Abstract
Purpose
While triple-negative breast cancer (TNBC) is negative for estrogen receptor alpha, a substantial proportion of carcinomas express estrogen receptor beta (ERβ); consequently, estrogen actions and metabolism may be relevant in this cancer subtype.
Methods
A cohort of 81 TNBC patients from Tohoku University Hospital, Japan were characterised with regard to the expression of estrogen receptor beta and enzymes known to modulate levels of estrogens in breast and other tissues (Aromatase, 17-beta- Hydroxysteroid dehydrogenases 1, 2 and 6). This was done at the protein level by means of immunohistochemistry. As this cohort has been previously characterised for androgens, this also allows for comparison between the expressions of estrogen-related proteins and of androgen-related proteins. Preliminary mechanistic studies in cell culture were also undertaken.
Results
17βHSD2 was detected in the highest number of cases followed by 17βHSD1, 17βHSD6 and aromatase. When comparing the expression of ERβ with that of the enzymes, it was positively correlated with the expression of 17βHSD6 (p < 0.05) and trended towards correlation with dual expression of 17βHSD1 and 2 (p < 0.07). 17βHSD1 was associated with significantly reduced tumour volume (p = 0.0025), while ERβ was associated with a trend towards reduced lymphovascular invasion, (p < 0.061). Interestingly, in survival analysis, 17βHSD6 expression was the only one of these five factors that influenced survival, with positive samples being associated with longer disease-free survival compared to those that were negative for 17βHSD6 (p < 0.05). In assessing associations with expression of proteins in the androgenic pathway, expression of aromatase appeared to be associated with androgenic pathways in TNBC patients (p < 0.05). Due to this association and the potential relevance to androgen-directed therapies in TNBC, we evaluated this interaction in vitro. We observed androgen-dependent upregulation of aromatase and ERβ in a subset of AR expressing TNBC cell lines (MDA-MB-453, SUM-185-PE and MFM-223).
Conclusion
Overall this study suggests the presence of, and a potential protective effect of estrogens in TNBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of breast cancers are estrogen dependent, and estrogen-based therapies have been used with reasonable success in many patients expressing estrogen receptor α (ERα) in carcinoma cells. At this juncture, the presence or the absence of ERα in carcinoma cells is considered one of the four markers used in standard pathology practice to classify breast cancers into groupings which both predict clinical outcome and indicate potential therapies. The remaining three markers are the progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), as well as the proliferation marker Ki-67 [1]. This grouping is often described as the ER/PR/Her2 classification, and this has led to any breast cancers lacking ERα being described as ER-negative breast cancer, or in the case of those lacking also PR and HER2, triple-negative breast cancer (TNBC).
However, this description of TNBC as being also estrogen receptor negative is potentially misleading and may lead to disregard for a major intracrine and steroid signalling pathway in these subsets of hard-to-treat breast cancer patients. TNBC, by definition, lacks ERα expression, but previous studies have documented that 25–50 percent of TNBC and up to 70 percent of ERα-negative cancers express ERβ in their carcinoma cells [2–5]. While numerous studies have documented the effects and interactions of ERα and ERβ in breast cancers, few have focused on the roles of ERβ in TNBC cases. This is despite the fact that the potential significance of ERβ in TNBC patients is all the more important due to the lack of current targeted and effective treatments. In the previous studies, there was no consensus on the effects of ERβ expression in TNBC [5–9], possibly due to the inherent complexity of ERβ transcription (reviewed in [10, 11]). However, recent studies including larger patient numbers demonstrated that ERβ expression was associated with clinical benefits in TNBC patients [5]. A biological role for ERβ in TNBC is supported by bioinformatics studies demonstrating its effects on a number of cellular processes, the net effect of which would be expected to be deleterious to the cancer and beneficial to the patient [12].
Taking into account the potential roles of ERβ in TNBC biology, it is apparent that intra-tumoral estrogen metabolism in the ERα negative and TNBCs may be a biologically relevant question to pursue. Therefore, in this study, we examined the status of multiple pathways of intra-tumoral estrogen synthesis including the typical estrone/estradiol synthesis pathways (aromatase (arom), 17-beta-Hydroxysteroid dehydrogenase types 1 and 2 (17βHSD1, 17βHSD2)), and the alternative pathways from androgens to estrogens (17-beta-Hydroxysteroid dehydrogenase type 6 (17βHSD6)) reported in prostate cancer patients (Supp. Figure 1). The latter may be particularly relevant given the parallels between breast and prostate cancers [13], particularly the triple-negative type, and the potential importance of androgens and androgen modulation in subsets of TNBC [14]. A suggestion of this potential interaction comes from the protective effect of ERβ1 in the histologically apocrine subtype of breast cancer [6] which is associated with high expression of androgen receptor (AR) and considered similar in nature to the molecular apocrine/LAR subtypes of ERα negative/TN breast cancers [15–17]. In addition to examining the estrogen generating intracrine landscape of TNBC, we also examined the statuses of ERβ and ERβ1 in TNBC specimens, as this receptor is a pivotal component in confirming the presence of a complete intracrine pathway. In an attempt to set these enzymes in the wider context of intracrine metabolism in the breast, we also examined possible interactions between their expression and that of androgens and related transcription factors in TNBC cases. Guided by the IHC analysis, we explored the mechanistic nature of correlations and interactions in a panel of TNBC cell lines.
