Abstract
Radial scars (RS’s) are benign breast lesions known to be associated with carcinomas and other high-risk lesions (HRL’s). The upgrade rate to carcinoma after core biopsy revealing RS is 0–40 %. We sought to determine the outcomes of RS with and without HRL diagnosed by core biopsy. Patients who underwent core biopsy revealing RS without carcinoma at our institution between 1/1996 and 11/2012 were identified from a surgical pathology database. Retrospective chart review was utilized to classify patients as RS-no HRL or RS-HRL. HRL was defined as ADH, LCIS, and/or ALH. We determined upgrade rate to carcinoma at surgical excision, and upgrade to HRL for RS-no HRL patients. Univariate analysis was performed to identify risk factors for upgrade in RS-no HRL patients. 156 patients underwent core biopsy revealing RS, 131 RS-no HRL (84 %), and 25 RS-HRL (16 %). The overall rate of upgrade to invasive carcinoma was 0.8 % (1/124). 1.0 % (1/102) of RS-no HRL and 13.6 % (3/22) of RS-HRL patients were upgraded to DCIS (P = 0.0023). The upgrade of RS-no HRL to HRL at excision was 21.6 % (22/102). By univariate analysis, RS-no HRL with radiologic appearance of a mass/architectural distortion had a significantly higher rate of upgrade to HRL or carcinoma compared with calcifications (P = 0.03). Excision of RS to rule out associated invasive carcinoma is not warranted, given a <1 % rate of upgrade at excision. However, excision to evaluate for non-invasive cancer or HRL may be considered to help guide clinical decision-making about use of chemoprevention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radial scar (RS) or complex sclerosing lesion is a rare, benign lesion of the breast with unknown etiology. Histologically, RS’s consist of a central fibroelastotic core with outwardly radiating ducts, creating a stellate appearance [1]. RS’s can be indistinguishable from invasive carcinoma by radiologic appearance alone, often presenting as a spiculated mass or architectural distortion [2–10]. As a result, core needle biopsy is commonly recommended for histologic confirmation of suspected RS’s.
For HRL’s such as atypical ductal hyperplasia (ADH), core biopsy alone is considered unreliable and surgical excision is recommended to rule out malignancy. RS’s are known to be associated with carcinoma and proliferative breast disease [2, 4, 7, 11–14], and have a 0–40 % reported rate of upgrade to malignancy at excision [15–25]. Given the lack of consistency in rate of associated malignancy, the role of surgical excision following core biopsy demonstrating RS remains controversial. In addition, factors predictive of upgrade for RS’s diagnosed by core biopsy have not been definitively identified in previous studies.
The purpose of this study was to evaluate the outcomes of RS’s without carcinoma diagnosed by core biopsy. We sought to determine the rate of upgrade to carcinoma upon surgical excision of RS’s with or without associated HRL on core biopsy. In addition, we analyzed factors predictive of an upgrade to carcinoma or HRL for patients diagnosed with RS without HRL on core biopsy.
Methods and patients
With approval from Massachusetts General Hospital/Partners Healthcare institutional review board, we identified patients from the surgical pathology department’s database who underwent a core biopsy at our institution revealing RS without carcinoma between 1/1996 and 11/2012. Retrospective medical record review was utilized to collect data on surgical, pathologic, and radiologic procedures and findings, as well as patient characteristics.
Core needle biopsies were performed with stereotactic, sonographic, or MRI guidance, and marker clips were placed routinely for each type of biopsy. Stereotactic biopsy was performed with a dedicated prone stereotactic biopsy table (MultiCare Platinum, Lorad). The Mammotome system with 11-gauge devices were used from 1997 to 2007, after which vacuum-assisted 7-gauge (EnCor, SenoRx) and 9-gauge (Eviva, Hologic) devices were used with at least four and six specimens routinely acquired, respectively, per institutional protocol. Additional specimens were acquired at the discretion of the performing radiologist. Specimen radiography and post-biopsy mammography were routinely performed for all patients. Ultrasound-guided biopsy was performed with the patient in a supine position using a 12.5-MHz linear array (iU22, Philips Healthcare). Fourteen-gauge core biopsy needles (Monopty, Bard) were used, and, according to institutional protocol, five specimens were routinely acquired. Additional specimens were acquired at the discretion of the performing radiologist. Mammography was routinely performed after biopsy for all patients to document the accuracy of clip deployment. We began performing MRI-guided biopsies at our institution in 8/2007, which were performed on a 1.5-T magnet with a 4-channel coil (GE Breast Array Coil, GE Healthcare) and an immobilization and biopsy system (Breast Immobilization and Biopsy Device MR-BI 160, NORAS MRI Products). During the study, a 9-gauge vacuum-assisted device (Suros ATEC, Hologic) was used. Post-biopsy mammography was routinely performed for all patients to document clip deployment. All percutaneous and excisional pathology specimens were reviewed by one of six pathologists specializing in breast pathology (with 10–30 years of experience). Pathology specimens at core biopsy were immediately placed in formalin, and all tissue specimens had histologic slides prepared and stained with hematoxylin and eosin according to institutional protocols. All patients who underwent excisional biopsy had the percutaneous biopsy site identified in the excisional biopsy specimen.
