Abstract
A proliferative marker, expressed as the percentage of cells in a cell cycle, has been developed and used as a discriminant of more aggressive malignant phenotypes in early breast cancer (BC). The marker is usually expressed by the immunohistochemical staining of the cell cycle antigen Ki-67. It has not, however, yet been definitely evaluated, due to methodological concerns, which specific Ki-67 cut-off provide the strongest prognostic information in resected BC. We conducted a meta-analysis to explore the prognostic value of different cut-off levels of Ki-67 in terms of overall survival (OS) and disease-free survival (DFS) in early BC. The databases of PubMed, the ISI Web of Science, EMBASE, SCOPUS, the Cochrane Central Register of Controlled Trials, and CINHAL were used to identify the relevant literature. Data from studies reporting a hazard ratio (HR) and a 95 % confidence interval (CI) calculated as a multivariate analysis were pooled in a meta-analysis, with metaregression used to test for trends in predefined subgroups. All the statistical tests were 2-sided. Forty-one studies encompassing 64,196 BC patients were included in the analysis. Overall, n = 25 studies were available for the OS analysis. The pooled HR for high versus low Ki-67 was 1.57 (95 % CI 1.33–1.87, P < 0.00001). Twenty-nine studies were available for the DFS analysis. The pooled HR for high versus low Ki-67 was 1.50 (95 % CI 1.34–1.69, P < 0.00001). When a cut-off of Ki-67 staining ≥ 25 % was used, the pooled HR for OS was 2.05 (95 % CI 1.66–2.53, P < 0.00001), which was significantly different to studies where the cut-offs chosen were <25 %. In ER+ tumors, the HR for high versus low Ki-67 was similar and significant (HR = 1.51, 95 % CI 1.25–1.81, P < 0.0001). We conclude that Ki-67 has an independent prognostic value in terms of OS in BC patients. The Ki-67 threshold with the greatest prognostic significance is as yet unknown, but a cut-off >25 % is associated with a greater risk of death compared with lower expression rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prognostic factors are the clinicopathological variables associated with final outcomes (usually overall survival [OS]) that are used to estimate the risk of death in early breast cancer (BC) after surgery. Among them, tumor size, nodal status, and histological grade are the variables previously validated in clinical practice [1–5]. These 3 parameters form the basis of the Nottingham Prognostic Index, which was derived from a retrospective, multivariate regression analysis and splits patients into good, moderate, and poor prognostic groups [6]. Proliferation markers, expressed as the percentage of cells in the cell cycle, have been developed and used as discriminants of more aggressive malignant phenotypes, and are usually expressed by the immunohistochemical (IHC) staining of the cell cycle antigen Ki-67. However, the optimal approach to the assessment and interpretation of Ki-67 in clinical practice is still a matter of debate among pathologists. In particular, the cut-off adopted for the discrimination of cancers with a good versus a poor prognosis is still widely discussed. Ideally, a prognostic variable should identify a disease with (or without) enough of a risk of death or relapse to require further adjuvant medical treatment to improve survival rates. This information could be particularly useful in low-risk disease candidates for chemotherapy (CT).
The first meta-analysis published on the issue was a review of 46 studies by de Azambuja et al., who collected the data of 12,000 patients [7]. Patients were regarded as presenting positive tumors for the expression of Ki-67/MIB-1 according to cut-off points defined by the authors. This meta-analysis concluded that a high Ki-67/MIB-1 labeling index confers a higher risk of relapse and a worse survival rate in patients with early BC. The limitation of this analysis is that a discriminant cut-off point was not established, and the majority of the included studies reported hazard ratios (HRs) calculated as a univariate analysis. As a consequence, a strong and true independent prognostic value of Ki-67 could not be established.
A more comprehensive, independent, prognostic validation of Ki-67 is thus required, particularly when it comes to evaluating clinical outcomes in ER+ BC. We report a systematic review and meta-analysis of the independent significance of Ki-67 expression in terms of clinical outcomes in early BC. We also assess, where possible, the influence of Ki-67 in ER+ BC and according to different cut-off points (10–20 % vs. 20–25 % vs. ≥25–30 %). Finally a metaregression analysis according to ER+ and nodal status was performed.