Methods
Patients
The cohort documented in this study consists of 81 TNBC invasive ductal carcinoma (IDC) patients drawn from the population of patients treated at Tohoku University Hospital, Sendai, Japan between 1998 and 2007. This cohort has been previously reported in respect to androgen signalling [18] and general clinicopathological data [19], but the only overlap between the previous report and this one is the use of historical androgen and clinicopathological data to assess the interaction of AR expression and the novel data on estrogen pathway presented here. As previously published, some of the samples in this cohort are IDC-DCIS cases in which both invasive and non-invasive carcinomas are visible within the same section. In these cases, we noted the expression of the markers separately for both the IDC and DCIS components of the samples in order to determine if they should be examined in DCIS samples (below). We also examined pure TNBC DCIS cases. The DCIS cohort was retrieved from two hospitals: St Luke’s Tokyo, and Tohoku Kosai Hospital, Sendai. Details of the clinicopathological information and androgen metabolism in this cohort have been described previously [20], but as with the IDC cohort, the only overlap between these two relates to the comparison of historical androgen data (AR expression) to that of the estrogen pathways examined in this study. Permission from the respective ethical review boards of all institutions was obtained for all samples.
Immunohistochemistry
Immunohistochemistry was performed as previously reported. In brief, primary antibodies directed against the N terminus of ERβ and thus non-specific to isoform (Genetex ERβ 14-C8, 1:1000); C terminus of the ERβ1 subtype, and thus specific to the full length ERβ1 isoform (Genetex, PPG5/10; 1:50) Aromatase (D.E. Evan/Novartis #667, 1:3000); 17βHSD6 (ProteinTech, HSD17β6, 11855-1-AP, 1:100); 17βHSD1 (Abnova 17βHSD1, H0003292-M03, 1:400); 17βHSD2 (Proteintech17βHSD2 10978-1-AP, 1:200); and FOXP1(Spring Bioscience, Clone Sp133, 1:200) were employed in 3 μm paraffin-embedded tissue sections of surgical pathology materials of breast cancer retrieved from the archives of the department of Anatomical Pathology. Following optimisation, citrate autoclave–based antigen retrieval was used in the cases of ERβ, ERβ1, 17βHSD1, 17βHSD6 and FOXP1. Antibodies have been previously validated by us [21, 22] or others [9], with the exception of 17βHSD6 which was optimised for the purposes of this study. Antibodies were visualised using a Histofine Kit appropriate for the species that the primary antibody was raised in, with DAB as a chromogen. Immunoreactivity was assessed by employing H-Score/Labelling index in the case of ERβ and ERβ1 and a semi-quantitative scale in the case of the cytoplasmic enzymes (0; 0% of carcinoma stained, 1; 1–50% of carcinoma stained, 2; >50% of carcinoma stained). Slides were scored by at least two of the authors, and in the case of any disagreement, the case was discussed and consensus reached.
Cell culture
The three different TNBC cell lines (MDA-MB-453, SUM-185-PE, and MFM-223) used in this study harboured relatively high AR expression, and were cultured in standard conditions. For the steroid treatments, we incubated the cells in dextran-coated charcoal-stripped FBS (foetal bovine serum) supplemented phenol red-free Leibovitz’s L-15 medium (Life Technologies), phenol red-free MEM medium (Life Technologies) or phenol red-free Ham’s F-12 medium (Pan-Biotech, Aidenbach, Germany) for 72 h. Following this period of steroid deprivation, we either treated the cells with vehicle, DHT (10 nM) or DHT(10 nM) and Bicalutamide (10 μM). Steroid hormone treatment was continued for 72 h after which cells were harvested, and RNA was extracted by means of the Trizol method.
Real-time PCR
Real-time PCR was performed on the Roche light-cycler machine using the Roche PCR master mix. Primers are given in Supplementary Table 1. Two housekeeping genes were employed to examine the data obtained (B-actin and RPL), and the gene with the least variability across the samples and between experimental conditions [23] was used to standardise the samples examined.
Statistical analysis
All statistical analysis was performed using JMP12.2.0. H score and Labelling index were classified as continuous variables, while the semi-quantitative proportional scoring of enzymes was classified as an ordinal variable. We do not have full data for all samples, and for this reason, numbers between different analyses may be slightly different. For all analyses, we tried to retain as much information as possible in the data and avoid dichotomisation. In some cases, this was, however, unavoidable, especially when multiple factors (>2) were being tested. We dichotomised ERβ at 10%; 17βHSD1, 2 and 6 at 50%; and aromatase at >0%. The cut points that were chosen as in the case of the receptor cutting at 10% seemed to give a natural separation into two populations (Fig. 2a), and in the case of the enzymes, they provided an even division of the data (Fig. 2g–i). In the case of ordinal variables being tested against continuous variables, ANOVA analysis was used, and the p value reported is that from the ANOVA. In the case of ordinal variables being tested against ordinal variables, Chi Squared analysis was used, and the p value reported was that of the Pearson’s test. Survival outcomes were visualised using Kaplan–Meier plots and tested via the log-rank test. Multivariate analysis was performed by parametric survival using the Weibull distribution. Other factors included were the clinicopathological parameters listed as well as the markers examined in this study. For cell culture work, changes in each cell line were analysed separately by ANOVA followed by Dunnet’s post hoc used in order to determine significant differences between individual groupings.
