Abstract
Purpose
Agents targeting the human epidermal growth factor receptor 2 (HER2) have improved outcomes of advanced HER2-positive breast cancer with durable responses. We evaluated first-line therapy long-term outcomes in patients responding for more than 1 year.
Methods
We retrospectively identified patients on first-line anti-HER2 therapy at The Royal Marsden Hospital for at least 1 year from 2001 to 2016. Demographics, disease characteristics, treatments and adverse events were recorded. Simple statistics, Fisher’s, Chi squared and log-rank tests were used.
Results
208 patients on treatment for at least 1 year had a median age of 54 years (31–88). 38.0% had de novo metastatic disease and 55.9% were ER positive. Of the relapsed cases, 54.4% previously had trastuzumab. At the time of presentation of metastatic disease, 27.4% of the entire cohort had pulmonary, 43.7% liver and 10.6% brain involvement. 97.1% received trastuzumab and 1.44% lapatinib; 33.2% pertuzumab and trastuzumab. 82.7% received chemotherapy (usually taxanes). 47.6% received maintenance endocrine therapy. Median progression-free survival was 39.5 months and overall survival 81.0 months. Overall response rate was 87.5%. Cardiotoxicity occurred in 4.8% of cases. Seven patients stopped treatment electively after 17–87 months and, so far, all remain in complete remission.
Conclusions
First-line anti-HER2 treatment is associated with median overall survival longer than 6 years in half of the patients free from disease progression after a year, but most still relapse eventually. Response prediction would be key to inform trial design and treatment decisions in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic treatments targeting the human epidermal growth factor receptor 2 (HER2) have significantly improved outcomes for patients with HER2-positive breast cancer and have altered its natural course [1]. These have also led to meaningful improvements in survival for advanced/metastatic disease [2].
HER2-directed agents including monoclonal antibodies such as trastuzumab and pertuzumab, drug-antibody conjugates including ado-trastuzumab emtansine (T-DM1) and tyrosine kinase inhibitors such as lapatinib and neratinib are now available for routine use and more agents are currently being investigated [3]. In the first-line setting along with chemotherapy, the overall response rate is 80% on dual anti-HER2 blockade and 69% on trastuzumab, with a median duration of response of 20.2 months and 12.5 months, respectively, and a complete remission rate of 5.5% and 4.2%, respectively [4, 5]. Response to anti-HER2 treatment is confirmed as frequently durable also in population-based studies [6, 7].
We reviewed and updated a database of patients on first-line anti-HER2 treatment at our Institution [8] and selected those in remission for more than 1 year to assess its long-term efficacy and cardiac safety and we made a preliminary assessment of patients electively stopping treatment following prolonged complete remission.
Methods
We retrospectively identified and reviewed the medical records of all patients with locally advanced or metastatic breast cancer who received first-line anti-HER2 treatment in the palliative setting from 01/10/2001 to 30/11/2016 at The Royal Marsden NHS Foundation Trust for at least 1 year.
Patients’ characteristics (including age and performance status), early and advanced stage tumour characteristics (including stage, grade, histology, receptor status) and treatment characteristics in the curative and palliative setting (including chemotherapy regimen, anti-HER2 regimen, number of cycles administered, endocrine treatment, dose reductions and delays, early discontinuation, use of growth factors, enrolment within clinical trials, treatment for central nervous system [CNS] metastatic disease, bone therapy, outcome of treatments in the curative setting) were extracted from our electronic medical records, along with data on clinical and radiological response. Based on international guidelines [9, 10], oestrogen receptor (ER) and progesterone receptor (PgR) were defined as negative in case of Allred score ≤ 2, whereas HER2 was defined as negative in case of score ≤ 1 on immunohistochemistry or single-probe average HER2 copy number < 4.0 signals/cell or dual-probe HER2/CEP17 ratio < 2.0 with an average HER2 copy number < 4.0 signals/cell on in situ hybridization.
According to the Food and Drug Administration definitions [11], progression-free survival (PFS) was calculated as the time from commencement of treatment until disease progression. Overall survival (OS) was calculated as time from commencement of treatment until death from any cause. Best responses were also recorded. Overall response rate (ORR) was calculated as the proportion of patients achieving partial response (PR) or complete response (CR) on systemic therapy; clinical benefit rate (CBR) was calculated as the proportion of patients achieving stable disease (SD), PR or CR. This analysis was approved as a service evaluation by The Royal Marsden Hospital/Institute of Cancer Research Committee for Clinical Research.
