Abstract
Background
Since concurrent malignancy may be associated with radial scars (RS) in up to 45% of RS diagnosed on core biopsy, surgical excision is usually advised. Recent very low upgrade rates have caused a re-evaluation of the need for routine surgery. We aimed to find subsets of RS at such low risk of upgrade, as to render imaging surveillance a plausible alternative to surgery.
Design
We performed a systematic review of the Pubmed, Cochrane and Embase databases, focusing on the following eligibility criteria: full papers, published after 1998, in English, included at least 5 RS, provided information on needle biopsy gauge and upgrade rates based on the excised lesion. For the meta-analysis, studies were grouped by the presence of histologic atypia and the core needle gauge. Study-specific and pooled upgrade rates were calculated for each subgroup.
Results
49 studies that included 3163 RS with surgical outcomes are included. There were 217 upgrades to malignancies, 71 (32.7%) invasive and 144 (66.4%) DCIS. The random-effects pooled estimate was 7% (95% CI 5, 9%). Among the pre-planned subgroups, in RS assessed by 14G NCB the upgrade rates were: without atypia − 5% (95% CI 3, 8%), mixed or presence of atypia not specified − 15% (95% CI 10, 20%), with atypia − 29% (95% CI 20, 38%). For RS assessed by a mix of 8-16G cores the respective upgrade rates were 2% (95% CI 1, 4%), 12% (95% CI 6, 18%) and 11% (95% CI 3, 23%) and for RS assessed by 8–11 vacuum assisted biopsies 1% (95% CI 0, 4%), 5% (95% CI 0, 11%) and 18% for the one study of RS with atypia assessed by VAB. Surgery after VAB excision showed no upgrades. The difference across all subgroups was statistically significant.
Conclusion
When stratified by atypia and biopsy gauge, upgrade rates in RS are consistent and predictable. RS assessed by VABs and lacking atypia have a 1% (95% CI 0, 4%) upgrade rate to DCIS. Other groups have upgrade rates of 2–28%. This risk may be reduced by VAB excision. The results of this meta-analysis provide a decision aid and evidence-based selection criteria for surgery after a needle biopsy diagnosis of RS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the turn of this century, NCBs have become the first line diagnostic modality for the histologic evaluation of breast lesions found on imaging. Their simplicity and close correlation with the final histology have reduced substantially the reliance on surgical biopsies. However, for several specific subsets of breast lesions, such as radial scars, core biopsies may not be fully representative of the entire process, such that immediate surgical excision discovers unsuspected concurrent invasive cancer or DCIS in over 40% of the cases. Atypical ductal hyperplasia, lobular carcinoma in situ, atypical lobular hyperplasia, papillary lesions, radial scars, cellular fibroepithelial lesions and flat epithelial atypia are among the diagnoses which when made on a needle core biopsy (NCB) have been associated with significant upgrade rates, leading to the standard recommendation for diagnostic surgical biopsy. The upgrade rates vary among these lesions, ranging from < 10% for radial scars and fibroepithelial lesions to > 40% for ADH. Although well intentioned and justified, the fact remains that for most women such surgery finds no malignancy. Attempts at finding subsets of women whose likelihood of an upgrade is sufficiently low as to forego surgical biopsy have had variable success and are ongoing.
Radial scars (RS) are benign, mostly asymptomatic breast lesions, common in well women. Because their spiculated outline on mammograms simulate invasive cancer, they are frequently evaluated after mammographic screening, where their prevalence is 5–6 per 10,000 mammograms [3, 22]. RS are diagnosed in 1–3.7% of core biopsies [18, 20] and account for 10% of all lesions undergoing diagnostic open biopsy [2, 11]. The detection of RS is expected to increase with the greater use of digital breast tomosynthesis, due to its improved visualization of architectural distortions [9, 24].
In a recent analysis of contemporary indications for open biopsy after screening [11], we found that while RS has a relatively high prevalence of 10%, its upgrade rate was only 12.2%, implying that the identification of effective risk stratification strategies for RS has the potential to have a significant impact in reducing benign open biopsies.