Materials and methods
The analysis in this paper was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [8].
Search methods and criteria for selecting studies for this review
An electronic search of PubMed, EMBASE, SCOPUS, the Web of Science, CINAHL, and the Cochrane Register of Controlled Trials was performed. The search terms included ((Ki[All Fields] AND 67[All Fields]) OR (“Ki-67 antigen”[MeSH Terms] OR (“Ki-67”[All Fields] AND “antigen”[All Fields]) OR “Ki-67 antigen”[All Fields] OR “mib 1”[All Fields]) OR (“Ki-67 antigen”[MeSH Terms] OR (“Ki-67”[All Fields] AND “antigen”[All Fields]) OR “Ki-67 antigen”[All Fields] OR “mib 1”[All Fields]) OR Ki-67[All Fields] OR “proliferative marker”[All Fields]) AND ((“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “cancer”[All Fields]) OR “breast cancer”[All Fields]) OR (“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “carcinoma”[All Fields]) OR “breast carcinoma”[All Fields])) AND (“mortality”[Subheading] OR “mortality”[All Fields] OR “survival”[All Fields] OR “survival”[MeSH Terms]) AND ((hazard[All Fields] AND (“Ratio (Oxf)”[Journal] OR “ratio”[All Fields])) OR HR[All Fields]) AND (multivariate[All Fields] OR (cox[All Fields] AND (“regression (psychology)”[MeSH Terms] OR (“regression”[All Fields] AND “(psychology)”[All Fields]) OR “regression (psychology)”[All Fields] OR “regression”[All Fields]))). The citation lists of the retrieved articles were screened manually to ensure the sensitivity of the search strategy.
The inclusion criteria for the primary analysis were as follows: 1) studies published as full articles, and in the English language, on (at least 10) adult patients with resected non-metastatic BC that reported either the prognostic impact of Ki-67 evaluated with IHC or the mRNA content in the RNA extracted from frozen or formalin-fixed paraffin-embedded (FFPE) tissue and 2) the availability of HRs and 95 % confidence intervals (CI) for OS- or BC-specific survival (BCSS). For a secondary analysis, studies providing HRs for disease-free survival (DFS) or relapse-free survival (RFS) were also included. Duplicate publications were excluded. Two reviewers (FP, MC) independently evaluated all the titles identified by the search strategy. The results were then pooled, and all potentially relevant publications were retrieved in full. The same two reviewers then evaluated the complete articles for eligibility. To avoid the inclusion of duplicated or overlapping data, we compared author names and the institutions where the patients were recruited. Then, if substantial doubts remained, the more recent study was included in the analysis.
Data extraction
The following details were extracted: name of the first author, type of study, year of publication, number of patients included in the analysis, rate of ER+ BCs, cut-off defining high Ki-67 expression, technique and antibody used for the Ki-67 staining, median follow-up, HRs for OS, DFS or BCSS as applicable, and the covariates used for the multivariate analysis of OS. The HRs were only extracted from multivariable analyses.
Data collection and statistical analysis
The meta-analysis was initially conducted for all the included studies for each of the endpoints of interest. OS was the primary outcome of interest and DFS the secondary outcome considered. “High” Ki-67 was defined according to the cut-off chosen by each author. Subgroup analyses were only conducted for ER+ BC, the different cut-offs adopted in the papers for the primary outcome (10–20 % vs. 20–25 % vs. ≥25 %, ≥20 % vs. <20 % and ≥25 % vs. <25 %), and if there were at least 3 papers for each subgroup. The extracted data were aggregated into a meta-analysis using the RevMan 5.3 software (Cochrane Collaboration, Copenhagen, Denmark). Estimates of HRs were weighted and pooled using the generic inverse variance and random effects model or the fixed effects model according to the heterogeneity [9]. Trend across subgroups was tested using metaregression with the rate of Ki-67 expression, ER+, and pN0 rates as the modifier of interest, treated as a continuous variable. The regression equation estimates the percentage of increased risk of death predicted at any given increase in Ki67, ER+, and pN0 rates of each study.
