Abstract
Purpose
Extending adjuvant endocrine therapy (ET) beyond 5 years has been shown to improve outcomes in breast cancer; however, limited data are available about if and why women pursue extended ET. The primary objective was to estimate the proportion of women who were willing to receive extended ET if recommended by their physician and secondarily, to determine what factors were associated with this decision.
Methods
This descriptive cross-sectional study surveyed 131 women with AJCC 7th Edition stages I–III breast cancer who had been taking adjuvant ET for 3–5 years. The survey inquired about the willingness to continue ET, quality of life (FACT-ES), and beliefs about medications (BMQ). Logistic regression was used to test for associations between clinical and disease factors, FACT-ES, BMQ, and the primary outcome.
Results
One hundred and twelve (85%) patients reported “moderate” (n = 30, 23%), “quite a bit” (n = 41, 31%), or “extreme” (n = 41, 31%) willingness to pursue extended ET; 19 (14%) patients were “not at all” or were “unlikely” to be willing to take extended ET. On univariate analysis, lower total and social well-being FACT-ES scores, and lower perceived necessity and higher concerns on BMQ were associated with lower willingness to pursue extended ET. On multivariable analysis, greater patient perception of necessity of ET was the only factor associated with willingness to pursue extended ET (OR 1.34, 95% CI 1.15–1.57, p = 0.0005).
Conclusions
Most women who have taken ET for multiple years report being willing to pursue extended ET if recommended. When discussing extended ET, the data from this study support exploring patients’ belief of medication necessity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nearly 250,000 women will be diagnosed with breast cancer in the United States each year, with hormone receptor (HR)-positive disease accounting for approximately 60–70% of cases [1]. There have been major advances in the treatment of HR-positive breast cancer, largely through the appropriate use of adjuvant chemotherapy and endocrine therapies (tamoxifen or an aromatase inhibitor, AI). At least 5 years of adjuvant endocrine therapy (ET) for HR-positive breast cancer in both pre- and post-menopausal women remains the standard of care [2,3,4].
Unique to HR-positive breast cancer, the risk of recurrence can persist many years after initial therapy. A meta-analysis of over 62,000 women followed 5–20 years after diagnosis confirmed that even small node negative, HR-positive tumors have a risk of recurrence beyond 5 years of 10–17% over 15 years (0.5–1% annual risk) and more advanced cancers have annual risks as high as 1.5–2.5% [5].
Multiple large randomized trials have observed improved disease-free and overall survival rates by extending tamoxifen therapy for 10 years [6, 7] or from the addition of an AI following tamoxifen [8, 9]. However, to date these trials have shown a relatively small absolute benefit in reducing distant recurrence rates (2–5%) or breast cancer mortality (0–3%) across trials.
Importantly, up to half of treated patients prematurely discontinue adjuvant ET before 5 years, primarily due to toxicity such as menopausal symptoms and arthralgias [10,11,12]. Although much less common, more serious adverse effects such as thromboembolic disease and endometrial cancer with tamoxifen and osteoporosis and fractures with AIs can occur and the cumulative risk of these effects appears to increase with extended therapy [7, 13, 14].
It is unknown what proportion of women will decline or accept extended ET and for what reasons. Risk of recurrence is likely to be only one factor considered by many women. For some women, their experience with symptoms, their attribution to ET, and the perceived impact of these symptoms on their quality of life (QOL) might influence their choice to continue treatment. For others, beliefs about medications or disease may have an impact on the decision to pursue extended therapy. It is necessary to examine factors that may influence the decision-making process to assist providers and patients when discussing extended ET.
Methods
Study design
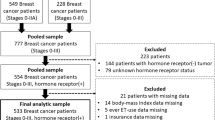
This was a single-institution cross-sectional survey to estimate the proportion of women willing to take extended ET and evaluate predictors of this acceptance. From July 2015 to May 2016, eligible outpatients attending the breast cancer clinics at the University of Michigan Rogel Cancer Center were recruited in person. Additionally, to enhance enrollment, patients in the University of Michigan Cancer Registry who met eligibility criteria but who did not have scheduled outpatient breast medical oncology clinic follow-up within 6 months of study initiation were identified and contacted by mail. To be eligible, women had to be able to read and speak English, be at least 18 years of age, and had to have initiated initial adjuvant ET with tamoxifen, letrozole, exemestane, or anastrozole for stage I–III HR-positive breast cancer at least 3 years before the date of enrollment, and report taking ET at their most recent office visit. Patients who had already made a definitive decision regarding the use of extended adjuvant ET according to the most recent office note or at the office visit during which they were approached about participation were excluded. The Institutional Review Board of the University of Michigan approved all study procedures.