Even contemporary reports of upgrade rates for RS have varied substantially, ranging from 0 to 28% [27]. This, combined with the lack of reliable predictive imaging characteristics of focal malignant change in RS, [15] has led to variation in practice, most centres recommending conservative surgical excision of RS diagnosed on NCB, while some now advocate observation [8,9,10].
Aims
We wished to identify subsets of women with a NCB diagnosis of RS who may safely avoid surgery. We hypothesised that much of the variance in the published estimates for upgrades of RS would be resolved if outcomes were stratified by the presence of atypia and the needle gauge of the core biopsy, as a surrogate for the extent of NCB sampling.
Materials and methods
Literature search and eligibility criteria
Using search terms “radial scar” or “complex sclerosing process” AND “breast” a search of the PUBMED, Embase and Cochrane databases was performed in September 2017 and repeated in October 2018 to identify primary studies that met pre-defined eligibility criteria, as follows: full papers, published after 1998, in English, included at least 5 RS diagnosed on NCB, provided information on biopsy gauge and upgrade rates of the excised lesion.
Study endpoints
The primary endpoint for this meta-analysis was the diagnosis based on pathologic evaluation of the excised lesion. Only invasive carcinoma or DCIS were included in the upgrade rate. As the principal decision being faced after a NCB diagnosis of RS is whether the area should be excised or not, we were only interested in conditions managed surgically. As such, lobular carcinoma in situ, atypical ductal hyperplasia or flat epithelial atypia were not included in the calculation of upgrade rates.
Extracted data
Study-specific descriptive information (clinical context, year of publication, country of origin) and quantitative data were extracted. This included the total number of RS diagnosed by NCB, the number of RS undergoing surgical excision, the needle gauge used for NCB and number of cores retrieved. For the subset of excised RS, the number showing histologic atypia on the NCB and the surgical pathology results, classified as the number of invasive cancers, DCIS or non-malignant findings were recorded. The presence of atypia, or lack thereof, was drawn directly from each study. If the study did not specify the presence of atypia or grouped all RS, the grouping “presence of atypia not specified or mixed results” was used. Information regarding needle gauge was drawn directly from each series. For studies that did not specify this information, the range of needle gauges used was used. When the same study presented data for RS assessed by various needle gauges or with varying histologic findings on NCB, the data were tabulated separately for each subset.
To address the heterogeneity in study designs, the findings were grouped into the following categories: (1a) RS assessed by 14G NCB, without atypia, (1b) RS assessed by 14G NCB, presence of atypia not specified or mixed results, (1c) RS assessed by 14G NCB, with atypia, (2a) RS assessed by a mix of 8-16G NCB, without atypia, (2b) RS assessed by a mix of 8-16G NCB, presence of atypia not specified or mixed results (2c) RS assessed by a mix of 8-16G NCB, with atypia, (3a) RS assessed by VAB 8-11G biopsies, without atypia, (3b) RS assessed by VAB 8-11G biopsies, presence of atypia not specified or mixed results, (3c) RS assessed by VAB 8-11G biopsies, with atypia, (4) RS undergoing surgery after VAB excision and (5) RS assessed by MRI guided 9-G VA biopsies.
We also evaluated series dealing with three special case scenarios, too few for meta-analysis, but nevertheless of practical, clinical interest. These included (i) RS ≤ 5 mm, without atypia or a papillary component, (ii) “Microscopic RS” defined as RS as an incidental histologic finding during evaluation of another target lesion with concordant imaging and (iii) “Mammographically occult RS” assessed by ultrasound guided 14-G NCB.
Statistical methods
Random-effects meta-analysis of proportions used the Der Simonian and Laird method to pool prevalence of upgrade to invasive carcinoma or DCIS following Freeman-Tukey Double arcsine transformation. Study upgrade rates were calculated using the number of lesions with malignant surgical outcomes and the number of women who underwent surgical biopsy as the denominator. The meta-analysis was stratified according to the subgroups described above and tests and measures of heterogeneity within and across subgroups were calculated. All summary measures (subgroup and overall) were reported with 95% exact confidence intervals.
We used Stata version 14.2 (StataCorp 2009; College Station, TX) for meta-analysis and the user written module metaprop.