Patients were classified as RS-no HRL or RS-HRL based on pathologic findings on core biopsy. Pathologic diagnoses of complex sclerosing lesions were classified as RS for the purpose of this study. HRL was defined as identification of ADH, lobular carcinoma in situ (LCIS), or atypical lobular hyperplasia (ALH). Core biopsies of RS containing flat epithelial atypia (FEA), and/or other types of epithelial atypia were excluded from the analysis.
Surgical excision is recommended per standard of care at our institution for patients with a core biopsy demonstrating RS. For this study, the upgrade rate to carcinoma was determined for RS-no HRL and RS-HRL patients who underwent surgical excision subsequent to core biopsy. Final pathology of the excisional biopsy specimen was utilized to determine upgrade to carcinoma, which included invasive ductal carcinoma (IDC), invasive lobular carcinoma (ILC), or ductal carcinoma in situ (DCIS). For RS-no HRL patients, we also determined the rate of upgrade to HRL at surgical excision using final pathology of the excised specimen.
Additional clinicopathologic data for the patient cohort was collected. Patient characteristics included age at core biopsy, history of breast cancer or concurrent contralateral breast cancer. Patients with a diagnosis of concurrent ipsilateral breast cancer were excluded from the analysis. Presentation prompting the core biopsy which resulted in a diagnosis of RS was classified as screening mammogram, screening MRI, or clinical detection. Clinical detection included a palpable change in the breast exam, breast pain, or bloody/non-bloody discharge. Radiologic appearance of the abnormality biopsied was classified as calcifications, architectural distortion/mass, or MRI enhancement. Core biopsies utilized in this study were stereotactic, ultrasound-guided, or MRI-guided. The most recent report from a visit with a primary care physician, breast radiologist, or breast oncologist was utilized to determine which patients were diagnosed with a new breast cancer during the period of follow-up after core biopsy revealing RS and subsequent surgical excision.
The R Project was utilized for statistical analyses, with a P value of <0.05 considered statistically significant. Univariate analysis was performed to identify risk factors for upgrade to carcinoma or HRL in RS-no HRL patients.
Results
Patient cohort
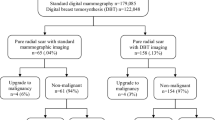
11,816 image-guided core biopsies were performed during the study period (1/1996–11/2012), from which we identified 180 (1.5 %) core biopsies corresponding to 179 patients revealing RS without carcinoma. 1 of the 180 RS patients was diagnosed with RS-no HRL, did not undergo surgical excision, and approximately 96 months later underwent a repeat core biopsy again revealing RS-no HRL in the same area of the breast. Only the later diagnosis was included in this analysis. 15 core biopsies contained RS with FEA and 8 contained other types of epithelial atypia, and were therefore excluded from this analysis.
Therefore, the study cohort included 156 patients with core biopsy revealing RS without carcinoma, 131 (84 %) RS-no HRL and 25 RS-HRL (16 %) (Table 1). Of the 25 RS-HRL patients, 88 % (22) had ADH, 12 % (3) ALH, and 0 % (0) LCIS in association with RS on core biopsy.
A total of 124 of the 156 patients (80 %) underwent surgical excision subsequent to the core biopsy revealing RS (Fig. 1). This includes 102 of the 131 (78 %) RS-no HRL patients and 22 of 25 (88 %) RS-HRL patients, who underwent excision at a median of 1.05 months (range 0.23–11.0) and 1.15 months (range 0.46–9.61) post-core biopsy, respectively.