As a great deal of the analysis performed was in the Chi squared format, we chose to use mosaic plots to represent the data. These are used for the visualisation of categorical variables and function as a graphical representation of contingency tables. In brief, they demonstrate the relative proportion of the whole population that resides in each category on the X axis by dividing the spacing on that axis accordingly. Then, the proportion of each category on the Y axis is shown within the individual X axis groupings, while the whole-population proportion is given in a bar to the right. Thus, it gives a visual representation of differences in proportions between groupings regardless of differences in size groupings on the X axis. We have also supplied the raw numbers in each category in order to make reading and interpreting these graphs as simple as possible.
Results
ERβ and estrogen metabolising enzymes in TNBC specimens and their relationship to clinicopathological factors
Immunoreactivities for ERβ and ERβ1 were examined in carcinoma cells of TNBC cases (Fig. 1) as well as in adjacent morphologically normal breast tissues where available, often in DCIS rather than IDC cases (Supplementary Fig. 2a–d). The patterns of ERβ and ERβ1 immunoreactivity and immunolocalisation were different between non-neoplastic and neoplastic epithelium, with non-neoplastic areas having a more intense and speckled appearance, with heterogeneity; but carcinoma cells had more uniform staining (Fig. 1, Supplementary Fig. 2). The enzymes (Arom, 17βHSD1, 17βHSD2, and 17βHSD6) were most predominantly found in the carcinoma cells of the breast (Fig. 1). The distribution and prevalence of all markers are given in Fig. 2 with ERβ, ERβ1 17βHSD1, 17βHSD2 and 17βHSD6 detectable at some levels in over half of the cohort and aromatase detectable in just less than half of the cohort. The immunoreactivities of ERβ and ERβ1 were closely related (Fig. 2c); however, ERβ1 was found in a greater proportion of samples than with ERβ possibly suggesting a differences in the sensitivities of the immunostaining.
Example images of IHC of ERβ, Aromatase, 17βHSD1, 17βHSD2 and 17βHSD6 in the cohort of TNBC patients IHC was performed as described in the text. To give readers a sense of the scoring system, we have included three samples here that cover the range of scoring, with details of each score being shown in the photomicrographs. a–c three different levels of ERβ expression, d–f the corresponding staining for ERβ1, g–i the immunoreactivity of aromatase, j–l the staining of 17βHSD1, m–o the staining of 17βHSD2, and p–r the staining of 17βHSD6. The scale bar represents 200 μM
Distribution of the expressions of ERβ, Aromatase, 17βHSD1, 17βHSD2 and 17βHSD6 in the cohort of TNBC patients The plots showing the distributions of the scoring of various values of ERβ, ERβ1, aromatase, 17βHSD1, 17βHSD2 and 17βHSD6. ERβ and ERβ1 were scored using the labelling index (a, b), and the results compared via a scatterplot (c). ERβ was further characterised using the gold standard H score method that indicated both prevalence and intensity (d). Li was, however, used to dichotomise at 10% where a positive/negative value assignment was necessary for the analysis (e). Only nuclear staining was taken into account by this method. All others were scored on a semi-quantitative scale: zero indicating no staining, 1 indicating <50% and 2 >50% (F-17βHSD6, G-Aromatase, H-17βHSD1, and I-17βHSD2). Sample sizes between markers may be variable due to missing or damaged samples. The colour intensity gives information if the pathway is associated with a more-potent estrogen environment or with a less-potent estrogen environment. Darker shades indicate increased estrogen. Canonical estrogen enzyme pathways are marked in red, and non-canonical in blue, and this colour scheme will be conserved throughout this paper
In order to further examine the potential correlation between the statuses of these factors and underlying biological processes, we evaluated the correlation between these factors and clinicopathological parameters of the patients (Table 1). In examining ERβ, its principal association was with reduced vascular invasion and a tendency towards reduced lymphatic invasion (p = 0.006 and p = 0.061, respectively). ERβ expression status also trended towards a positive association with EGFR expression (p = 0.08). The aromatase enzyme was associated with a lower level of Ki-67/Mib-1, but the correlation did not reach statistical significance (p = 0.12) and trended towards a positive association with CK5/6 (p = 0.08). In the analyses of the enzymes, possibly the most notable or significant interaction was between 17βHSD1 and tumour volume, where abundance of this enzyme was correlated with smaller tumour size (p = 0.0025), changes in histological grade (p = 0.02) and trended towards a correlation with EGFR expression (p < 0.08). 17βHSD2 did not associate with any of the clinicopathological factors examined, while 17βHSD6 only associated with lymphatic invasion (p = 0.03).
Differences between DCIS and IDC TNBC
All markers were also detected in a proportion of the DCIS cases examined with example histology shown in supplementary Fig. 2g–h. ERβ and ERβ1 appeared to increase between DCIS and IDC, although this trend was only associated with one set of DCIS samples and was non-significant (Figure S2I). The status of aromatase was not different between DCIS and IDC cases, but that of 17βHSD6 was significantly lower in TNBC IDC than in DCIS (p < 0.05, Figure S2 J, K). The statuses of 17βHSD1 and 2 did not appear significantly different between TNBC DCIS components and IDC components within the IDC tissues, and thus these enzymes were not examined in pure DCIS (data not shown).