Results
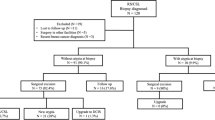
Our search strategy identified 271 citations; reduced to 88 by screening of titles. Review of abstracts and the full paper identified 51 papers as meeting eligibility criteria. These included 8 studies based solely on organised population-based screening programs. Combined, the eligible studies presented data for 3163 RS excised after NCB. The QUOROM diagram and Prisma checklist for this review are available in the appendix as supplementary information.
Overall, concurrent malignancy was documented in 217 cases (raw average 6.9%), of which 71 (32.7%) were invasive cancers and 144 (66.4%) DCIS (Table 1). The reported upgrade rates spanned a range of 0–45.4%.
Considering the entire cohort, as displayed in Fig. 1, there was evidence of significant heterogeneity between the groups (p = 0.000). This confirms there is likely to be significant variability between the studies overall, rendering a pooled analysis inappropriate. Study design and patient numbers varied among the studies. Study-specific data, upgrade rates, and estimated pooled upgrade rates, are displayed in Fig. 1.
Overall meta-analysis results of radial scars excised after core biopsy. ES effect size. This is the malignant upgrade rate, expressed as a proportion. % weight = random effects weights applied to each study in the overall meta-analysis. The studies are grouped by the gauge of the core biopsy needle used (14G, mix of 8-16G and vacuum assisted biopsies 8-11G). Within each group, studies are stratified as follows: no atypia, presence of atypia not specified or mixed results and atypical. Two further groups are also included: RS proceeding to surgical exsion after a VAB excision and RS evaluated under MRI guidance by 9G VAB. All subgroups are listed in chronological order of year of publication. The diamonds at the end of each section represent the pooled estimates of upgrade for the subgroup of radial scars according to the meta-analysis with 95% confidence interval also being presented. While the overall pooled estimate of upgrade for all studies combined is 7%, (95% confidence interval 6, 9%), grouping by needle gauge and presence of atypia stratifies the cases into subsets with significantly different upgrade rates. A measure of heterogeneity (I^2) is provided by subgroup where there are > 3 studies, and overall
Within each subgroup and sorted in chronological order, a trend appeared towards lower upgrade rates in more recent series, possibly reflecting more comprehensive sampling over time.
Table 2 presents the raw numbers of cases used in this analysis, stratified by the planned subtypes. The number of series contributing to each sub-group are: 24 for RS assessed by 14G NCB, 33 for RS assessed 8-16G biopsies, 10 for RS assessed by 8-11G VABs, 3 for RS undergoing surgery after prior VAB excision and 2 for RS assessed by MRI guided VABs. Several series presented data for more than one pre-planned category.
Of the special subtypes, one study of each of the following groups was found: RS ≤ 5 mm without atypia [19]; mammographically occult, asymptomatic RS, found only on ultrasound without atypia [23] and microscopic or incidental RS [16], together representing an additional 103 RS.
A grand total of 3266 excised RS is therefore included in this evaluation.
Modelled estimates
Table 3 and Fig. 2 summarise the surgical outcomes in women with RS stratified by needle gauge and the presence of atypia. For women with RS assessed by 14G NCB, the meta-analysis includes 1143 RS presented in 24 studies. The pooled estimate of upgrade was 5% (95% CI 3%, 8%) without atypia, 15% (95% CI 10%, 20%) when the presence of atypia was not specified and 28% (95% CI 20%, 38%) when atypia was identified on the NCB. Please note, the upgrade rates in the meta-analysis are not the same as the averages of the raw data, because weights are applied to each study in a meta-analysis.
For women with RS assessed by a mix of 8-16G NCB, the meta-analysis includes 1991 RS presented in 21 studies. The pooled estimate of upgrade was 2% (95% CI 1%, 4%) without atypia, 12% (95% CI 6%, 18%) when the presence of atypia was not specified and 11% (95% CI 3%, 23%) when atypia was identified on the NCB.
For women with RS assessed by 8-11G VAB, the meta-analysis includes 248 RS presented in 10 studies. The pooled estimate of upgrade was 1% (95% CI 0%, 4%) without atypia, 5% (95% CI 0%, 11%) when the presence of atypia was not specified and 18% in the one study when atypia was identified on the NCB (not listed in the meta-analysis, as a single study).
