Abstract
Purpose
Though advanced and metastatic epidermal growth factor receptor 2 (HER2)-positive disease is not curable, a small proportion of patients with HER2-positive metastatic breast cancer remain in prolonged complete remission with anti-HER2 treatment. We hypothesized that some cases of HER2-positive metastatic breast cancer may be curable. In this large, multicenter retrospective study, we aimed to assess the long-term outcomes for patients with a durable response to trastuzumab.
Methods
We retrospectively evaluated the data of patients diagnosed with HER2-positive metastatic breast cancer who received trastuzumab for more than 2 years as the first-line treatment. Patients diagnosed between April 1, 2001 and December 31, 2014 at 19 institutions in Japan were included in the analysis. From 124 potential subjects, 16 were excluded and 108 were evaluated.
Results
The median follow-up length was 7.7 years. Disease progression occurred in 44/108 (40.7%) patients and 13/108 (12%) patients died. The median progression-free survival was 11.2 years, and as more than 80% of patients were alive 10 years after metastatic breast cancer diagnosis. Of the 108 patients, 57 achieved a clinical complete response. Trastuzumab therapy was interrupted for 27 (47.4%) of these patients (based on the doctor’s recommendation for 19 patients, owing to adverse events for 4 patients, owing to unknown reasons for 3 patients, and at the request of 1 patient). Disease progression occurred in 4 of the 27 patients after the interruption of trastuzumab treatment. The median duration of trastuzumab therapy for all 27 patients was 5.1 years (0.9–9.3 years).
Conclusion
We found that some patients showed no evidence of disease after the interruption of trastuzumab therapy. Discontinuation of maintenance trastuzumab in this patient population after a limited time should be explored cautiously while awaiting a global collaborative effort for a randomized trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human epidermal growth receptor 2 (HER2) gene is upregulated in approximately 15% of breast cancer cases [1] and adjuvant trastuzumab can improve overall survival (OS) in this patient population. Current guidelines for HER2-positive metastatic breast cancer recommend indefinite uninterrupted continuation of HER2-targeted therapy in the absence of toxicity [2, 3]. Though advanced and metastatic HER2-positive disease is not curable, a small proportion of patients with HER2-positive metastatic breast cancer remain in prolonged complete remission with anti-HER2 treatment [4, 5]. The frequency of durable remission in this patient population is unknown; however, some patients remained alive for several years according to trastuzumab treatment, suggesting that a cure, or at least long-term control, may be possible.
The CLEOPATRA trial was a randomized phase III study that examined the 3-agent combination of trastuzumab, pertuzumab, and docetaxel compared with the combination of trastuzumab and docetaxel for patients with HER2-positive metastatic breast cancer. The addition of pertuzumab to trastuzumab and docetaxel resulted in a median OS of 56 months compared with 41 months in patients who received trastuzumab and docetaxel [6]. Sixty-seven (16.6%) of the 402 patients in the trial continued pertuzumab and trastuzumab treatment with a median follow-up of 49.5 months (range 0–75 months), while 6 (1.5%) patients continued trastuzumab treatment with a median follow-up 50.6 months (range 0-69 months) [6].
Studies evaluating long-term survival on trastuzumab maintenance therapy are limited, and the factors associated with long-term survival among patients with HER2-positive metastatic breast cancer remain unknown. A few published studies and case reports in the literature have, because of late relapses, cautioned oncologists against discontinuing maintenance trastuzumab even after 2–3 years in remission [7, 8]. With the observation of prolonged remission in patients with HER2-positive metastatic breast cancer, the optimal duration of maintenance trastuzumab has become an important and relevant clinical question worldwide.
We hypothesized that some cases of HER2-positive metastatic breast cancer may be curable and initiated a large, multicenter retrospective study to assess the long-term outcomes for patients with a durable response to trastuzumab. In addition, factors that could be associated with long-term tumor remission under trastuzumab were identified through exploratory analysis.
Methods
Participants
We retrospectively evaluated patients diagnosed with HER2-positive metastatic breast cancer who received trastuzumab as the first-line treatment for more than 2 years. Patients diagnosed with metastatic breast cancer between April 1, 2001 and December 31, 2014 at 19 institutions in Japan were included in this study. The presence of distant metastases was defined based on appropriate imaging and/or histological findings. The study was organized by the Breast cancer study group of the Japan Clinical Oncology Group, which includes 40 clinical institutions in Japan. The 19 participating institutions provided datasets for patients with HER2 positive metastatic breast cancer without identifying information. The study was coordinated by the Tokai University School of Medicine and was approved by the institutional review board at the Tokai University School of Medicine, which waived the need for written informed consent because of the retrospective nature of the study.