Upgrade to carcinoma or HRL
The overall rate of upgrade to invasive carcinoma at surgical excision for the patient cohort was 0.8 % (95 % CI 0.02–4.41 %, 1/124), 1.0 % (95 % CI 0.02–5.34 %, 1/102) for RS-no HRL, and 0.0 % (95 % CI 0.0–15.4 %, 0/22) for RS-HRL patients (P = 0.64). 1 % (95 % CI 0.0–5.34 %, 1/102) of RS-no HRL patients and 13.6 % (95 % CI 2.91–34.9, 3/22) of RS-HRL patients were upgraded to DCIS at surgical excision (P = 0.0023). Clinical, pathologic, and radiologic data for the five patients upgraded to invasive carcinoma or DCIS at surgical excision are listed in Table 2. Of the four patients upgraded to grade 1 DCIS, 1 subsequently began tamoxifen, 2 were offered tamoxifen but opted not to take it, and 1 was already on tamoxifen due to a history of ipsilateral DCIS.
The rate of upgrade to HRL at surgical excision for RS-no HRL patients was 21.6 % (95 % CI 14.0–30.8 %, 22/102). Type of HRL detected at surgical excision was 36 % (8) ADH, 36 % (8) ALH, 23 % (5) LCIS, 5 % (1) ADH and ALH.
Of the 41 patients found to have HRL on core biopsy or subsequent surgical excision (and not upgraded to carcinoma), five began chemoprevention as a result of the finding, three were already on chemoprevention, two were unknown, and 31 did not begin chemoprevention.
Factors associated with upgrade for RS-no HRL patients
By univariate analysis, RS-no HRL patients with a radiologic appearance of a mass/architectural distortion by ultrasonography or mammography had a significantly higher rate of upgrade to HRL or carcinoma compared with calcifications (33 vs. 13 %, P = 0.03) (Table 3). Age at core biopsy, history of breast cancer or concurrent contralateral breast cancer, initial presentation, and type of core biopsy were not significant (P > 0.05) predictors of upgrade.
Breast cancer development
111 patients who underwent surgical excision without upgrade to invasive carcinoma or DCIS after core biopsy revealing RS had post-excision follow-up at our institution. Of the 111 patients included, 2.7 % (3) were on chemoprevention prior to the core biopsy with RS due to history of HRL or breast cancer, and 10.8 % (12) began chemoprevention subsequent to surgical excision of the RS. At a median post-excision follow-up of 45.5 months (range 1.2–158.6), three patients (1 RS-HRL and 2 RS-no HRL) were diagnosed with a new ipsilateral or contralateral breast cancer (Table 4).
33 patients did not undergo surgical excision at our institution due to co-morbidities, personal preference, transfer of care to another institution, or lack of follow-up. 3 RS-HRL and 22 RS-no HRL patients who did not undergo excision had follow-up at our institution, with a median of 79.2 months (range 5.51–142.9) post-biopsy follow-up. Of the 25 patients with follow-up information, 1 RS-HRL patient with a history of contralateral IDC was diagnosed with 0.3 cm grade 1 DCIS ipsilateral to the RS at 15.4 months post-biopsy.
Discussion
In this series of 156 patients, we found a <1 % rate of upgrade to invasive carcinoma at surgical excision following core biopsy revealing RS with or without an associated HRL. Fourteen percent of RS’s with HRL on core biopsy were upgraded to DCIS at excision, and 22 % of RS’s without HRL on core biopsy were found to be associated with HRL at excision. Therefore, excision to rule out invasive carcinoma is not warranted. Excision to evaluate for non-invasive cancer or an associated HRL may be considered to help predict future risk of breast cancer and guide clinical decision-making regarding the potential benefit of chemoprevention.
Core needle biopsy has become the standard procedure for evaluation of suspicious breast lesions. Surgical excision is commonly recommended when core biopsy results in the diagnosis of a high-risk breast lesion to rule out the possibility of an associated malignancy [26]. Although rates of upgrade to carcinoma vary, HRL’s such as ADH or lobular neoplasia (ALH or LCIS) are generally considered to have an unacceptably high rate of malignancy underestimation and thereby warrant surgical excision [27–31].