Patients were recruited by mail using the modified Dillman method or at clinic visits [15]. Those recruited in conjunction with clinic visits provided written informed consent and then completed the survey immediately following the clinic visit or returned the survey by mail within 30 days of the clinic visit. Those who were recruited by mail were sent an introductory letter, informed consent document, and the survey. If they were interested in participating, they were asked to return the completed survey; informed consent was implied for those who returned the survey. Patients who did not return the survey within 3 weeks were sent a postcard asking them to participate. If the survey was not received within an additional 6 weeks, the information (letter, informed consent, and survey) was sent one additional time, to increase participation rates. If there was still no response, no further contact was made with the potential participant. All patients who provided informed consent in the clinic or who were contacted by mail about study participation were provided with a $2 bill [16].
Patients were informed that their responses would be kept confidential from their medical care providers. To reduce the effect of observer bias, all patients completed the survey independently; research assistants were available for questions if needed.
Measures
Data including sociodemographic information (age, menopausal status, partnered status, educational attainment, employment status, income) and breast cancer and treatment characteristics (AJCC 7th Edition TNM stage, HER2 status, tumor grade, prior local and systemic treatments including contralateral prophylactic mastectomy, and 21 gene recurrence score [RS] [17]) were collected from patients and/or the electronic medical record. The survey included investigator-developed sections (Online Appendix 1) as well as established psychometric tools and consisted of 107 items. Prior to enrollment of patients, semi-structured interviews and assessment of candidate items in the final survey were pretested during two focus groups (approximately 15 participants each) with the intent to improve readability and content and to confirm appropriate themes. The focus groups were audio recorded and analyzed by two different researchers (KCK and DLB). The content of the survey was adjusted accordingly.
The first question of the written survey was “If in meeting with your doctor, he or she recommends continuing hormonal therapy for longer than 5 years (up to 10 years), how willing would you be to continue taking your current hormonal therapy?” (Online Appendix 1). Responses were on a 5-point Likert-scale ranging from “Not at all” to “Extremely.”
To assess general beliefs about medications, the Beliefs about Medicines Questionnaire Specific (BMQ-S) was used [18,19,20,21]. The BMQ-S compromises two five-item scales assessing patients’ beliefs about the necessity of a prescribed medication and their concerns about the potential adverse consequences of taking it. The BMQ has been tested in a wide variety of patient populations and is a valid and reliable measure of medication beliefs. The BMQ-S uses a 1 to 5 Likert-scale from “strongly disagree” to “strongly agree.” Scores obtained for the individual items within each scale are summed to give a total score for the each of the necessity (α = 0.87) and concerns (α = 0.78) scales, ranging from 5 to 25 [20]. A higher score on BMQ-S-necessity indicates stronger beliefs about the necessity of treatment, and a higher score on BMQ-S-concerns indicates stronger concerns about treatment. A necessity-concerns differential score is calculated by subtracting the specific-concerns scale from the specific-necessity scale (range − 20 to 20) [18, 20, 21]. A positive differential score indicates stronger necessity beliefs than concerns, and a negative score indicates the opposite.
Each patient’s risk perception was assessed with the question, “Over the next 5 years, how likely do you think your breast cancer will come back?” and asked how much lower she thought it would be if she took an additional 5 years of ET. These questions used an ordinal likelihood measure with differing quantities of absolute percent risk (0–1%, 2–5%, 6–10%, 11–25%, 26–50%, 51–100%). The ordinal measure was chosen based on prior risk perception work that suggested that a percent likelihood measure, such as a visual analogue scale from 0 to 100%, has a higher degree of variance compared to ordinal or comparative risk likelihood measures [22]. The follow-up questions assessed each patient’s likelihood of continuing hormonal therapy if the expected degree of risk reduction was 1%, 5%, or 20%. In addition, we adapted questions from the Rakovitch Risk Perception (RRP) scale, a 9-item patient self-reporting scale that measures risk perception and risk aversive traits [23]. The risk perception component of the RRP scale includes four questions regarding risk perception, including developing local recurrence, developing distant recurrence, dying of breast cancer, and dying of something other than breast cancer. The risk aversive component of the RRP scale includes five questions unrelated to breast cancer, such as “I worry that I may have a stroke in the future.” All RRP scale items are scored on a 1 to 5 Likert-scale where larger numbers represent higher perception of risk perception and risk aversion.