Correlation of ERβ and estrogen metabolising enzymes in TNBC cases
ERβ was significantly correlated with 17βHSD6 in both DCIS and IDC TNBC (Pearson’s Chi Squared, p < 0.05, Fig. 3a, b) with no significant effect on 17βHSD1 or 2 (Data not shown, Fig. 3c). However, double-positive cases of 17βHSD1 and 2 trended towards statistical association with positive expression of ERβ (Pearson χ 2 test p = 0.13, Fig. 3d). The expressions of aromatase and 17βHSD1 were not significantly correlated with that of ERβ (Data not shown).
Association of estrogen metabolism with steroid receptors The interactions between estrogenic steroid metabolism pathways and ERβ (a–d) or AR (e–f) tested as shown. The association of ERβ with 17βHSD6 was examined in DCIS and IDC and a positive association was found (a, b). Positive associations between ERβ expression and canonical estrogen pathways were more tenuous with no correlation found for 17βHSD1 (c) or 17βHSD2 (Data not shown); however, there was an interaction between ERβ and their combined expression (d). Despite a lack of receptor association between ERβ and AR (data not shown), AR expression was significantly associated with aromatase expression, particularly that of high aromatase expression (e). A similar association with the alternate 17βHS6 pathway was not observed, although there is some evidence of a trend (f). All statistics are from Pearson’s Chi squared analysis. Canonical estrogen enzyme pathways are marked in red and non-canonical in blue, and the graphs shown are mosaic plots. These are used for the visualisation of categorical variables and function as a graphical representation of contingency tables. A full description of these is given in the accompanying text of this article. To aid in the understanding of these, the raw numbers of samples in each grouping are also given on the graph
Correlation of estrogen pathways, androgen intracrine-metabolising pathways and other regulators of hormone-dependent signalling in TNBC
In correlating the status of AR with the markers in this study individually, only one was associated AR, aromatase (p < 0.01, Fig. 3e, f). However, when analysing by the enzyme/receptor enzyme status, AR-positive cases were significantly associated with increased numbers of ERβ/aromatase-positive samples (p < 0.001, Fig. 4a). These results were also pronounced when the status was tentatively classified according to AR/FOXA1 status (data not shown, FOXA1 a marker of luminal like differentiations in TNBC). The same was not true for ERβ/17βHSD1 or ERβ/17βHSD6 and AR expressions (Fig. 4b, c). Other related intracrine enzymes were also significantly associated in sequential estrogen synthesis pathways in IDC samples and trended towards similar association in DCIS and IDC (17βHSD6 and 5αR1 and aromatase and 17βHSD5 and, Fig. 4d–f).
Association of androgen pathways with receptor-enzyme estrogen pathways As androgen receptor is a potential factor maintaining luminal differentiation in triple-negative breast cancer cells, we were interested to investigate if AR expression correlated with combined expression of ERβ receptor and enzymes (a–c). While both the aromatase/ERβ (a) and 3βdiol/ERβ (b) pathways showed a pattern of co-ordinated enzyme-receptor expression in AR-positive samples, this was only significant for aromatase and was not observed for 17βHSD1 (c) or 17βHSD2 (data not shown). In addition, we were interested in examining the overlap in expressions between enzymes that form Estradiol (Adione → 17βHSD5 → Testosterone → Aromatase → Estradiol) (d) and those that form 3β diol (Testosterone → 17βHSD6 → DHT → 5αR1 → 3βdiol) (e, f) in order to see if complete pathways were present. For IDC, both these pathways proved positively and significantly correlated (d, e), and a similar trend was seen for 17βHSD5 and aromatase (f). All statistics are from Pearson’s Chi squared analysis. Canonical estrogen enzyme pathways are marked in red and non-canonical in blue, the graphs shown are mosaic plots. These are used for the visualisation of categorical variables and function as a graphical representation of contingency tables. A full description of these is given in the accompanying text. To aid in the understanding of these, the raw numbers of samples in each grouping are also given on the graph
In addition to associations with the expression of AR, we also examined the relationships between ERβ1 status and the forkhead factor FOXP1, as reported in ERα-positive breast cancer [24, 25]. However, no correlation between FOXP1 and ERβ1 status was found, although FOXP1 was detected in 25% of TNBC IDC examined (data not shown).
Associations of ERβ and enzyme expressions with clinical outcome of TNBC patients
ERβ status did not confer significant effects on disease-free or overall survival of the patients but the statuses of both 17βHSD6 and 17βHSD2 showed separation on DFS and OS curves (Fig. 5a–d). When dichotomised, this was significant for 17βHSD6, where any expression was associated with a beneficial effect on disease-free survival of TNBC patients (Log rank test p = 0.025, Fig. 5e). Similar results were seen for overall survival, but they did not reach significance (Log rank test p = 0.07, Fig. 5f). The interaction of 17βHSD6 and disease-free survival remained significant in a subsequent multivariate analysis including clinicopathological factors and other enzyme expressions. In this analysis, 17βHSD2 approached significance depending on the other factors in the model.