Descriptive analysis method was used to summarize the data using counts and percentages for categorical variables and for the continuous non-normal variables using median and range or interquartile range. Chi squared and Fishers exact test were used to compare outcomes in different patient groups. The Kaplan–Meier method was utilized for the calculation of OS from date of treatment to death or last follow-up date. The Kaplan–Meier method was also used for the calculation of PFS from treatment to disease recurrence or death; progression-free and lost to follow-up patients were censored at last follow-up date. Median time to event was reported with 95% confidence interval and patient groups were compared using log-rank test.
Results
Two hundred and eight patients with HER2-positive advanced or metastatic breast cancer treated with first-line anti-HER2 treatment for at least 1 year were identified from the hospital database.
Baseline characteristics are reported in Tables 1 and 2. The median age at start of treatment was 54 years. One hundred and forty-three patients (68.7%) had a metastatic disease biopsy and 80 patients (55.9%) had ER-positive cancer on metastatic specimen. Seventy-nine patients (38.0%) were diagnosed with de novo metastatic disease, whereas 129 patients (62.0%) had previous early breast cancer. Sixty-six patients (50.8%) with relapsed disease had received chemotherapy in the adjuvant setting and 34 (26.1%) in the neoadjuvant setting. Fifty patients (54.4%) had already received trastuzumab in the curative setting but only one patient had received pertuzumab.
Eastern Cooperative Oncology Group (ECOG) performance status was 0–1 in 202 patients (97.1%). Metastatic disease involved the bones in 105 patients (50.5%), the lungs in 57 (27.4%), the liver in 91 (43.7%) and the brain in 22 (10.6%) before commencing anti-HER2 therapy. One hundred and seventy-two patients (82.7%) received chemotherapy along with anti-HER2 therapy, which included pertuzumab in 69 patients (33.2%) and trastuzumab in 202 patients (97.1%). Chemotherapy agents included docetaxel in 77 patients (35.6%), paclitaxel in 59 patients (28.4%), capecitabine in 13 patients (6.2%) and vinorelbine in 10 patients (4.8%). Two patients received single-agent T-DM1 and three patients had lapatinib alone. In 23 patients (11.1%), trastuzumab was given with endocrine treatment alone and in 7 patients it was given as a single agent. Ninety-nine patients (47.6%) received maintenance endocrine therapy following induction with chemotherapy. Bisphosphonates were given in 102 patients (49.0%). While on first-line therapy, 23 patients (11.1%) underwent whole brain radiotherapy, ten (4.8%) brain surgery, 15 (7.2%) stereotactic radiotherapy and one patient received intrathecal chemotherapy.
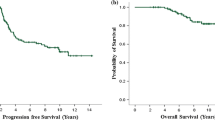
As shown in Table 3, in the overall cohort the median PFS was 39.5 months (95% CI 31.0–44.0) (Fig. 1). 52.5% of patients remained in remission at 24 months of follow-up and 29.4% at 5 years. The median OS was 81.0 months (95% CI 58.0–90.0) (Fig. 2), with 59.2% of patients alive at 5 years. Responses are presented in Table 4. 44 patients (21.1%) achieved complete remission, 138 (66.3%) partial response and 26 (12.5%) stable disease as best response on systemic treatment. In the overall cohort, ORR was 87.5%. Disease progression occurred in 108 patients (51.9%) during follow-up.
As shown in Table 5, cardiac toxicity was reported in 10 patients (4.8%). No deaths related to trastuzumab were observed. Out of 177 patients receiving concurrent chemotherapy, fifty-one patients (28.8%) required dose interruptions and 54 (30.5%) dose reductions. Forty-three patients (24.3%) received granulocyte-colony stimulating factor support.
Fifteen patients (7.2%) had to stop the anti-HER2 treatment because of toxicity, including cardiac toxicity in six cases, hypersensitivity in one case and owing to coincidental multiple spinal surgeries requiring prolonged hospital admissions in another centre for severe symptomatic spondylolisthesis in another case.