Summary of proportion of malignant upgrades for each subgroup of radial scars. ES effect size (the upgrade rate expressed as a proportion), % weight = random effects weights. This plot presents the pooled estimates of upgrade and 95% confidence intervals for each subgroup of RS and also the overall upgrade rate, presented in Fig. 1. Heterogeneity (I^2) is statistically significant across all subgroups (71.5%, p < 0.001). As subgroup 3c included only one study, summary analysis is not applicable
Table 3 and Fig. 2 also present the surgical outcomes for women undergoing surgery after VAB excision of RS. The presence of atypia in the VAB excision was not specified. The meta-analysis includes 57 such RS, presented in 3 studies. No upgrades were found in any of these 3 series (95% CI 0%, 3%).
The two series of RS evaluated by MRI guided biopsies, mostly by VABs, showed a pooled upgrade rate of 24% (95% CI 11, 39%).
Overall, there is a statistically significant difference across subgroups (p < 0.001). Within the subgroup assessed by 14G NCB, there was a statistically significant difference between the outcomes when stratified by the presence of atypia, as evident by the absence of overlapping confidence intervals. This is also true for RS assessed by 8-16G biopsies without atypia, versus those with atypia, but the more heterogeneous subgroup assessed by 8-16G biopsies shows overlapping confidence intervals with both other subgroups. For RS assessed by 8-11G VABs, the upgrade rate for several studies was zero, regardless of whether atypia was present or not specified. It is therefore unlikely that a statistically significant difference between such subgroups would be detected.
As shown in Table 4, Among the special case scenarios, for which relatively fewer cases have been reported, no upgrades were recorded in the following groups: RS ≤ 5 mm lacking atypia or an accompanying papillary component, “microscopic” RS found incidentally on histology when another lesion was targeted and successfully biopsied, and finally, in mammographically occult RS, found only on ultrasound examinations.
Discussion
Practice patterns for biopsy and management of RS are evolving. Whereas early series reported malignant upgrades in 25–45% of RS, some recent series have found far lower upgrades. Already one of the common borderline lesions, the detection of RS is likely to increase further with the growing use of tomosynthesis, providing further interest in non-surgical management of these lesions [24]. However, the wide range of reported upgrade rates, the persistence of a significant risk of undiagnosed malignancy even in contemporary practice and the lack of predictive imaging characteristics of foci of malignant change pose formidable barriers to the routine non-surgical management of RS.
We have focused on atypia and the extent of sampling as two plausible factors to account for the substantial variation in reported upgrade rates. Our meta-analysis of 49 series captures data for 3163 women, evaluated in the planned subgroup analysis. When stratified according to these key variables, the estimates for upgrade rates are more consistent within each subgroup, than in the whole dataset and statistically significant differences in the upgrade rates are evident. Within the subset assessed by 14G NCB, the presence of atypia is confirmed as a significant predictor of a malignant upgrade. Some heterogeneity remains among RS without atypia assessed by 14G NCB, the chief outlier being the series by Jackman in 1999, with a 40% upgrade rate, likely due to the small sample size of only 5 cases. As expected, there remains significant heterogeneity within the subgroup assessed by 8-16G NCBs, this group capturing a diverse mix of biopsy modalities. The upgrade rates were significantly lower for the group assessed by the 8-11G VABs than those assessed by smaller biopsies, but the low upgrade rates among all subsets of this group preclude assessment of the impact of histologic atypia.
Outside of intentional VAB excisions, the group with the lowest upgrade rate is RS without atypia assessed by VA biopsies. The upgrade rate for this group was 1% (95% CI 0–4%), comprising two cases of DCIS among 122 lesions.
The next lowest upgrade rate of 2% (95% CI 1–4%) was among RS without atypia sampled via 8-16G NCB. This group comprised 1263 RS, amongst which on excision 29 cases of DCIS and 10 invasive cancers were documented.
Two subgroups each had upgrade estimates of 5%. These included (i) RS without atypia assessed by conventional 14G NCB that included 30 cases of DCIS and 18 invasive cancers among 828 RS; and (ii) RS assessed by VAB, when the presence of atypia was not specified, comprising of 4 cases of DCIS and 2 invasive cancers among 115 RS.