The need for surgical excision when core biopsy reveals a RS remains unclear. Previous studies have reported a rate of upgrade to carcinoma at surgical excision ranging from 0 to 40 % [15–25]. The wide variability in malignancy underestimation is likely due to small, retrospective cohorts and differences in standard clinical practice regarding use of surgical excision for RS. A recent study surveying 477 members of the American Society of Breast Surgeons demonstrated that only 57 % of respondents recommended routine excision of biopsy-proven RS's [32]. Forty percent of the respondents reported use of selective excision, with criteria based on radiologic–pathologic correlation (71 %), lesion size (53 %), and the coexistence of atypia (37 %).
The argument in favor of excision of RS’s diagnosed by core biopsy is based on the finding of a high rate of upgrade to malignancy or HRL, as demonstrated in a number of previous studies [20, 22–25]. In a series of 62 RS’s without atypia, Linda et al. found an 8 % rate of upgrade to malignancy (3 % invasive carcinoma, 5 % DCIS), and demonstrated that mammographic and sonographic features are insufficient to predict which lesions will have associated malignancy [20]. The authors concluded that all RS's diagnosed at imaging-guided core biopsy should be surgically excised. Douglas-Jones et al. evaluated the false negative rate of needle core biopsy for the pre-operative diagnosis of RS, and found that 3.9 % (11/281) contained carcinoma at surgical excision [22]. A review of 118 patients with biopsy-proven RS diagnosed in the Welsh Breast Screening Programme revealed an upgrade to carcinoma or HRL of 26 % (25/95), thereby warranting surgical excision [23].
Others argue that routine imaging follow-up is sufficient given the low rate of upgrade to malignancy for biopsy-confirmed RS’s [15, 16, 18]. In 2008, Resetkova et al. analyzed 80 cases of RS with or without atypia and reported a 0 % rate of upgrade to carcinoma [18]. In their evaluation of a mammographic screening population, Cawson et al. also reported a 0 % rate of upgrade to carcinoma for mammographically detected RS's without atypia, and suggested that these patients can be safely managed with regular mammographic surveillance rather than excision [16]. Utilizing one of the largest cohorts in the literature, Brenner et al. reported an 8 % (13/157) malignancy underestimation rate (3 % invasive carcinoma, 5 % DCIS) for RS's with (28 %) and without (4 %) atypia [15]. The authors suggest that excision of RS's can be avoided when certain criteria are met, including no associated atypical hyperplasia at core biopsy, ≥12 biopsy specimens collected, and concordant histologic and mammographic findings. Finally, a study by Rajan et al. recommended use of mammotome excision to avoid surgical excision for the management of RS without atypia [33].
In our series, we found a <1 % overall rate of upgrade to invasive carcinoma for RS's diagnosed on core biopsy, which is relatively low compared with previous reports. One patient with RS-no HRL on core biopsy was upgraded to grade 1 ILC at excision. An additional four cases of RS (3 with HRL and 1 without HRL) were upgraded to grade 1 DCIS. Review of mammographic imaging and histologic findings from core biopsy suggested radiologic–pathologic discordance for the upgrade to ILC. Therefore, based on this series, one may consider forgoing surgical excision and instead utilizing close follow-up if there is radiologic–pathologic concordance and if the goal of excision is to rule out associated invasive carcinoma rather than detect HRL or non-invasive breast cancers.
The rate of upgrade to DCIS in our series was 14 % for RS-HRL patients and 1 % RS-no HRL patients. Importantly, the rate of upgrade to invasive carcinoma was <1 % for RS with and without HRL. A higher rate of upgrade to invasive or non-invasive breast cancer for RS with associated HRL has been similarly demonstrated in previous studies, which is expected given the known association of HRL’s with carcinoma [15, 19, 24]. Core biopsies resulting in a diagnosis of RS with an associated HRL may therefore warrant excision to rule out non-invasive cancer and to better estimate a patient’s risk of developing breast cancer. Patients with high-risk breast lesions diagnosed by core biopsy and/or surgical excision have consistently been shown to have an increased risk of future breast cancer compared to patients with benign breast biopsies [34].
Findings from our series demonstrated that RS’s without HRL on core biopsy are frequently associated with HRL’s at surgical excision. We found a low rate of associated invasive or non-invasive cancer (2.0 %, 2/102) at excision of RS’s without HRL on core biopsy, however, 22 % (22/102) were upgraded to HRL at excision. Therefore, 22 patients would have missed the opportunity for counseling regarding chemoprevention had they not undergone surgical excision for RS. Andacoglu et al. similarly demonstrated a high rate of upgrade to HRL (22.4 %) at surgical excision of RS without atypia [24]. Based on these findings, surgical excision of RS's diagnosed by core biopsy without HRL may be considered, if the goal is to identify associated high-risk breast lesions for which chemoprevention or high-risk surveillance may be indicated.
