Abstract
Purpose
Despite the recent expansion in the use of immunotherapy for many cancer types, it is still not a standard treatment for breast cancer. Identifying differences in the immune systems of breast cancer patients compared to healthy women might provide insight into potential targets for immunotherapy and thus may assist its clinical implementation.
Methods
Multi-colour flow cytometry was used to investigate myeloid and lymphoid populations in the peripheral blood of breast cancer patients (n = 40) and in the blood of healthy age-matched women (n = 25). We additionally performed functional testing to identify immune suppressive mechanisms used by circulating CD14+ myeloid cells from breast cancer patients.
Results
Our results show that breast cancer patients have significantly elevated frequencies of cells with the monocytic myeloid-derived suppressor cell (mMDSC) phenotype CD14+ HLA-DR−/low compared with healthy women (p < 0.01). We also observed higher levels of earlier differentiated T cells and correspondingly lower levels of T cells in later stages of differentiation (p < 0.05). These disease-associated differences could already be detected in early-stage breast cancer patients in stages 1 and 2 (n = 33 of 40) (p < 0.05). Levels of circulating T cells correlated with certain clinical features and with patient age (p < 0.05). Functional tests showed that CD14+ myeloid cells from breast cancer patients more potently suppressed autologous T cell proliferation than CD14+ cells from healthy women (p < 0.01). Subsequent investigation determined that suppression was mediated in part by reactive oxygen species, because inhibiting this pathway partially restored T cell proliferation (p < 0.01).
Conclusion
Our results highlight the potential importance of cells with mMDSC phenotypes in breast cancer, identifiable already at early stages of disease. This may provide a basis for identifying possible new therapeutic targets to enhance anti-cancer immunity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the leading causes of death in women worldwide [1], but despite steady advances in treatment and the promise of more effective immunotherapies, clinical outcomes remain suboptimal. In contrast to lung, melanoma and other types of cancer [2,3,4,5], immunomodulatory or cellular immunotherapy is not yet a routine form of treatment for breast cancer. Nonetheless, it is evident that the immune system does indeed play an important role in disease progression. This assertion is largely based on the results of studies which have shown a very close link between parameters of the immune system and the prognosis of breast cancer patients [6, 7]. Consequently, there is now growing interest in exploring the potential use of immunotherapy in treating breast cancer—early results from clinical trials evaluating immunotherapeutic agents such as vaccines and immune checkpoint inhibitors have shown promise in this approach [8,9,10]. Because breast cancer comprises a heterogeneous collection of diseases, identifying the patient groups which will benefit from particular forms of immunotherapy will be of key importance. Furthermore, identifying the barriers which reduce the efficacy of immunotherapy will be required to more accurately design effective treatment strategies. To this end, the use of blood-based biomarkers rather than tumour tissue biomarkers provides a less invasive approach with the additional advantage of allowing multiple sampling over time.
Interactions between the immune system and cancer can be complex and hard to define, resulting either in tumour suppression, tumour promotion, or both. The immune system is capable of recognising and combating cancer through effector cells such as cytotoxic T cells. This process may be sufficient to result in tumour elimination, but on the other hand it can select tumour cells which resist immune attack [11]. In the latter scenario, the immune system is not only ineffective at providing tumour protection, but may even contribute to disease progression. This is in part due to the ability of tumours to re-programme immune cells so that they suppress anti-tumour immune functions, for example in the case of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous group of myeloid cells which have been shown to impair anti-tumour immune responses and which commonly expand in response to pro-inflammatory signals [12]. They are characterised as myeloid cells of granulocytic and monocytic origin, although no unequivocal phenotype that can be used to definitively characterise MDSCs has yet been identified. Much effort is currently being expended in determining approaches to inhibit the suppressive activity of these cells, for example, using certain tyrosine kinase inhibitors [13]. One of the major mechanisms by which MDSCs suppress beneficial immune responses is by impairing T cell function through the release of reactive oxygen species, production of suppressive soluble molecules or through arginine starvation; these mechanisms could also be susceptible to therapeutic blockade to reduce suppressor function [14,15,16,17]. This would be desirable because high levels of MDSCs are associated with poor patient prognosis in a range of cancer types [18, 19]. Despite an increasing number of studies on the clinical relevance of MDSCs in human cancers including breast cancer [20,21,22,23], surprisingly there are only a few studies examining the clinical role of peripheral MDSCs of monocytic origin in breast cancer to date [23, 24].