Among RS without atypia, there was a small but step wise decline in upgrade rates with increasing needle gauge, with an upgrade of 5% (95% CI 3, 8%) among RS assessed by 14G cores to 2% (95% CI 1, 4%) for those assessed by 8-16G NCB and 1% (95% CI 0, 4%) for VABs. As discussed above, RS assessed by a mix of 8-16G needle biopsies also had a low upgrade of 5% (95% CI 0, 11%).
In all other subgroups, the upgrade estimates exceeded 10% and were as high as 28% when sampled via 14G cores and showed atypia. The upgrades in all these groups included invasive cancers as well as DCIS.
Concurrent malignancy in a RS may be focal and the correlation of extent of sampling with upgrade rates has been noted previously [15]. Because VABs produce larger volume samples, they lessen the potential for under sampling and diagnostic errors. Among other borderline lesions, the use of VABs has decreased the underestimation of ADH and DCIS [6, 13, 25]. Our analysis now confirms its improved sensitivity for the evaluation of RS. The present conclusions are concordant with previous reports emphasising the correlation between upgrade rates and biopsy modality, extent of sampling (> 12 cores), radiology pathology concordance and absence of atypia [5, 22, 29]. We note significant disparity among the studies in the number of core samples retrieved, ranging from 1 to 32.1 and up to 48 for VAB excision [28]. While data on the number of cores retrieved are not available for several series, in general, mores samples are obtained from VA biopsies. The larger gauge of the VAB excisions, combined with the greater number of samples lead to a larger proportion of lesions being evaluated by VABs, leaving less of the lesion unexamined. This more comprehensive evaluation reduces the chances of an upgrade on excision. Our findings are consistent with Ferreira’s 2017 logistic regression [12]. They reported that the use of VAB reduced the upgrade rate by 87%, or 3 times less than that of 14G NCB. They also found the presence of atypia was the only significant predictor of malignancy in RS, increasing the upgrade rate by10 times. In that study the presence of calcifications tripled the upgrade rate, while each unit increase in the number of cores reduced the risk by 0.8.
A prior review of RS included an analysis of 20 excision studies [21], finding an overall upgrade rate of 10.4%; 7.5% for those without atypia and 26% in RS with atypia.
A threshold for an acceptable level of risk has not been explicitly agreed upon in this field, but the precedent of BIRADs category 3 lesions, as used in the American College of Radiology, exists. For BIRADs category 3 lesions, the risk of malignancy is likely to be less than 2% and short-term (6 months) imaging surveillance is the accepted management recommendation. Applying this standard, RS without atypia assessed by VABs have a sufficiently low upgrade rate to be considered for short term surveillance, rather than surgery, however the 95% confidence interval for the pooled estimate of 1% upgrade rate was 0–4%. The fact that the upgrades in this group were to DCIS rather than to invasive cancer may provide a further impetus to a non-surgical approach. The 2% (95% CI 1,4%) estimated upgrade rate for RS without atypia, assessed by cores of 8-16G suggests this group may also be considered for non-surgical management. There is an overlap between this group and RS assessed by VABs. We note the upgrade rate for RS without atypia assessed by 14G NCB was 5% (95% CI 3,8%), suggesting that the lower upgrade rate in the 8-16G mixed modality group is attributable to the inclusion of cases assessed by larger caliber biopsies. In predicting the risk of an upgrade, when specific data about the biopsy modality, needle gauge and atypia are available, figures for the relevant specific subgroup should be used, reserving the heterogeneous 8-16G category for situations when the specific parameters cannot be ascertained.
While the small number of reported series precludes meta-analysis and requires caution, a non-surgical approach may also be considered for lesions in three other small special case scenarios. These included RS of ≤ 5 mm without atypia, mammographically occult but sonographically detected RS without atypia and incidental/microscopic RS without atypia. Despite the small number of reports addressing these subgroups, prior estimates indicate that up to 30.2% of all RS found on NCB are incidental to the lesion being targeted [7]. A non-surgical approach for these lesions is likely to have a significant impact in reducing benign open biopsy rates.
The upgrade rates for all other planned subgroups were ≥ 5%. We do not espouse any arbitrary risk threshold for avoiding surgery, but using this meta-analysis as a decision aid, the likelihood of concurrent malignant change in each specific clinical context can be predicted and the options and risks discussed with the patient. If observation is chosen, the need for continued, long term imaging surveillance should be emphasized.