Publication bias was evaluated using Begg’s test (rank regression), Egger’s test (linear regression), and funnel plot. Rosenthal’s fail-safe N test was used to compute the number of missing studies (with a mean effect of zero) that would need to be added to the analysis to yield an overall nonsignificant effect (P > 0.05). A higher N meant more robust results. Heterogeneity was assessed with the Cochran Q and I 2 statistics. All the statistical tests were 2-sided, and statistical significance was defined as P being less than 0.05.
Results
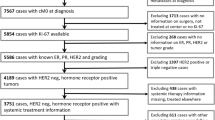
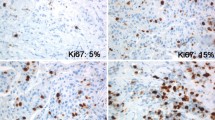
Figure 1 shows the flow diagram of the studies included in our meta-analysis. Forty-one studies published between 1996 and 2015, covering 64,196 patients, were included [10–50]. Table 1 presents the studies’ main data. The population of patients in each study varied from 92 to 20,023 cases, and the follow-up time ranged from 28 to 188 months. The MIB-1 antibody was applied to detect Ki-67 expression with IHC methods in n = 23 studies. The IHC methods were adopted in all trials except 1 that used a TMA-based analysis. Scoring system was lacking in almost all trials except in n = 11 where Ki-67 was evaluated after count in at least 250 (n = 1), 250–500 (n = 1), 500 (n = 4), 1000 (n = 3), and 2000 (n = 2) nuclei. Cut-off chosen were >5 % (n = 3); >10–11 % (n = 9); >14–15 % (n = 5); >20 % (n = 15); >25 % (n = 1); >30 % (n = 3); different cut-offs were tested in n = 3 trials and in n = 2 studies and the cut-off was not reported. More than 50 % of papers adopted Ki-67 cut-offs ≥14 %, the value first appeared to distinguish luminal B from luminal A BC as presented in the paper of Cheang et al. in 2009 [53]. In the papers presented before 2009, lower Ki-67 thresholds were, infact, used (Table 2).
Twenty-five and 27 publications had available data for the OS and DFS analyses, respectively.
Meta-analysis of overall survival
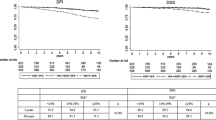
Overall, n = 25 studies were available for the OS analysis (in n = 6 studies, the BCSS rate was available instead of OS). The pooled HR for high versus low Ki-67 was 1.57 (95 % CI 1.33–1.87, P < 0.00001; Fig. 2). The heterogeneity was high (P < 0.00001, I 2 = 76 %), and so a random effects model was used.
Meta-analysis of disease-free survival
Overall, n = 29 studies were available for the DFS analysis (in n = 1 and n = 8 studies, event free survival (EFS) and RFS were reported instead of DFS). The pooled HR for high versus low Ki-67 was 1.50 (95 % CI 1.34–1.69, P < 0.00001; Fig. 3). The heterogeneity was high (P < 0.00001, I 2 = 82 %), and so a random effects model was used.
Sensitivity analysis
Twenty-three studies were considered in the subgroup analysis (n = 2 were excluded because they did not define the cut-off level for high Ki-67 expression). In studies where the cut-off value for Ki-67 was ≥10 and <20 % (n = 9 studies), the pooled HR for OS was 1.28 (95 % CI 1–1.64, P = 0.05; Fig. 4). The heterogeneity was high (P = 0.0003, I 2 = 72 %), and so a random effects model was used. In n = 10 studies, the cut-off for high Ki-67 was ≥20 but <25 %, and the pooled HR for OS was 1.44 (95 % CI 1.13–1.83, P = 0.004; Fig. 4). The heterogeneity was high (P = 0.01, I 2 = 58 %), and so a random effects model was used. Finally, where the cut-off used to split high versus low Ki-67 was ≥25 % (n = 5 studies), the pooled HR for OS was 2.05 (95 % CI 1.66–2.53, P < 0.00001; Fig. 4) with low heterogeneity (P = 0.83). The difference between the last subgroup and the others was statistically significant (P = 0.01; Fig. 4). The difference between the 2 subgroups that used the lower cut-offs (10–20 and 20–25 %) was not significant (P = 0.56).