Factors predictive of upgrade to malignancy or HRL for RS's diagnosed by core biopsy have not been definitively identified in previous reports. The presence of an associated HRL or atypia on core biopsy, core needle gage, number of samples obtained at core biopsy, as well as patient age and menopausal status have been suggested as potential predictive factors [15, 19, 24]. We were unable to evaluate risk factors of upgrade to carcinoma for RS's with HRL due to the small number of events in this series, but did analyze factors associated with upgrade to carcinoma or HRL for RS's without HRL on core biopsy. Interestingly, we found that RS's with a radiologic appearance of a mass or architectural distortion (by mammography or ultrasound) were more likely to be upgraded to carcinoma or HRL compared with RS's presenting as calcifications (33 vs. 13 %, respectively). This suggests a target cohort of patients who could potentially benefit from surgical excision of RS diagnosed by core biopsy. Although we did not evaluate core needle gauge or number of samples obtained as risk factors, we did analyze type of core biopsy performed (stereotactic, ultrasound-guided, or MRI-guided) and found no statistically significant difference in risk of upgrade. In addition, we did not find patient age, history of breast cancer, concurrent contralateral breast cancer, or type of presentation (screening mammogram, screening MRI, or clinical detection) to significantly predict upgrade to carcinoma or HRL for RS's without HRL on core biopsy.
The utility of breast ultrasound or MRI to evaluate the necessity of surgical excision for RS's has been suggested [35–37]. Linda et al. evaluated the utility of breast MRI to rule out malignancy in patients with HRL diagnosed at core biopsy, concluding that patients with biopsy-confirmed RS's without suspicious MRI findings can safely undergo follow-up instead of excision [35]. Similarly, Perfetto et al. suggest that breast MRI could be used to decide between follow-up or surgical excision of suspected RS's given the finding that MRI enhancement rate and time–intensity curve are useful in the differential diagnosis between benign and malignant breast lesions [36]. Cawson et al. analyzed 75 consecutive mammographic screen-detected RS's, and found that sonographic differences could help discriminate between RS's and carcinomas prior to performing a core biopsy [37]. In our series, 47 % (73/156) of patients underwent an ultrasound and 17 % (27/156) underwent a breast MRI as part of their diagnostic work-up prior to core biopsy; patients did not routinely undergo evaluation with ultrasonography or MRI after a biopsy demonstrating a RS. Due to the low incidence of upgrade to carcinoma in our series, we were unable to conclude whether ultrasound or MRI helps distinguish RS's associated with malignancy.
It has been speculated that RS's may be a precursor or risk factor for breast cancer [38–43]. Recently, Aroner et al. utilized data from the Nurses’ Health Studies to perform an updated analysis on the association between RS's and risk of breast cancer [43]. The authors confirmed their previous findings, reporting that RS was associated with a nearly twofold increased risk of breast cancer even after accounting for concurrent proliferative disease. In our series, at a median of 46 months follow-up, three patients (3 %) who underwent surgical excision following core biopsy revealing RS were diagnosed with a new breast cancer. Of note, two of these three patients had history of breast cancer and one had history of atypia. Due to the low number of events in our series, we are unable to determine whether RS is an independent risk factor for development of breast cancer.
The efficacy of chemoprevention with tamoxifen, raloxifene, and exemestane in reducing future risk of breast cancer for patients with atypical breast lesions has been established in numerous previous studies [34, 44–46]. Despite this, a relatively low percentage (4–20 %) of high-risk women elect to take chemoprevention [34, 47]. In our series, only 5 of 36 (14 %) patients with HRL on core biopsy and/or excision who were eligible for chemoprevention elected to begin taking it.