We collected data for 124 patients diagnosed with metastatic breast cancer. Sixteen patients were excluded because (i) progression-free survival (PFS) was less than 2 years with trastuzumab (n = 13) or (ii) no survival data were available (n = 3). Thus, 108 patients were evaluated.
Staging and pathology review
Metastatic disease was confirmed by histopathological analysis if specimens were available. Primary tumors were histologically classified using the World Health Organization criteria [9]. Based on imaging data, clinical complete response (cCR) was defined according to the decision of the physician at each institution. In some patients, metastatic sites were removed by surgery or were treated with radiation therapy. A patient was considered to have HER2-positive disease if the primary tumor or a metastatic tumor had a score of 3+ on HER2 immunohistochemical analysis or if fluorescence in situ hybridization revealed amplification of the HER2 gene. A patient was considered to have hormone receptor-positive disease if at least 1% of the tumor cells were positively stained for the estrogen receptor (ER) or the progesterone receptor on immunohistochemical analysis.
Statistical methods
Median values and standard deviations were used to summarize the age at diagnosis. Frequencies and proportions were used to present the categorical clinical characteristics. PFS was defined as the time interval from the diagnosis of metastatic breast cancer until the first progression of the cancer or the last follow-up date, whichever occurred first. OS was defined as the length of time from the diagnosis of metastases to death or to the last follow-up date if patients were alive at the last follow-up. Data on patients who were alive at the last follow-up were censored in the OS analyses. PFS and OS were estimated using the Kaplan–Meier product limit method. Kaplan–Meier curves were used to present PFS and OS for patients in each group. Log-rank was used to assess the effects of treatment and other predictive factors. The analyses were performed using SPSS ver. 23 (SPSS Inc., Chicago, IL). Two-sided p values of <0.05 were considered statistically significant.
Results
Clinical characteristics
Overall, 108 patients were diagnosed with HER2-positive metastatic breast cancer and received trastuzumab for more than 2 years as the first-line treatment (Table 1). The median follow-up period was 7.7 years. Disease progression occurred in 44/108 (40.7%) patients and 13/108 (12%) patients died. Twelve (11%) patients had local metastases only, while 96 (89%) had distant metastases. The median PFS was 11.2 years (Fig. 1a), and the median OS was not calculated, as more than 80% of patients were alive 10 years after the diagnosis metastatic breast cancer (Fig. 1b). Of the 108 patients, 34 (31.5%) had ER-positive primary tumors, while 66 (61.1%) had ER-negative tumors. Metastasis was confirmed pathologically for 36 patients (48.5%) in this dataset.
Clinical complete response
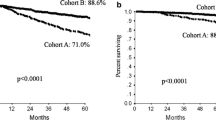
Among the 108 patients diagnosed with HER2-positive metastatic breast cancer, 57 (52.8%) had cCR, which was confirmed by imaging for 49 patients, surgery for 5 patients, and radiation therapy for 3 patients. Patients with cCR have a better prognosis than those with partial response or stable disease (p = 0.003) (Fig. 2a). The characteristics of the patients with cCR are shown in Table 2. Of these 57 patients, 13 (22.8%) had ER-positive primary tumors, while 37 (64.9%) had ER-negative tumors. Among the patients with cCR, 34 (59.6%) were confirmed pathologically. Trastuzumab therapy was interrupted for 27 (47.4%) patients before disease progression (19 patients at the recommendation of the doctor, 4 patients owing to adverse events, 3 patients for unknown reasons, and at the request of 1 patient). The median duration of trastuzumab therapy for these 27 patients was 5.1 years (0.9-9.3 years). Patients for whom trastuzumab therapy was interrupted before disease progression were not significantly different in terms of PFS from those who continued the trastuzumab therapy (Fig. 2b). Figure 3 shows the clinical course for patients with interrupted trastuzumab therapy before progression. The blue bar shows the duration of trastuzumab therapy and the orange bar shows the period after the interruption of therapy. Four patients experienced disease progression after the interruption of trastuzumab therapy, and 21 patients did not. Two patients did not have date of interrupted trastuzumab in this dataset. The patients were followed-up for a median duration of 3.9 years (0–8.9 years) after the interruption of trastuzumab therapy.
Progression-free survival after diagnosis of metastatic disease according to response. a Progression-free survival after diagnosis of metastatic disease according to response in all patients. b Progression-free survival after diagnosis of metastatic disease according to continuation or interruption of trastuzumab in patients with clinical complete response. CR complete response, PR partial response, SD stable disease
Discussion
In this large multicenter cohort of durable cCR patients with HER2-positive metastatic breast cancer treated with trastuzumab therapy, some patients showed no evidence of disease after the interruption of trastuzumab therapy. In patients who received trastuzumab as the first-line therapy for more than 2 years, the median PFS was 11.2 years and 80% of patients were alive after 10 years.