If we grouped studies with a cut-off of Ki-67 >20 % versus those with a cut-off <20 %, the HRs were 1.31 and 1.64 (P = 0.005 and <0.00001, respectively; data not shown), although the difference among these 2 subgroups was not significant (P = 0.12). If we split the studies according to cut-offs < vs ≥25 %, the HRs were 1.38 and 2.05 (P = 0.0004 and 0.00001, respectively), and the difference among these subgroups was statistically significant (P = 0.005; Fig. 5).
In 6 studies where only ER+ tumors were analyzed, the HR for high versus low Ki-67 was significant (HR = 1.51, 95 % CI 1.25–1.81, P < 0.0001) and the heterogeneity was low (P = 0.28, I 2 = 20 %; Fig. 6).
Metaregression analysis confirmed that any increased risk of death due to high Ki-67 level is not dependent and related with rates of ER+ status in each study and rates of pN0 BCs (P = 0.38 and P = 0.31). On the contrary, the regression equation confirmed that for any 10 % increase of Ki-67 level there is a significant 19 % increase in the risk of death (P = 0.05).
We also considered any potential publication biases in the studies analyzed for the primary endpoint. Note that only 3 studies lie to the left of the funnel and 1 to the right (Fig. 7). Moderate asymmetry was observed upon visual inspection of funnel plots; however, quantitative assessment by Begg’s test (P = 0.39) and Egger’s test (P = 0.00003) suggested that there was only modest publication bias. Rosenthal’s fail-safe N was 297, meaning that 297 ‘null’ studies would need to be located and included in order for the combined 2-tailed P value to exceed 0.05. Therefore, the result was relatively robust.
Discussion
Prognostic factors play an important role in the decision-making process concerning adjuvant treatment in medical oncology. Genomic tools are now available to estimate the prognosis in the initial stages, with low and intermediate risks of relapse; and quantify the added benefit of adjuvant CT when associated with endocrine therapy. The prognostic role of Ki-67 staining in pathology reports is still a matter of debate, and is not conventionally accepted. This meta-analysis shows that a high Ki-67 cut-off level (at least 10 %), evaluated using IHC methods, is associated with more than 50 % risk of death among patients with early BC, particularly in those with ER+ disease, where the risk of death increases by a similar magnitude. Furthermore, a higher Ki-67 labeling index is associated with a greater risk of recurrence (64 % increased risk). The value of the association between the level of Ki-67 and prognosis examined in this paper was only evaluated in studies that calculated HRs using a multivariate Cox regression analysis, where Ki-67 was adjusted with respect to common prognostic variables (e.g., stage, grade, and ER status). The prognostic value of Ki-67 is also confirmed in ER+ BC studies, which is information that could potentially aid decision making about postoperative treatment. Metaregression also validated that Ki-67 level is not influenced by ER expression and nodal status. Indeed, Ki-67 becomes valuable when clinicians estimate a prognosis and have to decide the value of adjuvant CT in early-stage disease, particularly in luminal B BC.
In 2008, a similar meta-analysis was published by Stuart-Harris and colleagues [51], who analyzed the independent prognostic value of Ki-67 for OS and DFS in 13 and 14 studies, respectively. The HRs in that research were 1.73 and 1.84, and both were significant. However, that review only covered trials published up to 2004. In this paper, we now include both more and recent trials, adding further information to current knowledge. Indeed, our study includes an assessment of the cut-off point that is potentially able to separate high versus low-risk patients. A proliferative marker like Ki-67 is useful, for example, in distinguishing luminal A-like from luminal B-like tumors, but the appropriate cut-off point is still a matter of debate among oncologists. At the 2015 St. Gallen Breast Cancer Conference, a median cut-off value within the range of 20–29 % above or below which the disease can be defined as “luminal B-like” was proposed and accepted by the majority of panelists. However, a fifth of them did not consider Ki-67 to be a useful marker with which to distinguish luminal A-like from luminal B-like tumors [52].