Our series of 156 patients (124 with surgical excision) represents one of the larger cohorts of patients diagnosed with RS by core biopsy and assessed for subsequent upgrade to carcinoma or HRL. In addition, our study includes long-term follow-up (median 46 months) for patients with RS who underwent excision to evaluate future breast cancer incidence. Our study has a number of limitations, including bias inherent to any retrospective study. This may be particularly relevant regarding the selection of patients who did and did not undergo excision following core biopsy; overall 80 % of patients underwent excision in this cohort. In addition, we were unable to evaluate lesion size, core needle gauge, or number of specimens collected per core biopsy as risk factors for upgrade. Finally, a small number of patients (8) did not undergo follow-up at our institution after surgical excision and therefore were not evaluable for subsequent breast cancer development.
In conclusion, RS's with or without HRL diagnosed by core biopsy are associated with a <1 % rate of upgrade to invasive carcinoma at surgical excision. Therefore, excision to rule out invasive carcinoma is not warranted. However, excision may be considered to evaluate for the presence of an HRL, which could provide valuable information regarding the potential role for chemoprevention to reduce the risk of future breast cancer.
References
Rosen P (ed) (1997) Radial sclerosing lesions. Rosen’s breast pathology. Lippincott-Raven, Philadelphia
Douglas-Jones AG, Pace DP (1997) Pathology of R4 spiculated lesions in the breast screening programme. Histopathology 30(3):214–220
Mitnick JS, Vazquez MF, Harris MN, Roses DF (1989) Differentiation of radial scar from scirrhous carcinoma of the breast: mammographic–pathologic correlation. Radiology 173(3):697–700
Ciatto S, Morrone D, Catarzi S, Del Turco MR, Bianchi S, Ambrogetti D, Cariddi A (1993) Radial scars of the breast: review of 38 consecutive mammographic diagnoses. Radiology 187(3):757–760
Alleva DQ, Smetherman DH, Farr Jr GH, Cederbom GJ (1999) Radial scar of the breast: radiologic–pathologic correlation in 22 cases. Radiographics 19 Spec No: S27–35; discussion S36–37
Finlay ME, Liston JE, Lunt LG, Young JR (1994) Assessment of the role of ultrasound in the differentiation of radial scars and stellate carcinomas of the breast. Clin Radiol 49(1):52–55
Hassell P, Klein-Parker H, Worth A, Poon P (1999) Radial sclerosing lesions of the breast: mammographic and pathologic correlation. Can Assoc Radiol J 50(6):370–375
Wallis MG, Devakumar R, Hosie KB, James KA, Bishop HM (1993) Complex sclerosing lesions (radial scars) of the breast can be palpable. Clin Radiol 48(5):319–320
Cohen MA, Sferlazza SJ (2000) Role of sonography in evaluation of radial scars of the breast. AJR Am J Roentgenol 174(4):1075–1078. doi:10.2214/ajr.174.4.1741075
Cawson JN, Nickson C, Evans J, Kavanagh AM (2010) Variation in mammographic appearance between projections of small breast cancers compared with radial scars. J Med Imaging Radiat Oncol 54(5):415–420. doi:10.1111/j.1754-9485.2010.02194.x
Kennedy M, Masterson AV, Kerin M, Flanagan F (2003) Pathology and clinical relevance of radial scars: a review. J Clin Pathol 56(10):721–724
Philpotts LE, Shaheen NA, Jain KS, Carter D, Lee CH (2000) Uncommon high-risk lesions of the breast diagnosed at stereotactic core-needle biopsy: clinical importance. Radiology 216(3):831–837
Frouge C, Tristant H, Guinebretiere JM, Meunier M, Contesso G, Di Paola R, Blery M (1995) Mammographic lesions suggestive of radial scars: microscopic findings in 40 cases. Radiology 195(3):623–625
Sloane JP, Mayers MM (1993) Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology 23(3):225–231
Brenner RJ, Jackman RJ, Parker SH, Evans WP 3rd, Philpotts L, Deutch BM, Lechner MC, Lehrer D, Sylvan P, Hunt R, Adler SJ, Forcier N (2002) Percutaneous core needle biopsy of radial scars of the breast: when is excision necessary? AJR Am J Roentgenol 179(5):1179–1184. doi:10.2214/ajr.179.5.1791179