Survival curves associated with 17βHSD6 expression Of all the proteins examined, 17βHSD6 was the only one associated with significant effects on survival (Log-Rank test). When graphed, both 17βHSD2 and six showed separation of the survival curves based on raw data (a–d). To test the trends we observed, we dichotomised on the basis of any enzyme expression for both these proteins, but 17βHSD6 was the only one approaching significance. Positive samples (dark blue line, 17βHSD6 expression >0) were associated with significantly better disease-free survival outcomes (e). A similar pattern was seen in overall survival, but this was not significant (f). This effect remained in a multivariate analysis of all the estrogenic enzymes examined as well as Ki-67, lymph node, and other known clinicopathological parameters. See text for details
Androgen regulation of estrogen pathways in TNBC cell lines
Given the marked interaction of AR and the positivities of both ERβ and aromatase detected in clinical cases of TNBC, we examined whether androgen treatment in TNBC cell lines could regulate both ERβ and aromatase expressions. Results demonstrated variable effects across the different cell lines with androgen treatment. Using β-actin as a housekeeping gene, the expression of ERβ and aromatase appeared to be androgen regulated in the MFM-223 cell line (Fig. 6a, b).
Responsiveness of Aromatase and ERβ to Androgen in TNBC cell lines Due to correlations between AR expression and Aromatase/ERβexpression, we analysed if these factors were regulated by androgens in a luminally differentiated AR expressing panel of TNBC cell lines (SUM-185-PE, MFM-223 and MDA-MB-453). Interestingly, regulation of these estrogen pathway proteins was not uniform across the cell lines. The only significant and androgen-dependent result was the upregulation of both ERβ and aromatase following androgen treatment (DHT 10 nM) in the MFM-223 cell lines and suppression of this upregulation by co-administration of androgen antagonist Bicalutamide (10 μM). Data are shown as mean, and the SEM. Statistics used are based on ANOVA followed by Dunnets Post Hoc to determine differences between the groups
Discussion
The central finding of this paper is that ERβ is expressed in a majority of TNBC cases, and in many settings, it is expressed alongside enzymes that can make some form of ERβ ligand. While ERβ and AR expression were not strongly correlated, there was an association between androgen pathways and the presence of the estrogen-producing enzyme aromatase. Interestingly, when it came to an examination of the effects of these factors on biological outcomes, the most significant factor appeared to be the expression of the 17βHSD6 enzyme, which steroidogenically is thought to mainly act by producing the selective receptor beta antagonist 3βdiol from DHT. This work shows that, despite their conceptualisation as a ‘triple-negative’ cancer, TNBC cases still retain many relevant steroid pathways that should be examined further.
Various studies have reported mixed results regarding the correlation between ERβ expression and survival in TNBC patients, including better [5, 6], worse [7, 8] or no effects [9] on clinical outcome. One potential reason for this discrepancy is the nature of the antibodies used. For instance, different ERβ forms were reported to exert different context-dependent effects on survival outcomes of the patients [9]. However, this is not true in all the cases, as many used one of the two antibodies as we used in this study, and we saw a close relationship between the expression patterns of ERβ non specified and ERβ1 in our study. Results of a recent meta-analysis study indicated that overall, ERβ appears to exert positive effects upon survival or clinical outcomes in ERα-negative cases [26]. Dependent on this, the possibility of ERβ agonism as a combination therapy in TNBC has been proposed [27].
One alternate possible resolution is that the inherent variability of TNBC suggests that estrogen actions through ERβ could be beneficial only in some patients. It is possible that the relative proportions of TNBC subgroups could vary between cohorts which could account for the discrepancies reported. The effects of ERβ agonists have been evaluated in TNBC cell lines; although there was no effect on cell proliferation, there was inhibition of invasive ability in the basal subtype of TNBC cell lines (HCC1806, HCC1937) [28]. Furthermore, induced ERβ expression in a commonly used TNBC model (MDA-MB-231) resulted in an inhibition of cell proliferation [4]. Results of our present study are partially supportive of the results of the majority above, suggesting that the presence of an estrogen pathway could be associated with clinicopathological parameters related to better prognosis or clinical outcome such as smaller tumour size and lack of lymph vascular invasion.
Despite the growing body of literature examining the potential roles of estrogen signals through ERβ in breast cancer in general and TNBC in particular, few have examined the corresponding pathways of estrogenic steroid metabolism in TNBC [29]. One of the strengths of this study is that we examined both the receptor and intratumoral estrogen-synthesising and -metabolising enzymes in the same tissue. This allowed us to demonstrate significant correlations between the receptor and the enzymes, suggesting functional estrogen pathways in TNBC. Beyond this, we also examined the potential interactions of estrogen pathways in TNBC with the other main steroid pathway suggested to be dominant in a subset of TNBCs, namely that of the androgen receptor. AR status in carcinoma cells was significantly correlated with 17βHSD6, the principal enzyme in an alternative pathway that was reported to generate ERβ-specific ligands from the degradation of the potent androgen DHT [30]. The functionality of this ligand has been shown previously in breast cancer cell lines [23].This suggests a possible interplay between AR signalling and ERβ signalling as reported in prostate cancer. In further support of this idea, previous studies in breast cancer also demonstrated that the ligand above was at least present in the breast cancer local environment, suggesting that this pathway is active in breast tissues [31]. Despite these comments, a note of caution should be raised. Although we were able to show a direct relationship between ERβ expression and 17βHSD6 expression, we only observed a trend towards significance in their correlation with AR. We were also underpowered to split the samples into survival outcomes based on 17βHSD6/ERβ expression. It is possible that, as we have shown with other steroidogenic enzymes [32], the 17βHSD6 enzyme may have a non-steroidogenic role. We, however, think this evidence is sufficient to suggest the possibility of the pathway as a research avenue that should be pursued.