In the present study, we assessed circulating populations of myeloid and lymphoid cells in female breast cancer patients, with particular emphasis on cells with monocytic MDSC-like (mMDSC) phenotypes. Due to medical advances, breast cancer is now commonly diagnosed at an early stage. As such we considered it important to additionally determine if alterations in the immune system occur in early disease development (stages 1 and 2 [25]). This may highlight which populations of immune cells could be targeted for effective immunotherapy in particular patient subgroups. To complement this observational approach, circulating myeloid cells were also examined for their ability to suppress T cell activation and proliferation. The aim of this study was to identify disease-associated alterations in breast cancer patients and to uncover suppressive mechanisms used by circulating myeloid cells, which together may provide valuable information for targeted immunotherapy approaches in future.
Materials and methods
Samples
Blood samples from 40 breast cancer patients (age range 36–81 years, median age 61) were recruited locally at the Tübingen University Women’s Hospital between 2014 and 2016. The cohort included 35 patients with primary tumours and five patients with metastatic disease. Patient tumours were classified according to TNM staging (tumour size (T), nodal status (N) and metastasis (M)). Blood was drawn upon diagnosis, prior to surgery and before receiving any treatment. Apart from the diagnosis of breast cancer, patients did not have any other serious health problems. Detailed characteristics of this patient cohort are summarised in Table 1. Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA-blood using Ficoll–Hypaque gradient centrifugation and stored in a viable state in liquid nitrogen. In addition, we included a control group of 25 age-matched healthy women (age range 36–84 years, median age 61). PBMCs of this control cohort were obtained from routine blood donations at the Tübingen University Hospital.
All patients gave their written informed consent for the storage and scientific analysis of their biomaterial. The use of the samples was approved by the University of Tübingen Ethics Committee (ethics approval number 626/2016BO2).
Immunophenotypic analysis of circulating blood myeloid cells and T cells
Flow cytometry was used to phenotype blood myeloid cells (including monocytes, monocytic MDSCs and dendritic cells (DCs)) and lymphoid populations (including differentiation stages of CD4+ and CD8+ T cells) as previously described [26] using the following antibody panels (online resource 1). For the gating of mMDSCs, lineage-negative events were selected before exclusion of CD14-negative cells. This population was then used to gate HLA-DR-positive or HLA-DR-negative events using an HLA-DR-negative internal reference population. To gate DCs, lineage-negative events were first gated, followed by CD14-negative and HLA-DR-positive cells. From this population, myeloid DCs (mDCs) were identified based on CD11c positivity, while plasmacytoid DCs (pDCs) were identified as CD123-positive cells. All gating steps, including those for T cells, are illustrated in online resource 2. For the establishment of antibody panels, fluorescence minus one controls were used. Due to the limited availability of patient material, we could not perform multiple testing of the same sample, but consistency in machine performance was achieved by using cytometer setup and tracking (CST) beads before and after each sample measurement. Furthermore, repeated measurements of the same batch of a biological control donor were used in each run to confirm consistency in measurement conditions.
T cell/monocyte co-culture suppression assays
The suppressive capacity of CD14+ myeloid cells from breast cancer patients on autologous proliferating T cells was assessed using monocytes isolated from whole PBMC by magnetic cell sorting with human CD14 MicroBeads (Miltenyi Biotech, Teterow, Germany). The isolated CD14+ monocytes were co-cultured with CD14-depleted PBMC at a ratio of 1:1.5 (CD14-depleted PBMC:monocytes) for 5 days in IMDM with GlutaMAX (Life Technologies, Darmstadt, Germany) containing 10% FCS (SERATEC, Göttingen, Germany). CD14-depleted PBMC without the addition of monocytes were included as a positive control. All experiments were performed in 96-well U-bottom plates (Greiner Bio-One, Frickenhausen, Germany) containing a total of 0.25 × 106 cells per well (i.e. 0.1 × 106 CD14-depleted PBMC and 0.15 × 106 monocytes to give a ratio of 1:1.5). In order to assess the degree of T cell proliferation, CD14-depleted PBMCs were stained with CFSE (Invitrogen, San Diego, USA) according to our previous protocol [27] but with the following modifications: cells were incubated with CFSE staining solution for 5 min at room temperature in the dark and then washed with 10 mL PB buffer (5% FCS in PBS) to stop the labelling reaction. T cells were activated with CD3/CD28 T cell activator Dynabeads (Invitrogen, San Diego, USA) (1.5 µL/well). The 1:1.5 ratio (CD14-depleted PBMC:monocytes) was chosen based on a prior study [28] and on preliminary experiments which showed a concentration-dependent relationship between CD14+ myeloid cells and T cell suppression. Following the 5-day culture period, flow cytometry was used to characterise cell phenotypes and to assess the extent of T cell proliferation. The following antibodies were used: CD3-A700 (BD Pharmingen, Heidelberg, Germany), CD4-APC (Milteny Biotech), CD8-Pacific Blue and CD14-APC-H7 (both from BD Pharmingen). Suppression assays were performed with five patient samples (four with primary early-stage disease, one metastatic) randomly selected from the patient cohort. All experiments were performed using triplicate cultures to ensure consistency in results.