Survivor concerns were analyzed using the Assessment of Cancer Survivors Concerns (ACS), a 5-item patient self-rating scale that measures fears about recurrence and health in cancer survivors [24]. The ACS uses a 1 to 4 rating from “not at all” to “very much.” Construct validity was examined in multiple group confirmatory factor analysis on short-term and long-term cancer survivors and showed internal consistency and validity. Assessment of mood was conducted via the Hospital Anxiety and Depression Scale (HADS) and QOL and symptom burden with the Functional Assessment of Cancer Therapy-Endocrine Subscale (FACT-ES) [25, 26]. Both the HADS and FACT-ES have been validated with good internal consistency and criterion validity in patients with breast cancer. The HADS is a 14-item patient self-assessment scale that can detect states of depression and anxiety. HADS uses a scale of 0–3, with 3 indicating higher symptom frequencies and each subscale (anxiety and depression) can range from 0 to 21. For the FACT-ES, a 56-item tool, the total score and the subscales (Physical, Social, Emotional, and Functional Well-Being; General; and Endocrine Symptom Scale) were analyzed. Higher values on the FACT-ES total and subscales reflect better functioning and fewer symptoms. It is a validated tool in women with breast cancer receiving endocrine treatments with good internal consistency (range α = 0.65–0.87) and test–retest reliability (r = 0.93).
Statistical methods
Sociodemographic information, clinical characteristics, and survey responses were summarized using descriptive statistics.
The primary objective of this cross-sectional study was to estimate the proportion of women who were not willing to receive extended ET, as assessed using the first question in the survey. The proportion of women and 95% exact binomial confidence interval (CI) for each of the 5 responses were calculated.
The sample size was based on the precision of the estimate for the primary aim. With 130 women, we expected to reasonably estimate the proportion of women unwilling to extend ET (i.e., the largest expected exact binomial 95% confidence interval length being 16.4% if as many as 30% were unwilling to extend ET).
The exploratory analyses examined whether patient-related factors (patient-reported ET adherence, beliefs about medications, concerns about cancer recurrence, risk perception, and psychosocial and physical symptom burden) were associated with willingness to accept or decline extended adjuvant ET. Validated questionnaires were scored according to previous published guidelines. Non-validated questionnaires were explored by question (e.g., the individual questions about risk perception). Other independent variables included in the analysis were age, marital status (married or in a committed relationship vs. other), education (at least some college vs. high school or less), employment (employed vs. unemployed), annual household income (> $60,000 vs. < $60,000), race (white vs. other), type of breast surgery (lumpectomy vs. mastectomy), contralateral prophylactic mastectomy (yes vs. no), prior treatment with chemotherapy (yes vs. no), and type of endocrine treatment (AI vs. tamoxifen). The exploratory objectives were examined univariably using Kruskal–Wallis or t-tests (depending on normality) and multivariably using multiple logistic regression with the binary outcome of willing (“moderately” to “extremely” on question 1) versus not willing (“not at all” to “unlikely”). Scientific hypotheses of confounding and univariable analysis were used to arrive at a final model.
Results
Patient sample
Of 253 eligible patients, 131 patients completed the survey including the first question, which was essential for evaluating the primary endpoint. The two patients who completed part of the survey but who did not complete the first question were excluded from the analysis. Among the analyzed patients, 32 (24%) patients were enrolled in the clinic and 99 (76%) were recruited via mail. As shown in Table 1, the mean age was 60, and 118 (90%) were non-Hispanic white. Ninety-one (69%) had Stage I, 35 (27%) had Stage II, and 5 (4%) had Stage III disease. Of the 85 patients who had the 21-gene RS assay performed, mean RS was 16.95 (standard deviation, SD 8.43); 45 (53%) had scores in the low range, 36 (42%) in the intermediate range, and 4 (5%) in the high range. Chemotherapy was administered to 53 (40%) of patients, and 92 (70%) had received radiation therapy. The cohort was evenly divided between current receipt of tamoxifen and an AI. Mean and median PRO scores for the entire cohort are noted in Supplementary Table 1.