Of particular interest, the presence of AR in TNBC cases was significantly correlated with the expression of aromatase and the dual expression of aromatase and ERβ. The wider implications of this finding remain unknown, beyond the suggestion that the expression of AR in carcinoma may be associated with a more differentiated state and hence possibly the preservations of a more functional set of steroid pathways. We have previously reported similar interactions between AR and androgenic enzyme expression in breast cancer cases [20, 33]. Previous studies in neural cells and testis also reported that aromatase expression could be androgen dependent [34, 35], while other studies reported the possible androgenic up-regulation of ERβ expression in breast cancer cell lines [36], thus providing some precedent for androgenic regulation of estrogen pathways.
In line with the findings/studies above, we demonstrated an androgen-dependent increase of both ERβ and aromatase expression in vitro in one of the three most widely used models of AR expressing TNBC. This particular cell line (MFM-223) is the only one of the three in the panel we used to demonstrate growth inhibition in response to androgens [37, 38], suggesting that this underlying difference in terms of up-regulated steroid pathways could possibly explain some of these divergent responses. However. it awaits further investigations for clarification.
Conclusions
To summarise, we examined the main form of the beta-type estrogen receptor ERβ1 and relevant steroid metabolising enzymes using immunohistochemistry in a wide range of TNBC specimens. Overall, estrogen pathways were associated with clinicopathological parameters related to better prognosis of the patients, and in these cancers, some aspects of estrogen metabolism may be under regulation by AR signalling. The presence of active estrogen pathways in triple-negative cancers further indicates the possible use of estrogen-manipulating agents in these cancers as a form of therapy. In particular, in the case of ERβ in TNBC, specific agonists with minimal side effects could provide a viable option in this difficult-to-treat cancer subtype.
References
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. doi:10.1093/annonc/mdt303
Marotti JD, Collins LC, Hu R, Tamimi RM (2010) Estrogen receptor-beta expression in invasive breast cancer in relation to molecular phenotype: results from the nurses’ health study. Mod Pathol 23(2):197–204. doi:10.1038/modpathol.2009.158
Guo L, Meng J, Yilamu D, Jakulin A, Fu M, Wang B, Abulajiang G (2014) Significance of ERbeta expression in different molecular subtypes of breast cancer. Diagn Pathol 9:20. doi:10.1186/1746-1596-9-20
Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE, Pockaj BA, Couch FJ, Olson JE, Reynolds C, Lingle WL, Spelsberg TC, Goetz MP, Ingle JN, Hawse JR (2014) ERbeta1: characterization, prognosis, and evaluation of treatment strategies in ERalpha-positive and -negative breast cancer. BMC Cancer 14(1):749. doi:10.1186/1471-2407-14-749
Wang J, Zhang C, Chen K, Tang H, Tang J, Song C, Xie X (2015) ERbeta1 inversely correlates with PTEN/PI3 K/AKT pathway and predicts a favorable prognosis in triple-negative breast cancer. Breast Cancer Res Treat. doi:10.1007/s10549-015-3467-3
Honma N, Saji S, Kurabayashi R, Aida J, Arai T, Horii R, Akiyama F, Iwase T, Harada N, Younes M, Toi M, Takubo K, Sakamoto G (2008) Oestrogen receptor-beta1 but not oestrogen receptor-betacx is of prognostic value in apocrine carcinoma of the breast. APMIS 116(10):923–930. doi:10.1111/j.1600-0463.2008.01122.x
Guo L, Zhu Q, Aisimutuola M, Yilamu D, Liu S, Jakulin A (2015) Expression and prognostic value of estrogen receptor beta in patients with triple-negative and triple-positive breast cancer. Exp Ther Med 9(6):2147–2150. doi:10.3892/etm.2015.2380
Hamilton N, Marquez-Garban D, Mah V, Fernando G, Elshimali Y, Garban H, Elashoff D, Vadgama J, Goodglick L, Pietras R (2015) Biologic roles of estrogen receptor-beta and insulin-like growth factor-2 in triple-negative breast cancer. BioMed Res Int 2015:925703. doi:10.1155/2015/925703
Wimberly H, Han G, Pinnaduwage D, Murphy LC, Yang XR, Andrulis IL, Sherman M, Figueroa J, Rimm DL (2014) ERbeta splice variant expression in four large cohorts of human breast cancer patient tumors. Breast Cancer Res Treat 146(3):657–667. doi:10.1007/s10549-014-3050-3
Smart E, Hughes T, Smith L, Speirs V (2013) Estrogen receptor beta: putting a positive into triple negative breast cancer? Horm Mol Biol Clin Investig 16(3):117–123. doi:10.1515/hmbci-2013-0042
Haldosen LA, Zhao C, Dahlman-Wright K (2014) Estrogen receptor beta in breast cancer. Mol Cell Endocrinol 382(1):665–672. doi:10.1016/j.mce.2013.08.005