In seven patients, the treatment was electively discontinued in the context of prolonged complete remission after 17, 41, 59, 66, 78, 85 and 87 months and at the time of updated data collection in June 2019 these patients were all still alive and in continuing remission for 79, 90, 92, 100, 123, 130 and 171 months. These seven cases are summarized in Table 6. On the other hand, of 140 patients in remission for more than 17 months, 45 (32.1%) eventually experienced disease progression.
Discussion
Treatments targeting HER2 have become important agents in the management of metastatic HER2- positive breast cancer and have altered significantly the natural course of this disease. [1] Patients may have dramatic responses to anti-HER2 treatment and achieve prolonged remissions. Our analysis adds to the findings previously reported by Yeo et al. [8] and contributes to the limited evidence on outcomes in long-term responders to anti-HER2 treatments. Our findings are consistent with a previously published series of long-term responders enrolled in the HER-OS registry which documented a progression-free rate of 47.1% at 5 years and a median time to progression of 4.5 years in a population of 268 patients free from disease progression for more than 2 years [7]. Our cohort had a similar age and performance status and a similar proportion of ER-positive cases. We included a higher proportion of patients with liver or CNS involvement which may explain the different efficacy outcomes in our series. Nevertheless, our findings support an active and potentially more effective treatment of CNS disease in HER2-positive breast cancer patients, especially in patients achieving a prolonged response to systemic therapy.
The median PFS in our analysis is almost twice as long as the median PFS documented in the experimental arm of the CLEOPATRA study. [4, 5] However, only one-third of our patients received pertuzumab, which became available in the United Kingdom in 2012 and publicly funded in 2013. Moreover, a higher proportion of patients in our series had received trastuzumab in the curative setting. Therefore, these findings suggest that patients responding to trastuzumab for more than 1 year have a good chance of long-term outcome lasting several years, even if they have previously received it as adjuvant treatment.
Our study raises the question of the optimal duration of anti-HER2 therapy in long-term responders with advanced/metastatic disease, given that almost one-third of our patients remained in remission at 5 years of follow-up. In early breast cancer, three randomized trials have explored different durations of trastuzumab in the adjuvant setting [12,13,14] and two out of three found that duration of 6 months failed to show non-inferiority compared with a year. No similar trials have been reported in advanced disease, and yet this issue is relevant regarding the debate on the increasing healthcare costs and the cost-effectiveness of anti-HER2 treatments [15]. Seven patients in our series had their treatment electively discontinued based on prolonged complete remission, and none of these have so far relapsed, suggesting that cures may sometimes be possible even in patients with metastatic disease. These prolonged remissions may be related either to a clearance of the HER2-positive clones or to immune surveillance. A randomized trial of discontinuation of anti-HER2 therapy following prolonged complete remission would be challenging to conduct owing to the small proportion of potentially eligible patients and potential concerns amongst both patients and health care professionals about those randomized to stop treatment. Nevertheless, at the very least a prospective national or international data base on patients electively stopping is strongly warranted.
Our analysis indicates also that long-term anti-HER2 treatment remains safe and well tolerated. Continuation of HER2-directed treatment can increase the risk of cardiotoxicity and regular monitoring of cardiac function with echocardiogram or multigated acquisition scan is mandatory [16]. The incidence of cardiotoxicity varies according to patient-related factors, such as previous chemotherapy, comorbidities and age [17, 18]. A 2012 meta-analysis of trials investigating trastuzumab documented and increased risk for severe heart failure of 2.5 versus 0.4% [relative risk (RR) 5.11, 90% CI 3.00–8.72] and reduction in left ventricular ejection fraction (RR 1.83, 90% CI 1.36–2.47) [19]. The subsequent APT study of adjuvant weekly paclitaxel chemotherapy plus trastuzumab in node-negative, HER2-positive tumours measuring up to 3 cm documented grade 3 left ventricular systolic dysfunction only in 0.5% of patients and asymptomatic LVEF decline in 3% [20]. Our analysis did not demonstrate any increased cardiac toxicity in patients receiving anti-HER2 therapy for more than 1 year, and the treatment was discontinued due to cardiac adverse events in only six patients, which suggests that most cases are reversible following dose interruptions and adequate medical therapy.