To investigate mechanisms potentially responsible for suppression, co-cultures were treated with inhibitors targeting candidate pathways previously suggested to be involved in mediating immune suppression. These were the reactive oxygen species (ROS) inhibitor superoxide dismutase (200 IU/mL) (Sigma-Aldrich, Steinheim, Germany), anti-TGFβ antibody (10 μg/mL) (R&D Systems, Wiesbaden, Germany) and the STAT-3 (signal transducers and activator of transcription 3) inhibitor AG490 (10 μmol/L) (Sigma-Aldrich).
Flow cytometry data analysis
Flow cytometry data analysis was performed using FlowJo software version 10.07 (Tree Star, Ashland, USA). Events not part of the main acquisition population were first excluded using a time-versus-side scatter gate. This was followed by removing cell doublets and subsequently the exclusion of dead cells (EMA-positive events) and cell debris with the use of a morphological gate. This was followed by gating for specific populations of interest according to the gating strategies shown in online resources 2 and 3. For assessing T cell proliferation in suppression assays, in a first step CD14+ cells were gated out to avoid contamination of the CFSE signal. CD3+ events were gated which was followed by gating both CD4+ and CD8+ populations. An index of CFSE mean fluorescence intensity was created for CD4+ and CD8+ populations for each condition relative to the corresponding positive control in order to determine the relative degree of T cell proliferation between experimental conditions.
Statistical analysis
Statistical analyses were performed using SPSS version 20 (IBM, Ehningen, Germany) and GraphPad Prism 6 (GraphPad Software, San Diego, USA). To compare two independent groups, non-parametric Mann–Whitney U tests were used. To compare changes in the same sample under different experimental conditions, Wilcoxon matched-pair tests were used (these statistical tests included values obtained from biological replicates to consider biological variation and technical replicates to account for measurement error). Correlations were calculated using Spearman correlation analysis. A value of p < 0.05 was considered statistically significant. Because this was an exploratory study, we aimed to reduce the chance of obtaining false-negative results. As such statistical analyses were not corrected using the Bonferroni method.
Results
T cell phenotypes in peripheral blood are associated with certain clinical features of breast cancer patients
Using flow cytometry, we measured myeloid cell populations and a spectrum of T cell populations from early to late stages of differentiation in the peripheral blood of 40 breast cancer patients. We characterised myeloid cells including monocytes, mDCs and pDCs (16 populations) and lymphoid cells including effector and memory T cells in both the CD4+ and CD8+ compartments (62 populations). These populations were investigated for association with clinical features of the breast cancer cohort; tumour characteristics such as pathological tumour size (pT), tumour grade, HER2 status, oestrogen (ER) and progesterone (PR) receptor expression and patient age were considered. We saw a number of correlations between T cell distribution and breast cancer patient clinical features (selected examples are shown in Fig. 1). For example, patients with larger tumours (pT2 vs. pT1) tended to have higher levels of earlier differentiated CD4+ T cell populations (CD45RA+ CD95− CD27+ CD28+) (p < 0.01), while CD4+ phenotypes at later differentiation stages (CD95+) tended to be present at lower levels in these patients (p < 0.01) (Fig. 1a). In addition, we observed that a number of later differentiated populations of CD8+ T cells (CD57+ and CD45RA+ CD95+ CD27− CD28−) were negatively associated with hormone receptor expression (Fig. 1b). We furthermore found a number of inverse correlations between patient age and the level of CD8+ T cells including naïve CD8+ T cells (CD8+ CD45RA+ CD95− CD27+ CD28 +) (p = 0.0001) and central memory phenotypes (CD8+ CD95+ CD45RA− CD27+ CD28+ and CD8+ CD95+ CD45RA− not gated for CD27 and CD28 expression) (p = 0.0167 and p = 0.0329) (Fig. 1c). We did not find any relationship between age and tumour characteristics (pT, tumour grade, HER2, ER and PR), suggesting that the associations between them and leukocyte levels are independent of age.