In recognising the value of VABs, some centres have suggested a repeat biopsy with a vacuum-assisted device, when high-risk borderline lesions such as RS, are identified on 14G NCB [28, 32]. In the UK screening Program, a creative solution has been implemented that replaces surgery with VAB excision, whereby lesions biopsied previously and found to be RS are re-booked for VAB excision [26]. Since the likelihood of malignancy is low, particularly in the absence of atypia in the initial biopsy, there are no oncologic objections to the piecemeal removal of such lesions and the experience to date has been reassuring. Our meta-analysis included 57 such lesions in 3 series, with no malignant upgrades.
Widening the VAB excision approach to RS with atypia may be more problematic. Such lesions are more likely to harbour undetected foci of DCIS and invasive cancer and the morcellation of cancers by VABs may compromise the histologic evaluation of tumour size and peritumoural lymphovascular invasion. Surgery would still be required for the evaluation of surgical margins.
Despite the general high negative predictive value of MRIs, we note the upgrade rate for MRI guided biopsies of RS was 24% (95% CI 11, 39%), similar to the upgrade rates of unselected series of RS.
The limitations of this study include the absence of information on radiology pathology (R-P) concordance and incomplete data on the number of cores retrieved or the proportion of the lesion left behind. In addition, patient level information regarding age, lesion size, symptoms or findings on clinical breast examination are unavailable. Each of these parameters has been highlighted previously in small studies as possible predictors of upgrade [1, 14, 31]. In a meta-analysis we are restricted by the information reported in eligible studies and the above data are not reported commonly. However, we posit that the biopsy modality may be a surrogate for the extent of sampling, as are the number of cores and the proportion of lesion left behind. We have attempted to present some information regarding the number of cores when available and have highlighted studies from population-based screening services, as distinct from other groups. R-P concordance has been emphasized as a way of enhancing diagnostic accuracy, one recent series finding an upgrade rate of only 2% in concordant cases [8]. We cannot vouch that R-P concordance has been achieved on all studies included, but note the increasing recognition of this factor, particularly in recent series.
The estimates for upgrades in this analysis have been based on excised RS. It is possible that the RS recommended for excision may have significantly different, potentially more worrisome features from those allocated to observation. We have no way of ascertaining if this is the case, but if true, this selection bias would exaggerate our estimates of upgrade rates in RS.
Finally, unlike the present analysis, some groups have included various atypical epithelial proliferations in their upgrade rates [17], finding non-malignant atypia in over 20% of excised RS. The reasoning for the inclusion of non-malignant atypia in upgrade rates is that long term follow up studies of women with RS indicate that the slightly increased risk of subsequent cancer is predicated on the nature of the coexisting proliferative disease [4, 30] and the specific type of the proliferative disease may impact clinical care, including chemoprevention. However, since the focus of the present analysis is on reducing non-malignant surgical biopsies, our estimates of upgrades relate specifically to invasive cancer and DCIS.
Conclusions
Upgrade rates for RS are predictable on the basis of the presence of atypia and core biopsy gauge. RS without atypia assessed by 8-11G VABs has an upgrade rate of only 1% (95% CI 0, 4%) and only to DCIS. Imaging surveillance may be a reasonable option for these patients. The upgrade rates for the other planned RS subgroups ranged between 2%-28%, but can be reduced by VAB excisions, as an alternative to surgery. The estimates from this meta-analysis can inform clinical care.