Willingness to pursue extended endocrine therapy
Most women (112/131) reported being “moderately” to “extremely” willing to pursue extended ET (“Moderately” to “Extremely”: 85%, 95%, CI 78–91%) (Fig. 1). Specifically, 30 (23%) patients reported “moderate,” 41 (31%) “quite a bit,” and 41 (31%) “extreme” willingness to pursue extended ET. Seven (5%) stated they were “not at all” willing and 12 (9%) were “unlikely” (“Not at all” to “Unlikely”: 14%, 95% CI 9–22%). There was no difference in willingness to pursue extended adjuvant ET between those enrolled in conjunction with a clinic visit and those recruited via mail (p = 0.16).
Willingness to pursue extended endocrine therapy. Proportion of patients with each response to question 1, “If in meeting with your doctor, he or she recommends continuing hormonal therapy for longer than 5 years (up to 10 years), how willing would you be to continue taking your current hormonal therapy?”
Patient thresholds for extended endocrine therapy and recurrence risk perception
Participants were asked about their willingness to take extended ET for a variety of absolute reductions in risk of recurrence, ranging from 1 to 20%. As shown in Fig. 2, 52% of patients were “moderately” to “extremely” likely to pursue extended ET for a 1% absolute reduction in risk of recurrence. In contrast for a 20% absolute reduction in risk, 89% of patients were at least moderately willing to take extended ET.
Patient thresholds for considering extended ET. Proportion of patients with each response to, “If you take an extra 5 years of hormonal therapy and it lowers your risk of cancer coming back by X%, how likely would you be to continue your hormonal therapy?” In the 3 questions, X was 1%, 5%, and 20%. Responses were grouped into “not at all” + “unlikely” and “moderately” + “quite a bit” + “extremely”
Patients were also asked about their perceived risk of recurrence. As shown in Table 2, the perceived risk of recurrence was not consistent with the pathologic stage for some patients. There was no statistically significant association between patients’ perceived risk of recurrence and their 21-gene RS (p = 0.37).
Association between clinical and patient-reported factors and willingness to pursue extended ET
Univariate analysis
Univariate analyses were performed to explore associations between clinical and patient-reported factors and willingness to take extended ET (“not at all”–“unlikely” vs. “moderately”–“extremely” likely) (Table 3). There was a trend toward a greater likelihood of being less willing to take extended ET and prior receipt of chemotherapy (63% vs. 37%, p = 0.05). There was no statistically significant difference between the two groups for any other clinicopathologic factor, including stage of disease, tumor size, tumor grade, type of surgery, receipt of radiation therapy, class of endocrine therapy prescribed, menopausal status, partnered status, education, employment, income, or race, although power was limited for these analyses.
A higher necessity belief was associated with more willingness to take extended ET (median BMQ-S Necessity Score, 7 vs. 9; p = 0.004). Conversely, a higher medication concern score was associated with less willingness to take extended ET (median BMQ-S Concerns Score, 9.5 vs. 6, p = 0.0007). Higher perception of risk of disease recurrence based on the question “Over the next 5 years, how likely do you think your breast cancer will come back?” was not associated with greater willingness to pursue extended ET (p = 0.5). Similarly, there was no statistically significant difference in willingness to take extended ET when risk was assessed using either the Rakovitch Risk Perception scale or the Assessment of Cancer Survivors scale (Supplementary Table 2).
Poorer QOL was associated with less willingness to pursue extended ET (median FACT-ES Total Score, 145.4 vs. 151.5, p = 0.045). Of the individual components of the FACT-ES, patients with lower scores on the social well-being component were less willing to pursue extended ET (median 19 vs. 24, p = 0.013). There were no statistically significant associations identified between the other components of the FACT-ES including the endocrine subscale, or between the anxiety or depression subscales of the HADS, and willingness to take extended ET (Supplementary Table 2).
Multivariate analysis
In the multivariable model, higher necessity belief in taking the medication was the only independent factor associated with willingness to take extended ET (BMQ-S Necessity Score, point estimate 1.32, 95% Wald Confidence Limit 1.09–1.59; p = 0.005) (Table 4). There was a trend toward both an increase in risk perception and an increase in quality of life and an increased willingness to take extended ET. Age, lymph node involvement, and stage of disease were not associated with willingness to take extended ET.
Discussion
In this cross-sectional study, we used both perceived risk of recurrence and a series of hypothetical scenarios to examine the associations between standard clinicopathologic risk factors and patient-reported symptoms and beliefs and willingness to take extended ET. Most women were “moderately” -to- “extremely” likely to pursue extended ET if recommended to do so by their physician. Notably, no clinicopathologic factor was associated with this decision. The only independent factor that was associated with willingness to take recommended extended ET was a belief that ET was necessary for risk reduction.