The question of the best cut-off point to adopt for Ki-67 in clinical practice is a matter of broad discussion, and a consensus is far from being reached on the original proposal of using a threshold ≥ 14 % to distinguish luminal B from luminal A tumors [53]. ESMO guidelines, for example, refer to a cut-off of 20 % for both Ki-67 and the progesterone receptor PgR to define luminal B-like, HER-2-negative BC that is suitable for adjuvant CT [54]. However, the guidelines state that laboratory-specific cut-off points can be used to distinguish between low and high values for Ki-67 and PgR. Quality assurance programs are also essential for laboratories reporting these results. Conversely, NCCN guidelines do not currently recommend the assessment of Ki-67 [55]. The meta-analysis in this paper shows that Ki-67 is best regarded as a continuum, because all cut-off points above 10 % are associated with a poorer prognosis. We cannot define a precise cut-off point above or below which the prognosis is very different. However, if we split studies with a cut-off >25 % and compare them with those that used a cut-off below this figure, the prognostic information is greatest in the former (HR = 2.05 vs. 1.38) and the difference between them is significant. Alternatively, if we split studies with a cut-off ≥ vs. <20 %, both groups are associated with a significantly increased risk of death (HR 1.64 vs. 1.31), although the subgroup difference is not significant. Attempts to standardize Ki-67 assessments were made by Dowsett et al. [56] and Polley et al., who evaluated the factors contributing to inter-laboratory discordance that can make it difficult to obtain a useful cut-off point for clinical decision making [57]. The same authors made similar attempts in 2015, and stated that before Ki-67 could be recommended for clinical use, further research was needed to standardize how it is assessed and correlate it with outcomes [58].
In addition to its prognostic importance, several authors have also demonstrated the value of Ki-67 for predicting the benefit of adjuvant therapy in high-risk luminal B-like and node-negative patients. Penault-Llorca showed that patients whose tumors had Ki-67 levels >20 % benefited from the addition of docetaxel (HR 0.51 vs. 1.03 for those with Ki-67 < 20 %) [59]. Similarly, Criscitiello and colleagues found a significant benefit from the addition of CT to endocrine therapy in luminal B-like BC with a Ki-67 > 32 % [60]. Viale et al. [11], through a centralized analysis of Ki-67 by IHC in tumor blocks of patients enrolled in the BIG 1-98 trial, confirmed the prognostic role of Ki-67 in a population treated with endocrine therapy alone. They also observed a greater benefit of letrozole compared to tamoxifen in BC patients whose tumors had Ki-67 levels >11 % (HR = 0.53 vs. 0.81 in those with Ki-67 ≤ 11 %). The significance of Ki-67 in highly proliferative tumors like triple-negative BC is, however, unknown, because almost all these BCs have a very proliferative phenotype.
Our meta-analysis has some intrinsic limitations. First, the population data were extracted from published papers and individual patient data were unavailable. Second, the cut-offs used in the series were chosen conventionally by the authors, and there is no definitive consensus among pathologists to date on the optimal Ki-67 threshold for accurately defining high versus low-risk patients, particularly in luminal B-like disease. Third, each cut-off interval defined in this meta-analysis (10–20, 20–25 and >25 %) was chosen somewhat arbitrarily, and the greater magnitude in studies with higher cut-offs does not mean that this represents the ideal point at which to split patients with the poorest prognosis. Also, scoring methods (nuclei counts) were not specified in almost all trials, and this methodology concern could have contributed to the observed differences and heterogeneity among cut-offs. Finally, Ki-67 cut-off, as calculated from this meta-analysis, does not permit to anticipate and suggest a better or lesser benefit from adjuvant therapy. It is merely a prognostic variable, which can aid the discussion and information with the patient to estimate his risk of relapse and death, but not the relative advantage with any systemic therapy. Probably, a Ki-67 labeling index should be used as a continuous score, with the highest values associated with the greatest risk of death, and this was confirmed by metaregression analysis. However, this meta-analysis confirms the independent prognostic significance of Ki-67 in early BC, in both pN0 and pN + stages, and particularly in the ER+ subgroup. An arbitrary cut-off of at least 25 % seems to be associated with better discriminatory power compared with lower cut-off points (10–20 and 20–25 %).