References
Andacoglu O, Kanbour-Shakir A, Teh YC, Bonaventura M, Ozbek U, Anello M, Ganott M, Kelley J, Dirican A, Soran A (2013) Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol 36:7–11. https://doi.org/10.1097/COC.0b013e3182354a3f
Aroner SA, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Rosner BA, Hankinson SE, Tamimi RM (2013) Radial scars and subsequent breast cancer risk: results from the Nurses’ Health Studies. Breast Cancer Res Treat 139:277–285. https://doi.org/10.1007/s10549-013-2535-9
Azavedo E, Svane G (1992) Radial scars detected mammographically in a breast cancer screening programme. Eur J Radiol 15:18–21
Berg JC, Visscher DW, Vierkant RA, Pankratz VS, Maloney SD, Lewis JT, Frost MH, Ghosh K, Degnim AC, Brandt KR, Vachon CM, Reynolds CA, Hartmann LC (2008) Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat 108:167–174. https://doi.org/10.1007/s10549-007-9605-9
Brenner RJ, Jackman RJ, Parker SH, Evans WP III, Philpotts L, Deutch BM, Lechner MC, Lehrer D, Sylvan P, Hunt R, Adler SJ, Forcier N (2002) Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol 179:1179–1184. https://doi.org/10.2214/ajr.179.5.1791179
Burbank F (1997) Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology 202:843–847. https://doi.org/10.1148/radiology.202.3.9051043
Cawson JN, Malara F, Kavanagh A, Hill P, Balasubramanium G, Henderson M (2003) Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer 97:345–351. https://doi.org/10.1002/cncr.11070
Conlon N, D’Arcy C, Kaplan JB, Bowser ZL, Cordero A, Brogi E, Corben AD (2015) Radial scar at image-guided needle biopsy: is excision necessary? Am J Surg Pathol 39:779–785. https://doi.org/10.1097/pas.0000000000000393
Dominguez A, Durando M, Mariscotti G, Angelino F, Castellano I, Bergamasco L, Bianchi CC, Fonio P, Gandini G (2015) Breast cancer risk associated with the diagnosis of a microhistological radial scar (RS): retrospective analysis in 10 years of experience. Radiol Med 120:377–385. https://doi.org/10.1007/s11547-014-0456-2
Falomo E, Adejumo C, Carson KA, Harvey S, Mullen L, Myers K (2018) Variability in the management recommendations given for high-risk breast lesions detected on image-guided core needle biopsy at U.S. Academic Institutions. Curr Probl Diagn Radiol. https://doi.org/10.1067/j.cpradiol.2018.06.004
Farshid G, Gill PG (2017) Contemporary indications for diagnostic open biopsy in women assessed for screen-detected breast lesions: a ten-year, single institution series of 814 consecutive cases. Breast Cancer Res Treat 162:49–58. https://doi.org/10.1007/s10549-016-4087-2
Ferreira AI, Borges S, Sousa A, Ribeiro C, Mesquita A, Martins PC, Peyroteo M, Coimbra N, Leal C, Reis P, Sousa JA (2017) Radial scar of the breast: Is it possible to avoid surgery? Eur J Surg Oncol 43:1265–1272. https://doi.org/10.1016/j.ejso.2017.01.238
Jackman RJ, Nowels KW, Rodriguez-Soto J, Marzoni FA Jr, Finkelstein SI, Shepard MJ (1999) Stereotactic, automated, large-core needle biopsy of nonpalpable breast lesions: false-negative and histologic underestimation rates after long-term follow-up. Radiology 210:799–805. https://doi.org/10.1148/radiology.210.3.r99mr19799
Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ (1999) Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med 340:430–436. https://doi.org/10.1056/nejm199902113400604
Krishnamurthy S, Bevers T, Kuerer H, Yang WT (2012) Multidisciplinary considerations in the management of high-risk breast lesions. AJR Am J Roentgenol 198:W132–W140. https://doi.org/10.2214/ajr.11.7799
Lee KA, Zuley ML, Chivukula M, Choksi ND, Ganott MA, Sumkin JH (2012) Risk of malignancy when microscopic radial scars and microscopic papillomas are found at percutaneous biopsy. AJR Am J Roentgenol 198:W141–W145. https://doi.org/10.2214/ajr.11.7712
Li Z, Ranade A, Zhao C (2016) Pathologic findings of follow-up surgical excision for radial scar on breast core needle biopsy. Hum Pathol 48:76–80
Lopez-Medina A, Cintora E, Mugica B, Opere E, Vela AC, Ibanez T (2006) Radial scars diagnosed at stereotactic core-needle biopsy: surgical biopsy findings. Eur Radiol 16:1803–1810. https://doi.org/10.1007/s00330-006-0196-3