Cawson JN, Malara F, Kavanagh A, Hill P, Balasubramanium G, Henderson M (2003) Fourteen-gauge needle core biopsy of mammographically evident radial scars: is excision necessary? Cancer 97(2):345–351. doi:10.1002/cncr.11070
Lopez-Medina A, Cintora E, Mugica B, Opere E, Vela AC, Ibanez T (2006) Radial scars diagnosed at stereotactic core-needle biopsy: surgical biopsy findings. Eur Radiol 16(8):1803–1810. doi:10.1007/s00330-006-0196-3
Resetkova E, Edelweiss M, Albarracin CT, Yang WT (2011) Management of radial sclerosing lesions of the breast diagnosed using percutaneous vacuum-assisted core needle biopsy: recommendations for excision based on seven years’ of experience at a single institution. Breast Cancer Res Treat 127(2):335–343. doi:10.1007/s10549-008-0119-x
Becker L, Trop I, David J, Latour M, Ouimet-Oliva D, Gaboury L, Lalonde L (2006) Management of radial scars found at percutaneous breast biopsy. Can Assoc Radiol J 57(2):72–78
Linda A, Zuiani C, Furlan A, Londero V, Girometti R, Machin P, Bazzocchi M (2010) Radial scars without atypia diagnosed at imaging-guided needle biopsy: how often is associated malignancy found at subsequent surgical excision, and do mammography and sonography predict which lesions are malignant? AJR Am J Roentgenol 194(4):1146–1151. doi:10.2214/AJR.09.2326
Londero V, Zuiani C, Linda A, Battigelli L, Brondani G, Bazzocchi M (2011) Borderline breast lesions: comparison of malignancy underestimation rates with 14-gauge core needle biopsy versus 11-gauge vacuum-assisted device. Eur Radiol 21(6):1200–1206. doi:10.1007/s00330-010-2053-7
Douglas-Jones AG, Denson JL, Cox AC, Harries IB, Stevens G (2007) Radial scar lesions of the breast diagnosed by needle core biopsy: analysis of cases containing occult malignancy. J Clin Pathol 60(3):295–298. doi:10.1136/jcp.2006.037069
Osborn G, Wilton F, Stevens G, Vaughan-Williams E, Gower-Thomas K (2011) A review of needle core biopsy diagnosed radial scars in the Welsh Breast Screening Programme. Ann R Coll Surg Engl 93(2):123–126. doi:10.1308/003588411X12851639107953
Andacoglu O, Kanbour-Shakir A, Teh YC, Bonaventura M, Ozbek U, Anello M, Ganott M, Kelley J, Dirican A, Soran A (2013) Rationale of excisional biopsy after the diagnosis of benign radial scar on core biopsy: a single institutional outcome analysis. Am J Clin Oncol 36(1):7–11. doi:10.1097/COC.0b013e3182354a3f
Bianchi S, Giannotti E, Vanzi E, Marziali M, Abdulcadir D, Boeri C, Livi L, Orzalesi L, Sanchez LJ, Susini T, Vezzosi V, Nori J (2012) Radial scar without associated atypical epithelial proliferation on image-guided 14-gauge needle core biopsy: analysis of 49 cases from a single-centre and review of the literature. Breast 21(2):159–164. doi:10.1016/j.breast.2011.09.005
Jacobs TW, Connolly JL, Schnitt SJ (2002) Nonmalignant lesions in breast core needle biopsies: to excise or not to excise? Am J Surg Pathol 26(9):1095–1110
Ingegnoli A, d’Aloia C, Frattaruolo A, Pallavera L, Martella E, Crisi G, Zompatori M (2010) Flat epithelial atypia and atypical ductal hyperplasia: carcinoma underestimation rate. Breast J 16(1):55–59. doi:10.1111/j.1524-4741.2009.00850.x
McGhan LJ, Pockaj BA, Wasif N, Giurescu ME, McCullough AE, Gray RJ (2012) Atypical ductal hyperplasia on core biopsy: an automatic trigger for excisional biopsy? Ann Surg Oncol 19(10):3264–3269. doi:10.1245/s10434-012-2575-0
Elsheikh TM, Silverman JF (2005) Follow-up surgical excision is indicated when breast core needle biopsies show atypical lobular hyperplasia or lobular carcinoma in situ: a correlative study of 33 patients with review of the literature. Am J Surg Pathol 29(4):534–543
Sohn V, Arthurs Z, Herbert G, Keylock J, Perry J, Eckert M, Fellabaum D, Smith D, Brown T (2007) Atypical ductal hyperplasia: improved accuracy with the 11-gauge vacuum-assisted versus the 14-gauge core biopsy needle. Ann Surg Oncol 14(9):2497–2501. doi:10.1245/s10434-007-9454-0