Shanle EK, Zhao Z, Hawse J, Wisinski K, Keles S, Yuan M, Xu W (2013) Research resource: global identification of estrogen receptor beta target genes in triple negative breast cancer cells. Mol Endocrinol 27(10):1762–1775. doi:10.1210/me.2013-1164
Risbridger GP, Davis ID, Birrell SN, Tilley WD (2010) Breast and prostate cancer: more similar than different. Nat Rev Cancer 10(3):205–212. doi:10.1038/nrc2795
McNamara KM, Moore NL, Hickey TE, Sasano H, Tilley WD (2014) Complexities of androgen receptor signalling in breast cancer. Endocr-Related Cancer 21(4):T161. doi:10.1530/erc-14-0243
Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R (2010) Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol 23(2):205–212
Tsutsumi Y (2012) Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol 42(5):375–386
Gatalica Z (1997) Immunohistochemical analysis of apocrine breast lesions. Consistent over-expression of androgen receptor accompanied by the loss of estrogen and progesterone receptors in apocrine metaplasia and apocrine carcinoma in situ. Pathol Res Pract 193(11–12):753–758. doi:10.1016/s0344-0338(97)80053-2
McNamara K, Yoda T, Miki Y, Chanplakorn N, Wongwaisayawan S, Incharoen P, Kongdan Y, Wang L, Takagi K, Mayu T, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Ohuchi N, Sasano H (2013) Androgenic pathway in triple negative invasive ductal tumours: its correlation with tumour cell proliferation. Cancer Sci 104(5):639–646. doi:10.1111/cas.12121
Miyashita M, Ishida T, Ishida K, Tamaki K, Amari M, Watanabe M, Ohuchi N, Sasano H (2011) Histopathological subclassification of triple negative breast cancer using prognostic scoring system: five variables as candidates. Virchows Arch 458(1):65–72. doi:10.1007/s00428-010-1009-2
McNamara KM, Yoda T, Nurani AM, Shibahara Y, Miki Y, Wang L, Nakamura Y, Suzuki K, Yang Y, Abe E, Hirakawa H, Suzuki T, Nemoto N, Miyashita M, Tamaki K, Ishida T, Brown KA, Ohuchi N, Sasano H (2014) Androgenic pathways in the progression of triple-negative breast carcinoma: a comparison between aggressive and non-aggressive subtypes. Breast Cancer Res Treat. doi:10.1007/s10549-014-2942-6
Ariga N, Moriya T, Suzuki T, Kimura M, Ohuchi N, Satomi S, Sasano H (2000) 17 beta-Hydroxysteroid dehydrogenase type 1 and type 2 in ductal carcinoma in situ and intraductal proliferative lesions of the human breast. Anticancer Res 20(2B):1101–1108
Takagi M, Miki Y, Miyashita M, Hata S, Yoda T, Hirakawa H, Sagara Y, Rai Y, Ohi Y, Tamaki K, Ishida T, Suzuki T, Ouchi N, Sasano H (2016) Intratumoral estrogen production and actions in luminal A type invasive lobular and ductal carcinomas. Breast Cancer Res Treat 156(1):45–55. doi:10.1007/s10549-016-3739-6
Kilic Y, Celebiler AC, Sakizli M (2014) Selecting housekeeping genes as references for the normalization of quantitative PCR data in breast cancer. Clin Trans Oncol 16(2):184–190. doi:10.1007/s12094-013-1058-5
Rayoo M, Yan M, Takano EA, Bates GJ, Brown PJ, Banham AH, Fox SB (2009) Expression of the forkhead box transcription factor FOXP1 is associated with oestrogen receptor alpha, oestrogen receptor beta and improved survival in familial breast cancers. J Clin Pathol 62(10):896–902. doi:10.1136/jcp.2009.065169
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, Banham AH (2008) Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor-beta in primary invasive breast carcinomas. Breast Cancer Res Treat 111(3):453–459. doi:10.1007/s10549-007-9812-4
Tan W, Li Q, Chen K, Su F, Song E, Gong C (2016) Estrogen receptor beta as a prognostic factor in breast cancer patients: a systematic review and meta-analysis. Oncotarget 7(9):10373–10385. doi:10.18632/oncotarget.7219
Lattrich C, Schuler S, Haring J, Skrzypczak M, Ortmann O, Treeck O (2014) Effects of a combined treatment with tamoxifen and estrogen receptor beta agonists on human breast cancer cell lines. Arch Gynecol Obstet 289(1):163–171. doi:10.1007/s00404-013-2977-7
Hinsche O, Girgert R, Emons G, Grundker C (2015) Estrogen receptor beta selective agonists reduce invasiveness of triple-negative breast cancer cells. Int J Oncol 46(2):878–884. doi:10.3892/ijo.2014.2778
Oduwole OO, Li Y, Isomaa VV, Mantyniemi A, Pulkka AE, Soini Y, Vihko PT (2004) 17beta-hydroxysteroid dehydrogenase type 1 is an independent prognostic marker in breast cancer. Cancer Res 64(20):7604–7609. doi:10.1158/0008-5472.can-04-0446
Weihua Z, Lathe R, Warner M, Gustafsson JA (2002) An endocrine pathway in the prostate, ERbeta, AR, 5alpha-androstane-3beta,17beta-diol, and CYP7B1, regulates prostate growth. Proc Natl Acad Sci USA 99(21):13589–13594
Honma N, Saji S, Hirose M, Horiguchi S, Kuroi K, Hayashi S, Utsumi T, Harada N (2011) Sex steroid hormones in pairs of tumor and serum from breast cancer patients and pathobiological role of androstene-3beta, 17beta-diol. Cancer Sci 102(10):1848–1854. doi:10.1111/j.1349-7006.2011.02018.x