Matrai C, D’Alfonso TM, Pharmer L, Drotman MB, Simmons RM, Shin SJ (2015) Advocating nonsurgical management of patients with small, incidental radial scars at the time of needle core biopsy: a study of 77 cases. Arch Pathol Lab Med 139:1137–1142. https://doi.org/10.5858/arpa.2014-0550-OA
Miller CL, West JA, Bettini AC, Koerner FC, Gudewicz TM, Freer PE, Coopey SB, Gadd MA, Hughes KS, Smith BL, Rafferty E, Specht MC (2014) Surgical excision of radial scars diagnosed by core biopsy may help predict future risk of breast cancer. Breast Cancer Res Treat 145:331–338. https://doi.org/10.1007/s10549-014-2958-y
Mooney KL, Bassett LW, Apple SK (2016) Upgrade rates of high-risk breast lesions diagnosed on core needle biopsy: a single-institution experience and literature review. Mod Pathol 29:1471–1484. https://doi.org/10.1038/modpathol.2016.127
Osborn G, Wilton F, Stevens G, Vaughan-Williams E, Gower-Thomas K (2011) A review of needle core biopsy diagnosed radial scars in the Welsh Breast Screening Programme. Ann R Coll Surg Engl 93:123–126. https://doi.org/10.1308/003588411x12851639107953
Park VY, Kim EK, Kim MJ, Yoon JH, Moon HJ (2016) Mammographically occult asymptomatic radial scars/complex sclerosing lesions at ultrasonography-guided core needle biopsy: follow-up can be recommended. Ultrasound Med Biol 42:2367–2371. https://doi.org/10.1016/j.ultrasmedbio.2016.06.004
Phantana-Angkool A, Forster MR, Warren YE, Livasy CA, Sobel AH, Beasley LM, Trufan SJ, Hadzikadic-Gusic L, Sarantou T, Voci AE, Sarma D, White RL Jr (2018) Rate of radial scars by core biopsy and upgrading to malignancy or high-risk lesions before and after introduction of digital breast tomosynthesis. Breast Cancer Res Treat. https://doi.org/10.1007/s10549-018-4973-x
Philpotts LE, Shaheen NA, Jain KS, Carter D, Lee CH (2000) Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology 216:831–837. https://doi.org/10.1148/radiology.216.3.r00se31831
Pinder SE, Shaaban A, Deb R, Desai A, Gandhi A, Lee AHS, Pain S, Wilkinson L, Sharma N (2018) NHS Breast Screening multidisciplinary working group guidelines for the diagnosis and management of breast lesions of uncertain malignant potential on core biopsy (B3 lesions). Clin Radiol 73:682–692. https://doi.org/10.1016/j.crad.2018.04.004
Rageth CJ, O’Flynn EA, Comstock C, Kurtz C, Kubik R, Madjar H, Lepori D, Kampmann G, Mundinger A, Baege A, Decker T, Hosch S, Tausch C, Delaloye JF, Morris E, Varga Z (2016) First International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res Treat 159:203–213. https://doi.org/10.1007/s10549-016-3935-4
Rajan S, Wason AM, Carder PJ (2011) Conservative management of screen-detected radial scars: role of mammotome excision. J Clin Pathol 64:65–68. https://doi.org/10.1136/jcp.2010.083485
Rakha EA, Ho BC, Naik V, Sen S, Hamilton LJ, Hodi Z, Ellis IO, Lee AH (2011) Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology 58:626–632. https://doi.org/10.1111/j.1365-2559.2011.03786.x
Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD (2006) Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer 106:1453–1461. https://doi.org/10.1002/cncr.21730
Sloane JP, Mayers MM (1993) Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology 23:225–231
Sohn VY, Causey MW, Steele SR, Keylock JB, Brown TA (2010) The treatment of radial scars in the modern era–surgical excision is not required. Am Surg 76:522–525
Acknowledgements
We acknowledge the BreastScreen Australia Clinical Advisory Committee for supporting this work. We thank the editors and anonymous reviewers of the journal whose suggestions have improved the presentation of our work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Gelareh Farshid and Elizabeth Buckley declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farshid, G., Buckley, E. Meta-analysis of upgrade rates in 3163 radial scars excised after needle core biopsy diagnosis. Breast Cancer Res Treat 174, 165–177 (2019). https://doi.org/10.1007/s10549-018-5040-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-5040-3