Association between peripheral T cells and breast cancer clinical features. Multi-colour flow cytometry was used to analyse immune cell phenotypes in the peripheral blood of breast cancer patients (n = 40). We found correlations between circulating immune cells and breast cancer clinical parameters. a Association between earlier and later differentiated CD4+ T cells and pathological tumour size (pT). b Association between later differentiated CD8+ T cells and progesterone receptor (PR) expression on breast tumours. c CD8+ T cell phenotypes and association with patient age. pT pathological tumour size, PR progesterone receptor, TEMRA terminally differentiated effector memory cells re-expressing CD45RA (phenotype: CD45RA+ CD95+ CD27− CD28−), CM central memory T cells (phenotype: CD95+ CD45RA− CD27+ CD28+), Naïve naïve T cells (phenotype: CD45RA+ CD95− CD27+ CD28+)
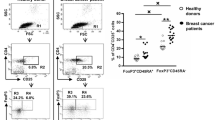
Breast cancer patients have elevated levels of CD14+ HLA-DR−/low MDSC phenotypes in the peripheral blood compared with healthy women
We next examined whether the levels of circulating lymphoid and myeloid cell populations differed between breast cancer patients and controls. We found that the frequencies of cells with the mMDSC phenotype CD14+ HLA-DR−/low was significantly higher in patients when assessing their levels as a percentage of total leukocytes (CD45+), and also relative to CD14+ cells (p = 0.0084 and p = 0.0105, respectively) (Fig. 2a). Importantly, when only looking at early-stage patients (stages 1 and 2, n = 33) in the cohort, we observed the same association (p = 0.0116 and p = 0.0151), indicating that the differences can already be detected at earlier breast cancer stages (online resource 4). These differences appeared to be specific for cells with a suppressor phenotype; we did not detect differences in the levels of CD14+ monocytes between breast cancer patients and healthy women (Fig. 2a, right panel), nor did we observe differences in mDCs or pDCs (Fig. 2b).
Frequencies of immune cell populations in breast cancer patients and healthy women. PBMCs from 40 breast cancer patients and 25 healthy age-matched control women were stained with panels of antibodies for lymphoid and myeloid cells and their levels assessed using multi-colour flow cytometry. a Frequencies of cells with the mMDSC phenotype CD14+ HLA-DR−/low (left-hand panel) and of CD14+ cells (right-hand panel) within CD45+ cells. b Frequencies of circulating myeloid dendritic cells (mDCs, left-hand panel) and plasmacytoid DCs (pDCs, right-hand panel) within CD45+ cells. c Levels of circulating CD4+ and CD8+ T cells as percentages of CD3+ cells. d Frequencies of earlier differentiated CD4+ T cells expressing CD45RA within CD3+ cells (left-hand panel); frequencies of later differentiated (CD45RA−) CD8+ CM cells within CD3+ cells (right-hand panel). BC breast cancer, PBMCs peripheral blood mononuclear cells, mMDSCs monocytic MDSCs, mDCs myeloid dendritic cells, pDCs plasmacytoid dendritic cells, CM central memory T cells
We also observed that populations of lymphoid cells were present at different levels between patients and controls (selected results are shown in Fig. 2). While the frequencies of circulating CD4+ and CD8+ T cells showed no difference between breast cancer patients and healthy women (Fig. 2c), the relative frequencies of several earlier differentiated T cell populations (CD4+ CD45RA+ CD95+ CD27+ CD28+) were elevated in breast cancer patients (p = 0.0076), which was again also true when only considering early-stage patients (p = 0.0026). In contrast, later differentiated T cells lacking the expression of CD45RA (e.g. central memory cells (CD8+ CD95+ CD45RA− CD27+ CD28+)) tended to be lower in breast cancer patients than healthy women (p = 0.0466) (Fig. 2d).
Circulating CD14+ myeloid cells from breast cancer patients suppress the proliferation of autologous T cells
Our results revealed that within CD14+ monocytes, cells with mMDSC phenotypes (CD14+ HLA-DR−/low) were elevated in breast cancer patients. Thus in order to model immune suppression that these cells may exert in vivo, we compared the suppressive potential of equivalent numbers of isolated CD14+ cells between breast cancer patients and healthy women. Because CD14+ HLA-DR−/low mMDSCs, but not the total levels of CD14+ monocytes, were elevated in breast cancer patients, we asked whether these cells with an mMDSC phenotype from breast cancer patients had suppressive properties.
To determine the suppressive capacity of circulating CD14+ HLA-DR−/low mMDSCs from early-stage breast cancer patients, we co-cultured isolated CD14+ cells with autologous CD14-depleted PBMCs. PBMCs were labelled with CFSE and stimulated with CD3/CD28 beads, with the degree of proliferation by CD4+ and CD8+ T cells measured by flow cytometry after 5 days of culture. We observed potent and consistent suppression of CD4+ and CD8+ T cell proliferation by CD14+ myeloid cells from breast cancer patients (n = 5) (p < 0.0001) (Fig. 3a). In preliminary experiments, we observed a concentration-dependent association between isolated CD14+ cells and T cell proliferation (data not shown). To investigate whether this suppressive capacity by circulating myeloid cells was specific to breast cancer patients, we tested the suppressive capacity of myeloid cells from healthy age-matched women (n = 4). We found that myeloid cells from healthy women could also suppress proliferating T cells, but the suppressive capacity was weaker when compared to breast cancer patients (p = 0.0037 for CD8+; trend for CD4+) (Fig. 3a).