Yoda T, Kikuchi K, Miki Y, Onodera Y, Hata S, Takagi K, Nakamura Y, Hirakawa H, Ishida T, Suzuki T, Ohuchi N, Sasano H, McNamara KM (2015) 11beta-Prostaglandin F2alpha, a bioactive metabolite catalyzed by AKR1C3, stimulates prostaglandin F receptor and induces slug expression in breast cancer. Mol Cell Endocrinol. doi:10.1016/j.mce.2015.07.008
McNamara KM, Yoda T, Miki Y, Nakamura Y, Suzuki T, Nemoto N, Miyashita M, Nishimura R, Arima N, Tamaki K, Ishida T, Ohuchi N, Sasano H (2014) Androgen receptor and enzymes in lymph node metastasis and cancer reoccurrence in triple-negative breast cancer. Int J Biol Mark. doi:10.5301/jbm.5000132
Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli CE (1994) Androgens regulate aromatase cytochrome P450 messenger ribonucleic acid in rat brain. Endocrinology 135(1):395–401
Maris P, Campana A, Barone I, Giordano C, Morelli C, Malivindi R, Sisci D, Aquila S, Rago V, Bonofiglio D, Catalano S, Lanzino M, Ando S (2015) Androgens inhibit aromatase expression through DAX-1: insights into the molecular link between hormone balance and Leydig cancer development. Endocrinology 156(4):1251–1262. doi:10.1210/en.2014-1654
Rizza P, Barone I, Zito D, Giordano F, Lanzino M, De Amicis F, Mauro L, Sisci D, Catalano S, Wright KD, Gustafsson JA, Ando S (2014) Estrogen receptor beta as a novel target of androgen receptor action in breast cancer cell lines. Breast Cancer Res 16(1):R21. doi:10.1186/bcr3619
Hackenberg R, Luttchens S, Hofmann J, Kunzmann R, Holzel F, Schulz KD (1991) Androgen sensitivity of the new human breast cancer cell line MFM-223. Cancer Res 51(20):5722–5727
Hickey TE, Robinson JL, Carroll JS, Tilley WD (2012) Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol 26(8):1252–1267
Acknowledgements
The authors would like to acknowledge all the members of their laboratories, whose informal input was extremely valuable. The authors would like to acknowledge the extremely capable assistance provided by both Katsuhiko Ono and Yoshiaki Onodera, in particular, and more generally by the staff at the Anatomical Pathology department of the Tohoku University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The Authors have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2016_4050_MOESM2_ESM.pdf
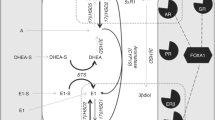
Supplementary material 2 (PDF 307 kb) Figure S1 Potential pathways of estrogen metabolism in TNBC. These figures show the enzymatic conversion of steroids (black lines) alongside the predominant or only enzymes known to catalyse that reaction (white box on top of black line). In addition, steroids as potential ligands of receptors are shown(grey dotted lines). While we and others have previously studied androgen metabolism pathways in detail (5αR1, 17βHSD5) estrogen metabolism pathways have been relatively neglected, even though these are only one enzymatic step from androgens. The presence of ERβ in TNBC tissue provides an important rationale for studying these enzymes. It should also be noted as in the arrows at the right that the presence or absence of these enzymes can determine if a carcinoma is androgen dominant or estrogen dominant. Estrogen metabolism will reduce the pool of circulating androgens and likewise androgen metabolism will limit the pool of circulating estrogens, thus these two pathways interact on multiple levels. Two pathways of estrogen metabolism are shown. In red, the canonical pathway of estrogen synthesis is given involving the non-reversible aromatization of C19 steroids to C18 steroids with the use of relatively low-potency androgens as estrogenic precursors. In blue the 3β-diol pathway is highlighted with the use of the most-potent androgen, DHT as a precursor to the production of an ERβ-selective C19 steroid, 3β-diol
10549_2016_4050_MOESM3_ESM.pdf
Supplementary material 3 (PDF 455 kb) Figure S2 Changes in expression between Normal, DCIS and IDC. IHC of histologically normal adjacent to the DCIS shown here is given in A–D. IHC immunoreactivity of ERβ1, aromatase and 17βHSD6 in DCIS is shown (E–H). Based on changes observed between normal and cancerous and DCIS and IDC components we examined the changes in expression of ERβ (I), aromatase (J) and 17βHSD6 (K) between TNBC DCIS and the levels observed in the IDC samples. In this analysis, we noted a significant decrease in the expression of 17βHSD6 (p < 0.001, Chi squared Pearsons) with no other significant changes observed on the basis of histology. The scale bar represents 200 μM
Rights and permissions
About this article
Cite this article
McNamara, K.M., Oguro, S., Omata, F. et al. The presence and impact of estrogen metabolism on the biology of triple-negative breast cancer. Breast Cancer Res Treat 161, 213–227 (2017). https://doi.org/10.1007/s10549-016-4050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-4050-2