The present data are derived from studies published mainly in the last decade that encompass more than 60,000 BC patients, and are more robust and up to date compared to the work examined in similar previous meta-analyses.
Using IHC to assess the Ki-67 labeling index is less expensive and more widely available than the genomic tests that are currently used to identify patients with high-risk lymph node-negative BC who may benefit from adjuvant CT. Some publications have already evaluated and identified the concordance between the Ki-67 labeling index and the results of the Oncotype Dx® associated risk of recurrence [61–64]. Recently, a Magee equation using histological variables including Ki-67 was able to predict correctly the final Oncotype Dx® score in a series of 283 case of BC [65]. Similarly IHC4 score, that considers ER, PgR, HER2, and Ki-67, was able to independently predict prognosis as well as Oncotype Dx® [66, 67]. Accordingly, the Ki-67 labeling index may be a useful marker where molecular assays are not technically feasible or available.
In conclusion, this meta-analysis of a series of more than 60,000 patients confirms that the proliferative marker Ki-67 has an independent prognostic value in terms of survival and relapse in patients with early-stage BC, and should be routinely assessed by pathologists.
A Ki-67 threshold of at least 25 % of immunostained cells is associated with the most powerful outcome prognostication; however, this not relieve the need for a prospective validation.
References
Page DL (1991) Prognosis and breast cancer. Recognition of lethal and favourable prognostic types. Am J Surg Pathol 15:334–349
Koscielny S, Tubiana M, Lê MG et al (1984) Breast cancer: relationship between the size of the primary tumour and the probability of metastatic dissemination. Br J Cancer 49:709–715
Rosen PP, Groshen S, Saigo PE et al (1989) Pathological prognostic factors in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma: a study of 644 patients with median follow-up of 18 years. J Clin Oncol 7:1239–1251
Carter CL, Allen C, Henson DE (1989) Relation of tumour size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63:181–187
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Galea MH, Blamey RW, Elston CE et al (1992) The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 22:207–219
de Azambuja E, Cardoso F, de Castro Jr G et al (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177e88
Aleskandarany MA, Green AR, Benhasouna AA et al (2012) Prognostic value of proliferation assay in the luminal, HER2-positive, and triple-negative biologic classes of breast cancer. Breast Cancer Res 14(1):R3
Viale G, Giobbie-Hurder A, Regan MM et al (2008) Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol 26(34):5569–5575
Bago-Horvath Z, Rudas M, Dubsky P et al (2011) Adjuvant sequencing of tamoxifen and anastrozole is superior to tamoxifen alone in postmenopausal women with low proliferating breast cancer. Clin Cancer Res 17(24):7828–7834
Boström P, Söderström M, Vahlberg T et al (2011) MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 11(11):348
Brennan DJ, Rexhepaj E, O’Brien SL et al (2008) Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clin Cancer Res 14(9):2681–2689
Brown RW, Allred CD, Clark GM et al (1996) Prognostic value of Ki-67 compared to S-phase fraction in axillary node-negative breast cancer. Clin Cancer Res 2(3):585–592
Cancello G, Maisonneuve P, Rotmensz N et al (2013) Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol 24(3):661–668
De Cicco C, Gilardi L, Botteri E et al (2013) Is [18F] fluorodeoxyglucose uptake by the primary tumor a prognostic factor in breast cancer? Breast 22(1):39–43