Suppressive capacity of circulating CD14+ myeloid cells from breast cancer patients. Isolated CD14+ myeloid cells from five breast cancer patients were cultured with autologous CD14-depleted PBMCs labelled with CFSE and stimulated with CD3/CD28 beads. Inhibitors against TGFβ (10 μg/mL), reactive oxygen species (ROS) (SOD; superoxide dismutase (200 IU/mL)) and STAT-3 (AG490, (10 μmol/L)) were added to the cultures to identify the mechanism(s) responsible for the ability of CD14+ myeloid cells to suppress autologous T cells. After 5 days of culture T cell proliferation was measured by flow cytometry. a Suppressive capacity of CD14+ myeloid cells from breast cancer patients (n = 5) and healthy women (n = 4) on autologous CD4+ and CD8+ T cells (****p < 0.0001, compared to control without monocytes). b Influence of inhibitors targeting TGFβ, STAT-3 and ROS on CD14+ -mediated T cell suppression in breast cancer patients (****p < 0.0001; ***p < 0.001; **p < 0.01, compared to untreated co-culture (w/o)). c Effect on T cell suppression by inhibiting ROS compared with targeting TGFβ or STAT-3. BC breast cancer, Control no monocytes added, w/o without treatment, ROS reactive oxygen species, TGFβ-Ab neutralising anti-TGFβ-antibody, STAT-3 signal transducer and activator of transcription 3
Circulating CD14+ myeloid cells from breast cancer patients suppress immune responses via reactive oxygen species
To investigate the mechanism(s) potentially responsible for the suppressive capacity of breast cancer CD14+ myeloid cells, we treated cells in the model previously used to investigate immune suppression with inhibitors targeting different myeloid-suppressor pathways, namely TGFβ (inhibitor: neutralising antibody), ROS (inhibitor: superoxide dismutase, SOD) and STAT-3 (inhibitor: AG490). We found that inhibition of ROS via SOD partially restored T cell proliferation (p < 0.0001 for CD8+ and p = 0.0002 for CD4+), while we also observed weak but statistically significant restoration for anti-TGFβ (p = 0.0067 for CD8+; CD4+ not significant) and AG490 (p = 0.0009 for CD8+ and p = 0.0002 for CD4+) (Fig. 3b). Compared with untreated cultures, those treated with SOD restored CD8+ T cell proliferation by 131% and CD4 73% on average. Treatment with anti-TGFβ and AG490 only led to a 34% (CD8) and 11% (CD4) and 44% (CD8) and 31% (CD4) proliferation increase compared with untreated cultures, respectively (Fig. 3c).
Discussion
Compared with some other cancer types [2,3,4,5], immunotherapy is not yet a routine form of treatment for breast cancer. To gain an indication of which immune cells could be targeted for effective immunotherapy, we considered it an important first step to identify possible alterations in the immune system of breast cancer patients compared with healthy age-matched women. Characterising such “signatures” of immune alteration on both a phenotypical and functional level might provide a means of identifying potential targets for immunotherapy. Due to public health campaigns and medical advances, breast cancer is now typically diagnosed at an early stage of disease. To account for this, we additionally examined if immune perturbations occur early in disease development by comparing the immune signatures between healthy women and early-stage breast cancer patients. To date, studies attempting to identify changes in the immune system in exclusively early-stage breast cancer are rare [29, 30].
Our results reveal that cells with the mMDSC phenotype CD14+ HLA-DR−/low are present at significantly higher frequencies in breast cancer patients than in healthy individuals. Despite the recent expansion in the study of MDSCs in later stage cancer patients [23, 24, 26, 31], there are no studies which have so far assessed whether these cells are clinically relevant in early-stage breast cancer. Thus this study is, to the best of our knowledge, the first to show that CD14+ HLA-DR−/low mMDSCs are elevated early in breast cancer progression (clinical stages 1 and 2). This finding suggests that immune suppression via elevated mMDSCs occurs early in the development of breast cancer, which may help protect tumour cells from immune attack. Interestingly, we found that total monocyte frequencies did not differ between breast cancer and healthy women, indicating the pool of CD14+ myeloid cells in breast cancer to be selectively driven towards MDSC differentiation, thereby leaving other populations of myeloid cells unaffected. Indeed, we did not observe levels of other myeloid cells, such as mDCs, to be altered in breast cancer. Collectively, our findings indicate the importance of mMDSCs (CD14+ HLA-DR−/low), already in earlier stages of breast cancer. Pending clinical follow-up will reveal if these cells can be used to predict patient survival as previously shown in other cancer types [18].