In addition, patients were asked about the threshold of benefit that would be required for them to consider taking extended ET. In this scenario, about half of patients were willing to take treatment for a 1% absolute benefit, and almost three-quarters were willing to consider treatment for a 5% benefit. Recent data from randomized clinical trials have demonstrated a benefit from extended ET for patients with HR-positive breast cancer, although the absolute benefits for most women have been relatively small. As providers discuss the merits of extended ET with patients, it will also be important to understand more about the patient perspective regarding extended treatment to maximize persistence with therapy for those most likely to benefit.
Based on the findings reported here, consideration of standard clinicopathologic characteristics alone may not be sufficiently persuasive for patients at high risk to agree to take extended ET. If results from newer predictive factors such as multiparameter genomic assays, circulating tumor cells, or other liquid biopsies are shown to be helpful in identifying those women most likely to benefit from extended adjuvant ET, additional research will be required to examine patient acceptance of these results and their impact on uptake of extended ET. In addition, more qualitative research is required to understand more about the necessity beliefs underlying patient decision making in this arena.
As has previously been reported, we identified substantial discordance between actual and perceived risk of disease recurrence. Of those patients with Stage I and Stage II disease, 49% and 35%, respectively, reported that they believed their risk of disease recurrence in the next 5 years was less than 1%, representing underestimation of risk. In contrast, 14% of patients with Stage I and 21% of patients with Stage II disease perceived that their risk of disease recurrence in 5 years was more than 11%, which likely represents overestimation of risk for the majority of those patients [5]. Therefore, when discussing extended ET with patients, providers cannot assume that patients have an accurate understanding of their risks of recurrence. Additional research to improve the effectiveness of patient-provider communication, including decision aids, about risk of recurrence and use of extended ET may also be beneficial [27, 28].
There are several limitations to this study, including that it is a relatively small, generally low-risk, well-educated cohort derived from a single academic institution. In addition, the sample was biased toward those more likely to tolerate ET, since eligible patients had to have reported taking ET for at least 3 years. This bias may have led to the lack of an identifiable association between patient-reported endocrine symptoms and willingness to take extended ET. Because 85% of patients were at least moderately willing to consider taking extended ET when recommended by their physician, this limited the statistical power to identify factors associated with unwillingness to continue therapy. Finally, although we attempted to evaluate patients before they had a comprehensive discussion about the risks and benefits of extended adjuvant ET, some patients may have already received information or guidance from their oncology providers or from other sources, such as their peers or the Internet.
For patients with HR-positive disease, there is a substantial risk of late disease recurrence [5]. However, data available to date do not define which patients are likely to benefit the most from extended ET. Given the high number needed to treat, this can lead to substantial overtreatment and result in unnecessary toxicity and negative impacts on quality of life. Greater availability of tools that provide physicians with more individualized data for patients, and a better understanding of patient perception of disease risk and factors that influence treatment decision making, are both necessary to optimize the use of extended ET.