Trastuzumab has been widely used for the treatment of patients with HER2-positive metastatic breast cancer for over 15 years, with significant benefits to survival [10]. Data from neoadjuvant studies show that a pathological complete response (CR) to neoadjuvant therapy occurs in 25–60% of patients [11]. These patients have the potential to be cured with only chemotherapy and anti-HER2 therapy. A small proportion of patients with HER2-positive metastatic breast cancer remain in prolonged complete remission with trastuzumab maintenance therapy. Gullo et al. [12] reported on 84 patients with HER2-positive metastatic breast cancer treated from May 2000 to March 2007. Thirteen (15%) achieved CR. As part of different institution practice, patients in Dublin continued taking trastuzumab until disease progression or for at least 5 years. In Milan trastuzumab was generally interrupted in CR patients within 2 years of achieving remission. All eight durable complete response (DCR) patients received trastuzumab together with their first chemotherapy for metastatic disease. The median duration of trastuzumab treatment for CR patients was 67 months (range 49 to 107+) in Dublin and 14 months (range 5–26) in Milan. The frequency of CR was very similar in the two countries (Milan 16% and Dublin 15%) [12]. These data indicate the potential of a cure in some subset of patients with HER2-positive metastases.
The optimal duration of trastuzumab treatment for patients with HER2-positive metastatic breast cancer who achieve CR is still unknown. Duration of trastuzumab treatment was not limited by national health insurance in Japan. In our study, trastuzumab therapy was interrupted for 27 (47.4%) patients after a median of 5.1 years (0.9–9.3 years). Of the 27 patients, 23 patients remain alive, continuously cancer free. In the Milan study, trastuzumab was interrupted in CR patients within 2 years of remission. After a median follow-up of 7 years (range 2.5–11.8 years), six of the 13 patients who achieved CR remain alive and have been continuously cancer free. Two other patients are alive and have been continuously free of metastatic cancer at 107 and 105 months, respectively, having received curative loco-regional therapy for new primary breast cancers. The remaining five patients have developed relapsed metastatic breast cancer, two while on maintenance trastuzumab [12]. In 2014, Witzel et al. [7] used data from the HER-OS registry to identify a cohort of 17 patients who benefited from a longer duration of trastuzumab maintenance therapy compared with interrupted trastuzumab. However, these 17 patients (6.4%) received lower doses of trastuzumab owing to therapy interruption, mostly because of other illnesses. The most frequent reasons for trastuzumab interruption were the patient’s wish (1.5%) or adverse cardiac events (1.1%).
Haq et al. [13] reported the results of a survey targeting oncologists. Of the 201 oncologists contacted, 47 responded to the online questionnaire by February 2015 (overall response rate of 23.4%). Regarding the duration of trastuzumab maintenance therapy in the metastatic breast cancer setting, 78% of respondents (n = 32) reported continuing trastuzumab until disease progression, while 4.9% (n = 2) continued trastuzumab until the development of side effects. Another 4.9% of respondents (n = 2) reported continuing trastuzumab for 2 years, and 12.2% (n = 5) reported continuing it indefinitely. Overall, 82.9% of respondents (n = 34) reported continuing trastuzumab till disease progression or the development of side effects in accordance with current guidelines [13]. In our study, trastuzumab therapy was interrupted based on the doctor’s recommendation for 19 patients, owing to adverse events for 4 patients, and upon request for one patient. The therapy was interrupted for unknown reasons for three patients. Of the 27 patients for whom trastuzumab therapy was interrupted, the median duration of the therapy was 5.1 years (0.9–9.3 years). Although some institutional guidelines [12] advocate 5 years of maintenance trastuzumab therapy, the effectiveness of the approach is still unknown. Long-term use of trastuzumab raises concerns about cardiotoxicity. An early pivotal trial showed a high incidence of cardiac events under trastuzumab, especially when combined with anthracyclines [14]. These adverse effects were mainly reversible. In studies of trastuzumab treatment beyond progression, cardiac events are uncommon and mostly asymptomatic [15, 16]. Only four patients discontinued trastuzumab because of adverse events in our study.
Data characterizing patients with HER2-positive metastatic breast cancer with favorable long-term outcomes are limited, and definitive patient characteristics are yet to be reported [4, 5]. However, retrospective studies have suggested favorable prognostic factors. These include younger age at diagnosis, good performance status, positive response to trastuzumab treatment, estrogen and progesterone receptor-positive disease, low burden of disease, metastasis to soft and bone tissues, surgical management with resection of the metastatic site, tumor metastases to nodes or local sites rather than to distant sites, and response to first-line taxane and trastuzumab [4, 5, 7].