Further to our findings on mMDSCs, we also found elevated levels of early differentiated T cells in breast cancer patients compared with healthy women. Higher levels of these cells indicates potential for the immune system to recognise novel or newly arising tumour antigens present in the tumour and thus mount an immune response against tumour cells. This anti-tumour potential might be counter-balanced by our finding of elevated mMDSCs, which may suppress the activity of beneficial T cells, for example by preventing their differentiation. Indeed, we found elevated levels of both mMDSCs as well as more immature T cells expressing CD45RA (which are not TEMRA cells) in breast cancer patients. This association suggests that mMDSCs may impair the maturation of T cells in cancer patients. This notion is supported in our prior study where patients with tumour antigen-reactive T cells experienced greater clinical benefit if they also had low MDSC levels [26]. In contrast to T cells in the periphery, T cells infiltrating the tumour (TILs) have frequently been shown to be associated with favourable prognoses in breast cancer [7, 29, 32, 33]. The relationship between peripheral and intra-tumoural TILs in breast cancer is not yet known, and thus we cannot judge whether our observation of altered levels of circulating T cells in breast cancer patients relates to the presence of T cells in the tumour. It is conceivable that higher levels of T cells in the blood may act to support the maintenance of intra-tumoural T cells, but such associations remain to be confirmed. It should certainly be considered that immune cells in the periphery may play different roles to those infiltrating the tumour, although there may be more transit in and out of tumours than previously appreciated [34].
Having observed elevated mMDSCs in patients, we then investigated whether CD14+ myeloid cells from breast cancer patients or healthy women are immunosuppressive. To better mimic immune suppression that these cells may exert in vivo, we compared the suppressive potential of equivalent numbers of isolated CD14+ myeloid cells between breast cancer patients and controls. We also aimed to determine potential mechanisms responsible for any immune suppression observed by inhibiting three of the pathways previously shown to be used by MDSCs in other cancer types [35,36,37], namely TGFβ, STAT-3 and ROS. Our results revealed that CD14+ myeloid cells from breast cancer patients are potent suppressors of autologous T cell proliferation, already in patients with early disease. Interestingly, CD14+ myeloid cells from healthy counterparts were also able to suppress autologous T cells, but not as profoundly as myeloid cells from breast cancer patients. This likely reflects normal levels of peripheral tolerance governed by mMDSCs in healthy individuals. The observation that myeloid cells in breast cancer patients more potently suppress immune responses may reflect our finding that cells with the CD14+ HLA-DR−/low mMDSC phenotype are present at higher levels in these patients. The potential association between CD14+ HLA-DR−/low mMDSCs and immune suppression is supported by other studies showing the suppressive features of this particular mMDSC phenotype in a number of other cancer types [28, 38,39,40]. However, as far as we are aware, this is the first study proposing their suppressive capacity in breast cancer patients. To more conclusively demonstrate the suppressive function of CD14+ HLA-DR−/low mMDSCs, future studies should additionally examine their suppressive function in an isolated system only containing these cells and activated CD4+/CD8+ T cells. This approach would isolate potential effects due to other cells present in circulating blood such as Tregs and other suppressive myeloid populations. However, while establishing the conditions for this study we observed a concentration-dependent association between T cell suppression and isolated CD14+ myeloid cells in the presence of all circulating mononuclear cells, suggesting the effects of other potentially suppressive cell types to be negligible. We chose to use a model including all circulating mononuclear cells to avoid working in a more artificial model with isolated cell types. We further found that inhibiting ROS partially reduced the suppressive effect of CD14+ myeloid cells from breast cancer patients, suggesting ROS as one of the suppressive mechanisms used in breast cancer mMDSC-mediated suppression of T cells, as in other cancer types [28, 41, 42]. However, inhibiting ROS could not completely restore T cell proliferation, suggesting that a combination of other suppressive mechanisms may be simultaneously used by these cells to suppress the immune system, or that ROS inhibition was incomplete. Further functional assays exploring a wider spectrum of candidate suppressive pathways may reveal more information regarding the mechanisms used by these cells to exert immune suppression in breast cancer.
This study shows that systemic immune alterations occur early in the development of breast cancer and that the identified differences between healthy women and breast cancer patients may serve as immunotherapy targets in future. Our results encourage the potential use of strategies targeting CD14+ HLA-DR−/low mMDSCs in breast cancer such as antioxidant treatment strategies. However, developing a more detailed picture of interactions between disease-associated factors and their effect on different immune cell populations (particularly MDSCs) will be crucial for the development of effective immunotherapeutic approaches.