References
DeSantis CE, Ma J, Goding Sauer A et al (2017) Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 67:439–448. https://doi.org/10.3322/caac.21412
Burstein HJ, Temin S, Anderson H et al (2014) Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J Clin Oncol 32:2255–2269. https://doi.org/10.1200/JCO.2013.54.2258
Burstein HJ, Lacchetti C, Anderson H et al (2016) Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol 34:1689–1701. https://doi.org/10.1200/JCO.2015.65.9573
Gradishar WJ, Anderson BO, Balassanian R et al (2016) Invasive breast cancer Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 14:324–354
Pan H, Gray R, Braybrooke J et al (2017) 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377:1836–1846. https://doi.org/10.1056/NEJMoa1701830
Gray R, Rea D, Handley K et al (2013) aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer. J Clin Oncol 31(18 Suppl):5. https://doi.org/10.1200/jco.2013.31.18_suppl.5
Davies C, Pan H, Godwin J et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816. https://doi.org/10.1016/S0140-6736(12)61963-1
Mamounas EP, Jeong J-H, Wickerham DL et al (2008) Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 Trial. J Clin Oncol 26:1965–1971. https://doi.org/10.1200/JCO.2007.14.0228
Jin H, Tu D, Zhao N et al (2012) Longer-term outcomes of letrozole versus placebo after 5 years of tamoxifen in the NCIC CTG MA.17 trial: analyses adjusting for treatment crossover. J Clin Oncol 30:718–721. https://doi.org/10.1200/JCO.2010.34.4010
Henry NL, Azzouz F, Desta Z et al (2012) Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol 30:936–942. https://doi.org/10.1200/JCO.2011.38.0261
Aiello Bowles EJ, Boudreau DM, Chubak J et al (2012) Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract 8:e149–e157. https://doi.org/10.1200/JOP.2012.000543
Crew KD, Greenlee H, Capodice J et al (2007) Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol 25:3877–3883. https://doi.org/10.1200/JCO.2007.10.7573
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Dowsett M, Forbes JF et al (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 386:1341–1352. https://doi.org/10.1016/S0140-6736(15)61074-1
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784. https://doi.org/10.1016/S0140-6736(11)60993-8
Dillman DA (1978) Mail and telephone surveys. Wiley, New York
Rookey BD, Le L, Littlejohn M, Dillman DA (2012) Understanding the resilience of mail-back survey methods: An analysis of 20 years of change in response rates to national park surveys. Soc Sci Res 41:1404–1414. https://doi.org/10.1016/j.ssresearch.2012.06.004
Sparano JA, Gray RJ, Makower DF et al (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373:2005–2014. https://doi.org/10.1056/NEJMoa1510764
Horne R, Weinman J, Hankins M (1999) The beliefs about medicines questionnaire: The development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 14:1–24. https://doi.org/10.1080/08870449908407311
Corter AL, Findlay M, Broom R et al (2013) Beliefs about medicine and illness are associated with fear of cancer recurrence in women taking adjuvant endocrine therapy for breast cancer. Br J Health Psychol 18:168–181. https://doi.org/10.1111/bjhp.12003
Emilsson M, Berndtsson I, Lötvall J et al (2011) The influence of personality traits and beliefs about medicines on adherence to asthma treatment. Prim Care Respir J 20:141–147. https://doi.org/10.4104/pcrj.2011.00005
Horne R, Weinman J (1999) Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 47:555–567
McQueen A, Swank PR, Bastian LA, Vernon SW (2008) Predictors of perceived susceptibility of breast cancer and changes over time: a mixed modeling approach. Heal Psychol 27:68–77. https://doi.org/10.1037/0278-6133.27.1.68
Rakovitch E, Franssen E, Kim J et al (2003) A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat 77:285–293
Gotay CC, Pagano IS (2007) Assessment of Survivor Concerns (ASC): a newly proposed brief questionnaire. Health Qual Life Outcomes 5:15. https://doi.org/10.1186/1477-7525-5-15
Stafford L, Judd F, Gibson P et al (2014) Comparison of the hospital anxiety and depression scale and the center for epidemiological Studies Depression Scale for detecting depression in women with breast or gynecologic cancer. Gen Hosp Psychiatry 36:74–80. https://doi.org/10.1016/j.genhosppsych.2013.08.010
Fallowfield LJ, Leaity SK, Howell A et al (1999) Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat 55:189–199
Collins ED, Moore CP, Clay KF et al (2009) Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol 27:519–525. https://doi.org/10.1200/JCO.2008.16.6215
Zdenkowski N, Butow P, Tesson S, Boyle F (2016) A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast 26:31–45. https://doi.org/10.1016/j.breast.2015.12.007
Funding
This study was funded by NLH who was a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (Grant Number CI-53-10) and by an American Cancer Society Research Scholar Grant (124654-RSG-13-240-01-PCSM).
Author information
Authors and Affiliations
Contributions
KCK: Conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing—original draft, review, and editing. KMK: Data curation, formal analysis, methodology, software, and writing—review and editing. DLB: Conceptualization, supervision, and writing—review and editing. JG: Conceptualization, supervision, and writing—review and editing. AFS: Writing—review and editing. DFH: Writing—review and editing. NLH: Conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, writing—original draft, review, and editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflicts of interest specific to the content of the submitted manuscript. NLH and DFH have conflicts of interest not related to the current manuscript which are documented on the signed Conflict of Interest Disclosure Forms submitted with the manuscript. All authors report no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kadakia, K.C., Kidwell, K.M., Barton, D.L. et al. Factors influencing the use of extended adjuvant endocrine therapy. Breast Cancer Res Treat 175, 181–189 (2019). https://doi.org/10.1007/s10549-019-05145-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05145-8