New agents with documented efficacy are currently being investigated in the management of advanced HER2-positive breast cancer [21]. Such compounds include Fc-engineered antibodies that can delay further disease progression in the context of heavily pre-treated disease [22] and are likely be tested in the near future in treatment-naïve patients. The roles of trastuzumab and pertuzumab are now very well established in the first-line setting; identifying predictors of long-term response to these would be important in selecting which patients might benefit from entry into future clinical trials assessing the long-term benefit of these newer agents in addition.
References
Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28(1):92–98
Chia SK, Speers CH, D’Yachkova Y, Kang A, Malfair-Taylor S, Barnett J et al (2007) The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 110(5):973–979
Ponde N, Brandao M, El-Hachem G, Werbrouck E, Piccart M (2018) Treatment of advanced HER2-positive breast cancer: 2018 and beyond. Cancer Treat Rev 67:10–20
Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M et al (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8):724–734
Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119
Gullo G, Zuradelli M, Sclafani F, Santoro A, Crown J (2012) Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol 23(8):2204–2205
Witzel I, Muller V, Abenhardt W, Kaufmann M, Schoenegg W, Schneeweis A et al (2014) Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer—results from the HER-OS patient registry. BMC Cancer 14:806
Yeo B, Kotsori K, Mohammed K, Walsh G, Smith IE (2015) Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast 24(6):751–757
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Food and Drug Administration (2007) Guidance for Industry Clinical Trial endpoints for the approval of cancer drugs and biologics
Earl HM, Hiller L, Vallier AL, Loi S, McAdam K, Hughes-Davies L et al (2019) 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 393:2599–2612
Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T et al (2013) 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 14(8):741–748
Mavroudis D, Saloustros E, Malamos N, Kakolyris S, Boukovinas I, Papakotoulas P et al (2015) Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 26(7):1333–1340
Durkee BY, Qian Y, Pollom EL, King MT, Dudley SA, Shaffer JL et al (2016) Cost-effectiveness of Pertuzumab in human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 34(9):902–909
Keefe DL (2002) Trastuzumab-associated cardiotoxicity. Cancer 95(7):1592–1600
Henry ML, Niu J, Zhang N, Giordano SH, Chavez-MacGregor M (2018) Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc Imaging 11(8):1084–1093
Soto-Perez-De-Celis E, Loh KP, Baldini C, Battisti NML, Chavarri-Guerra Y, De Glas NA et al (2018) Targeted agents for HER2-positive breast cancer in older adults: current and future perspectives. Expert Opin Investig Drugs 27(10):787–801
Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V et al (2012) Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006243.pub2/abstract
Dang C, Guo H, Najita J, Yardley D, Marcom K, Albain K et al (2016) Cardiac outcomes of patients receiving adjuvant weekly Paclitaxel and Trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2(1):29–36
Pernas S, Tolaney SM (2019) HER2-positive breast cancer: new therapeutic frontiers and overcoming resistance. Ther Adv Med Oncol 11:1758835919833519
Rugo HS, Im S-A, Wright GLS, Escriva-de-Romani S, DeLaurentiis M, Cortes J et al (2019) SOPHIA primary analysis: a phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2 + metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J Clin Oncol 37(15_suppl):1000
Acknowledgements
The authors wish to acknowledge the support of The Cridlan Trust and The Royal Marsden NIHR Biomedical Research Centre for Cancer.
Funding
No funding was required for this retrospective study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr Battisti has received travel grants from Genomic Health and speaker fees from Pfizer. Prof Smith attended an Advisory Board for Roche in 2014. Dr Ring has received advisory board fees from Roche, Novartis, Pfizer and Lilly and speaker fees from Novartis and Pfizer. Dr Tong has no conflict of interest.
Ethical approval
This research project has been reviewed and approved by The Committee for Clinical Review of The Royal Marsden NHS Foundation Trust. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for systemic anticancer treatment was obtained from all individual participants included in this retrospective analysis.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Battisti, N.M.L., Tong, D., Ring, A. et al. Long-term outcome with targeted therapy in advanced/metastatic HER2-positive breast cancer: The Royal Marsden experience. Breast Cancer Res Treat 178, 401–408 (2019). https://doi.org/10.1007/s10549-019-05406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05406-6