References
Breast cancer estimated incidence, mortality and prevalence worldwide in 2012. 2012. http://globocan.iarc.fr/old/FactSheets/cancers/breast-new.asp
Aerts JG, Hegmans JP (2013) Tumor-specific cytotoxic T cells are crucial for efficacy of immunomodulatory antibodies in patients with lung cancer. Cancer Res 73(8):2381–2388. https://doi.org/10.1158/0008-5472.CAN-12-3932
Malas S, Harrasser M, Lacy KE, Karagiannis SN (2014) Antibody therapies for melanoma: new and emerging opportunities to activate immunity (Review). Oncol Rep 32(3):875–886. https://doi.org/10.3892/or.2014.3275
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, Investigators K- (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372(21):2018–2028. https://doi.org/10.1056/NEJMoa1501824
Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, Margolin KA, Plimack ER, Lambert AM, Waxman IM, Hammers HJ (2015) Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 33(13):1430–1437. https://doi.org/10.1200/JCO.2014.59.0703
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, de Azambuja E, Quinaux E, Di Leo A, Michiels S, Piccart MJ, Sotiriou C (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: big 02-98. J Clin Oncol 31(7):860–867. https://doi.org/10.1200/JCO.2011.41.0902
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, Wolff AC, Wood WC, Davidson NE, Sledge GW, Sparano JA, Badve SS (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: eCOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966. https://doi.org/10.1200/JCO.2013.55.0491
Mittendorf EA, Clifton GT, Holmes JP, Schneble E, van Echo D, Ponniah S, and Peoples GE (2014) Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol 25(9):1735–1742. https://doi.org/10.1093/annonc/mdu211
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34(21):2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Emens L, Braiteh F, Cassier P, DeLord J, Eder J, Shen X, Xiao Y, Wang Y, Hegde P, Chen D, Krop I (2015) Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. Cancer Res. https://doi.org/10.1158/1538-7445
Domschke C, Schneeweiss A, Stefanovic S, Wallwiener M, Heil J, Rom J, Sohn C, Beckhove P, Schuetz F (2016) Cellular immune responses and immune escape mechanisms in breast cancer: determinants of immunotherapy. Breast Care (Basel). 11(2):102–107. https://doi.org/10.1159/000446061
Baniyash M (2016) Myeloid-derived suppressor cells as intruders and targets: clinical implications in cancer therapy. Cancer Immunol Immunother 65(7):857–867. https://doi.org/10.1007/s00262-016-1849-y
Heine A, Schilling J, Grunwald B, Kruger A, Gevensleben H, Held SA, Garbi N, Kurts C, Brossart P, Knolle P, Diehl L, Hochst B (2016) The induction of human myeloid derived suppressor cells through hepatic stellate cells is dose-dependently inhibited by the tyrosine kinase inhibitors nilotinib, dasatinib and sorafenib, but not sunitinib. Cancer Immunol Immunother 65(3):273–282. https://doi.org/10.1007/s00262-015-1790-5
Rodriguez PC, Ochoa AC (2008) Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev 222:180–191. https://doi.org/10.1111/j.1600-065X.2008.00608.x
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Yoshimura A, Muto G (2011) TGF-beta function in immune suppression. Curr Top Microbiol Immunol 350:127–147. https://doi.org/10.1007/82_2010_87
Kortylewski M, Yu H (2008) Role of Stat3 in suppressing anti-tumor immunity. Curr Opin Immunol 20(2):228–233. https://doi.org/10.1016/j.coi.2008.03.010
Shipp C, Speigl L, Janssen N, Martens A, Pawelec G (2016) A clinical and biological perspective of human myeloid-derived suppressor cells in cancer. Cell Mol Life Sci 73(21):4043–4061. https://doi.org/10.1007/s00018-016-2278-y
Kalathil SG, Thanavala Y (2016) High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother 65(7):813–819. https://doi.org/10.1007/s00262-016-1810-0
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ (2009) Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 58(1):49–59. https://doi.org/10.1007/s00262-008-0523-4
Choi J, Suh B, Ahn YO, Kim TM, Lee JO, Lee SH, Heo DS (2012) CD15 +/CD16low human granulocytes from terminal cancer patients: granulocytic myeloid-derived suppressor cells that have suppressive function. Tumour Biol 33(1):121–129. https://doi.org/10.1007/s13277-011-0254-6
Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X (2014) Noncanonical NF-kappaB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer. J Immunol. 193(5):2574–2586. https://doi.org/10.4049/jimmunol.1400833
Toor SM, Syed Khaja AS, El Salhat H, Faour I, Kanbar J, Quadri AA, Albashir M, Elkord E (2017) Myeloid cells in circulation and tumor microenvironment of breast cancer patients. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-017-1977-z
Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, Jernstrom H, Janols H, Wullt M, Bredberg A, Ryden L, Leandersson K (2015) Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE 10(5):e0127028. https://doi.org/10.1371/journal.pone.0127028
Number stages of breast cancer. 2014 http://www.cancerresearchuk.org/about-cancer/breast-cancer/stages-types-grades/number-stages Accessed 2017 June
Bailur JK, Gueckel B, Derhovanessian E, Pawelec G (2015) Presence of circulating Her2-reactive CD8 + T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res 17:34. https://doi.org/10.1186/s13058-015-0541-z
Larbi A, Cabreiro F, Zelba H, Marthandan S, Combet E, Friguet B, Petropoulos I, Barnett Y, Pawelec G (2010) Reduced oxygen tension results in reduced human T cell proliferation and increased intracellular oxidative damage and susceptibility to apoptosis upon activation. Free Radic Biol Med. 48(1):26–34. https://doi.org/10.1016/j.freeradbiomed.2009.09.025
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 70(11):4335–4345. https://doi.org/10.1158/0008-5472.CAN-09-3767
Boniface JD, Poschke I, Mao Y, Kiessling R (2012) Tumor-dependent down-regulation of the zeta-chain in T-cells is detectable in early breast cancer and correlates with immune cell function. Int J Cancer 131(1):129–139. https://doi.org/10.1002/ijc.26355
Poschke I, De Boniface J, Mao Y, Kiessling R (2012) Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int J Cancer 131(7):1611–1620. https://doi.org/10.1002/ijc.27410
Su Z, Ni P, She P, Liu Y, Richard SA, Xu W, Zhu H, Wang J (2017) Bio-HMGB1 from breast cancer contributes to M-MDSC differentiation from bone marrow progenitor cells and facilitates conversion of monocytes into MDSC-like cells. Cancer Immunol Immunother 66(3):391–401. https://doi.org/10.1007/s00262-016-1942-2
Ingold Heppner B, Untch M, Denkert C, Pfitzner BM, Lederer B, Schmitt W, Eidtmann H, Fasching PA, Tesch H, Solbach C, Rezai M, Zahm DM, Holms F, Glados M, Krabisch P, Heck E, Ober A, Lorenz P, Diebold K, Habeck JO, Loibl S (2016) Tumor-infiltrating lymphocytes: a predictive and prognostic biomarker in neoadjuvant-treated HER2-positive breast cancer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-15-2338
Menard S, Tomasic G, Casalini P, Balsari A, Pilotti S, Cascinelli N, Salvadori B, Colnaghi MI, Rilke F (1997) Lymphoid infiltration as a prognostic variable for early-onset breast carcinomas. Clin Cancer Res. 3(5):817–819
Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA (2016) Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med 22(4):433–438. https://doi.org/10.1038/nm.4051
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25(18):2546–2553. https://doi.org/10.1200/JCO.2006.08.5829
Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A (2014) Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res 20(15):4096–4106. https://doi.org/10.1158/1078-0432.CCR-14-0635
Vasquez-Dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, Pardoll D, Kim Y (2013) STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 123(4):1580–1589. https://doi.org/10.1172/JCI60083
Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA (2012) Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 18(19):5212–5223. https://doi.org/10.1158/1078-0432.CCR-12-1108
Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB (2010) Immunosuppressive CD14 + HLA-DRlow/- monocytes in prostate cancer. Prostate 70(4):443–455. https://doi.org/10.1002/pros.21078
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S (2013) Increase in CD14 + HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 62(8):1421–1430. https://doi.org/10.1007/s00262-013-1447-1
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, Yancey D, Dahm P, Vieweg J (2008) Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 14(24):8270–8278. https://doi.org/10.1158/1078-0432.CCR-08-0165
Mao Y, Poschke I, Wennerberg E, Pico de Coana Y, Egyhazi Brage S, Schultz I, Hansson J, Masucci G, Lundqvist A, Kiessling R (2013) Melanoma-educated CD14 + cells acquire a myeloid-derived suppressor cell phenotype through COX-2-dependent mechanisms. Cancer Res 73(13):3877–3887. https://doi.org/10.1158/0008-5472.CAN-12-4115
Acknowledgements
This work was supported by a grant from the German Research Foundation (DFG Pa 361/22-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Speigl, L., Burow, H., Bailur, J.K. et al. CD14+ HLA-DR−/low MDSCs are elevated in the periphery of early-stage breast cancer patients and suppress autologous T cell proliferation. Breast Cancer Res Treat 168, 401–411 (2018). https://doi.org/10.1007/s10549-017-4594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4594-9