Niell B, Specht M, Gerade B, Rafferty E (2012) Is excisional biopsy required after a breast core biopsy yields lobular neoplasia? AJR Am J Roentgenol 199(4):929–935. doi:10.2214/AJR.11.8447
Nizri E, Schneebaum S, Klausner JM, Menes TS (2012) Current management practice of breast borderline lesions—need for further research and guidelines. Am J Surg 203(6):721–725. doi:10.1016/j.amjsurg.2011.06.052
Rajan S, Wason AM, Carder PJ (2011) Conservative management of screen-detected radial scars: role of mammotome excision. J Clin Pathol 64(1):65–68. doi:10.1136/jcp.2010.083485
Coopey SB, Mazzola E, Buckley JM, Sharko J, Belli AK, Kim EM, Polubriaginof F, Parmigiani G, Garber JE, Smith BL, Gadd MA, Specht MC, Guidi AJ, Roche CA, Hughes KS (2012) The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat 136(3):627–633. doi:10.1007/s10549-012-2318-8
Linda A, Zuiani C, Furlan A, Lorenzon M, Londero V, Girometti R, Bazzocchi M (2012) Nonsurgical management of high-risk lesions diagnosed at core needle biopsy: can malignancy be ruled out safely with breast MRI? AJR Am J Roentgenol 198(2):272–280. doi:10.2214/AJR.11.7040
Perfetto F, Fiorentino F, Urbano F, Silecchia R (2009) Adjunctive diagnostic value of MRI in the breast radial scar. Radiol Med (Torino) 114(5):757–770. doi:10.1007/s11547-009-0405-7
Cawson JN (2005) Can sonography be used to help differentiate between radial scars and breast cancers? Breast 14(5):352–359. doi:10.1016/j.breast.2005.01.003
Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ (1999) Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med 340(6):430–436. doi:10.1056/NEJM199902113400604
Sanders ME, Page DL, Simpson JF, Schuyler PA, Dale Plummer W, Dupont WD (2006) Interdependence of radial scar and proliferative disease with respect to invasive breast carcinoma risk in patients with benign breast biopsies. Cancer 106(7):1453–1461. doi:10.1002/cncr.21730
Berg JC, Visscher DW, Vierkant RA, Pankratz VS, Maloney SD, Lewis JT, Frost MH, Ghosh K, Degnim AC, Brandt KR, Vachon CM, Reynolds CA, Hartmann LC (2008) Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat 108(2):167–174. doi:10.1007/s10549-007-9605-9
Manfrin E, Remo A, Falsirollo F, Reghellin D, Bonetti F (2008) Risk of neoplastic transformation in asymptomatic radial scar. Analysis of 117 cases. Breast Cancer Res Treat 107(3):371–377. doi:10.1007/s10549-007-9569-9
Bunting DM, Steel JR, Holgate CS, Watkins RM (2011) Long term follow-up and risk of breast cancer after a radial scar or complex sclerosing lesion has been identified in a benign open breast biopsy. Eur J Surg Oncol 37(8):709–713. doi:10.1016/j.ejso.2011.04.011
Aroner SA, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Rosner BA, Hankinson SE, Tamimi RM (2013) Radial scars and subsequent breast cancer risk: results from the Nurses’ Health Studies. Breast Cancer Res Treat 139(1):277–285. doi:10.1007/s10549-013-2535-9
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N (1998) Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 90(18):1371–1388
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC (1999) The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes of raloxifene evaluation. JAMA 281(23):2189–2197
Vogel VG, Costantino JP, Wickerham DL, McCaskill-Stevens W, Clarfeld RB, Grant MD, Wolmark N (2010) Carcinoma in situ outcomes in National Surgical Adjuvant Breast and Bowel Project Breast Cancer Chemoprevention Trials. J Natl Cancer Inst Monogr 2010(41):181–186. doi:10.1093/jncimonographs/lgq041
Ropka ME, Keim J, Philbrick JT (2010) Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 28(18):3090–3095. doi:10.1200/JCO.2009.27.8077
Conflicts of interest
The authors have no conflicts of interest or financial disclosures to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miller, C.L., West, J.A., Bettini, A.C. et al. Surgical excision of radial scars diagnosed by core biopsy may help predict future risk of breast cancer. Breast Cancer Res Treat 145, 331–338 (2014). https://doi.org/10.1007/s10549-014-2958-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2958-y