenc F, Dirix LY, Chan S (2020) Abstract PD14–08: Primary efficacy results from AIPAC: A double-blinded, placebo controlled, randomized multinational phase IIb trial comparing weekly paclitaxel plus eftilagimod alpha (soluble LAG-3 protein) vs. weekly paclitaxel plus placebo in HR-positive metastatic breast cancer patients. In: Virtual San Antonio Breast Cancer Symposium
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL, Wolmark N et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15(7):2483–2493
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384(9938):164–172
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H et al (2020) Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 6(5):676–684
Cardoso F, Bardia A, Andre F, Cescon DW, McArthur HL, Telli ML, Loi S, Cortes J, Schmid P, Harbeck N et al (2019) KEYNOTE-756: randomized, double-blind, phase 3 study of pembrolizumab vs placebo combined with neoadjuvant chemotherapy and adjuvant endocrine therapy for high-risk, early-stage estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS601
Loi S, McArthur HL, Harbeck N, Pusztai L, Delaloge S, Letrent K, Chen T, Li B, Tatsuoka K, Zardavas D et al (2020) A phase III trial of nivolumab with neoadjuvant chemotherapy and adjuvant endocrine therapy in ER+/HER2- primary breast cancer: checkmate 7FL. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS604
Pusztai LHH et al (2020) Abstract No. CT011: evaluation of durvalumab in combination with olaparib and paclitaxel in high-risk HER2- stage II/III breast cancer: results from the I-SPY 2 TRIAL. AACR. https://doi.org/10.1158/1538-7445.AM2020-CT011
Tolaney SM, Jerusalem G, Salgado R, Liu X, Chen T, Zhang H, Roberts M, Zardavas D, Prat A (2020) A phase II trial of nivolumab (NIVO) + palbociclib (PAL) + anastrozole (ANA) in postmenopausal women and men with estrogen receptor (ER)+/human epidermal growth factor 2 (HER2)- primary breast cancer (BC): checkmate 7A8. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS1105
Author information
Authors and Affiliations
Contributions
All authors (MK, JEM, KK) contributed equally to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MK and JEM have no disclosures to report. KK declares the following potential conflicts of interest: Medical Advisor—Immunomedics, Pfizer, Novartis, Eisai, Eli-Lilly, Amgen, Immunomedics, Merck, Seattle Genetics, and Astra Zeneca; Institutional Support—Immunomedics, Novartis, Incyte, Genentech/Roche, Eli-Lilly, Pfizer, Calithera Biosciences, Acetylon, Seattle Genetics, Amgen, Zentalis Pharmaceuticals, and CytomX Therapeutics; Speakers Bureau—Eli-Lilly; Spouse—Array Biopharma, Pfizer, Grail.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kearney, M.R., McGuinness, J.E. & Kalinsky, K. Clinical trial data and emerging immunotherapeutic strategies: hormone receptor-positive, HER2− negative breast cancer. Breast Cancer Res Treat 189, 1–13 (2021). https://doi.org/10.1007/s10549-021-06291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06291-8