Our study has several limitations. First, as a retrospective evaluation of data collected from an existing dataset, this study suffers from biases associated with any retrospective study, such as an inherent selection bias. Second, our study reflects treatment practices before pertuzumab and trastuzumab emtansine (T-DM1) became available. None of the patients included in this retrospective study received these drugs, which have improved survival outcomes dramatically and changed the landscape of treatment for HER2-positive metastatic breast cancer. As our dataset was collected before these drugs became available, our study cannot evaluate durable CR in patients who receive pertuzumab or T-DM1.
Conclusions
In conclusion, we found that some patients showed no evidence of disease after the interruption of trastuzumab therapy. In patients who received trastuzumab as the first-line therapy for more than 2 years, the median PFS was 11.2 years and 80% of patients remained alive after 10 years. Interruption of maintenance trastuzumab in this patient population after a limited time should be explored cautiously while awaiting a global collaborative effort for a randomized trial.
References
Iwamoto T, Fukui N, Kinoshita T et al (2015) Comprehensive prognostic report of the Japanese Breast Cancer Society registry in 2006. Breast Cancer 23(1):62–72
Cardoso F, Fallowfield L, Costa A et al (2011) Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22(Suppl 6):vi25–vi30
Giordano SH, Temin S, Kirshner JJ et al (2014) Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2078–2099
Harano K, Lei X, Gonzalez-Angulo AM et al (2016) Clinicopathological and surgical factors associated with long-term survival in patients with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 159:367–374
Yardley DA, Tripathy D, Brufsky AM et al (2014) Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer 110:2756–2764
Swain SM, Baselga J, Kim SB et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372:724–734
Witzel I, Muller V, Abenhardt W et al (2014) Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—results from the HER-OS patient registry. BMC Cancer 14:806
Beda M, Basso U, Ghiotto C, Monfardini S (2007) When should trastuzumab be stopped after achieving complete response in HER2-positive metastatic breast cancer patients? Tumori 93:491–492
The World Health Organization (1983) Histological typing of breast tumors. Neoplasma 30:113–123
Tripathy D, Kaufman PA, Brufsky AM et al (2013) First-line treatment patterns and clinical outcomes in patients with HER2-positive and hormone receptor-positive metastatic breast cancer from registHER. Oncologist 18:501–510
Clavarezza M, Puntoni M, Gennari A et al (2016) Dual block with lapatinib and trastuzumab versus single-agent trastuzumab combined with chemotherapy as neoadjuvant treatment of HER2-positive breast cancer: a meta-analysis of randomized trials. Clin Cancer Res 22:4594–4603
Gullo G, Zuradelli M, Sclafani F et al (2012) Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol 23:2204–2205
Haq R, Gulasingam P (2016) Duration of trastuzumab in patients with HER2-positive metastatic breast cancer in prolonged remission. Curr Oncol 23:91–95
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
von Minckwitz G, du Bois A, Schmidt M et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03-05 study. J Clin Oncol 27:1999–2006
Tripathy D, Slamon DJ, Cobleigh M et al (2004) Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 22:1063–1070
Acknowledgements
We would like to thank the following individuals for providing us with data on patients: Nobusuke Sasada; Hiroshima University Hospital, Mayumi Ishida; National Kyushu Cancer Center, Fukuoka, Japan, Keisei Anan; Kitakyushu Municipal Medical Center; Yasuyuki Kojima, St Marianna University School of Medicine, Hideaki Shigematsu; National Hospital Organization Kure Medical Center; and Hideaki Komatsu, Iwate Medical University School of Medicine. We would also like to thank Editage for providing editorial assistance.
Funding
This research is partially supported by the Practical Research for Innovative Cancer Control (15ck0106049h0002, 17ck0106307h0001) from the Japan Agency for Medical Research and Development, AMED, National Cancer Center Research and Development Fund (26-A-4).
Disclosure
Shigehira Saji received a research grant from Chugai Pharmaceutical Co. Ltd. Yutaka Tokuda received a research grant from Chugai Pharmaceutical Co., Ltd, Eisai Co. Ltd, and Novartis Pharma L.K. Hiroji Iwata holds honorarium in Chugai Pharmaceutical Co. Ltd. Norikazu Masuda holds honoraria in Chugai Pharmaceutical Co. Ltd and AstraZeneca. The other authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niikura, N., Shimomura, A., Fukatsu, Y. et al. Durable complete response in HER2-positive breast cancer: a multicenter retrospective analysis. Breast Cancer Res Treat 167, 81–87 (2018). https://doi.org/10.1007/s10549-017-4489-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4489-9