Dolled-Filhart M, McCabe A, Giltnane J et al (2006) Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res 66(10):5487–5494
Dumontet C, Krajewska M, Treilleux I et al (2010) BCIRG 001 molecular analysis: prognostic factors in node-positive breast cancer patients receiving adjuvant chemotherapy. Clin Cancer Res 16(15):3988–3997
Ermiah E, Buhmeida A, Abdalla F et al (2012) Prognostic value of proliferation markers: immunohistochemical ki-67 expression and cytometric s-phase fraction of women with breast cancer in Libya. J Cancer 3:421–431
Gamucci T, Vaccaro A, Ciancola F et al (2013) Recurrence risk in small, node-negative, early breast cancer: a multicenter retrospective analysis. J Cancer Res Clin Oncol 139(5):853–860
Gluz O, Nitz UA, Harbeck N et al (2008) Triple-negative high-risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: results of WSG AM-01 trial. Ann Oncol 19(5):861–870
Inwald EC, Klinkhammer-Schalke M, Hofstädter F et al (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139(2):539–552
Jacquemier J, Boher JM, Roche H et al (2011) Protein expression, survival and docetaxel benefit in node-positive breast cancer treated with adjuvant chemotherapy in the FNCLCC-PACS 01 randomized trial. Breast Cancer Res 13(6):R109
Jacquemier J, Charafe-Jauffret E, Monville F et al (2009) Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res 11(2):R23
Jung SY, Jeong J, Shin SH et al (2010) The invasive lobular carcinoma as a prototype luminal A breast cancer: a retrospective cohort study. BMC Cancer 3(10):664
Jung SY, Kim HY, Nam BH et al (2010) Worse prognosis of metaplastic breast cancer patients than other patients with triple-negative breast cancer. Breast Cancer Res Treat 120(3):627–637
Kim RG, Kim EK, Kim HA et al (2011) Prognostic significance of molecular subtype in T1N0M0 breast cancer: Korean experience. Eur J Surg Oncol 37(7):629–634
Kwon JH, Kim YJ, Lee KW et al (2010) Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carcinoma of 1 cm or less. BMC Cancer 15(10):557
Lee JA, Kim KI, Bae JW et al (2010) Triple negative breast cancer in Korea-distinct biology with different impact of prognostic factors on survival. Breast Cancer Res Treat 123(1):177–187
Lee KH, Im SA, Oh DY et al (2007) Prognostic significance of bcl-2 expression in stage III breast cancer patients who had received doxorubicin and cyclophosphamide followed by paclitaxel as adjuvant chemotherapy. BMC Cancer 12(7):63
Li FY, Wu SG, Zhou J et al (2014) Prognostic value of Ki-67 in breast cancer patients with positive axillary lymph nodes: a retrospective cohort study. PLoS ONE 9(2):e87264
Lin CH, Lu YS, Huang CS et al (2011) Prognostic molecular markers in women aged 35 years or younger with breast cancer: is there a difference from the older patients? J Clin Pathol 64(9):781–787
Loussouarn D, Campion L, Leclair F et al (2009) Validation of UBE2C protein as a prognostic marker in node-positive breast cancer. Br J Cancer 101(1):166–173
Meattini I, Desideri I, Saieva C et al (2014) Impact of sentinel node tumor burden on outcome of invasive breast cancer patients. Eur J Surg Oncol 40(10):1195–1202
Michalides R, van Tinteren H, Balkenende A et al (2002) Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br J Cancer 86(3):402–408
Naoi Y, Kishi K, Tanei T et al (2011) Development of 95-gene classifier as a powerful predictor of recurrences in node-negative and ER-positive breast cancer patients. Breast Cancer Res Treat 128(3):633–641
Niikura N, Masuda S, Kumaki N et al (2014) Prognostic significance of the Ki67 scoring categories in breast cancer subgroups. Clin Breast Cancer. 14(5):323–329.e3
Nishimura R, Osako T, Okumura Y et al (2010) Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med 1(5):747–754
Rocca A, Bravaccini S, Scarpi E et al. Benefit from anthracyclines in relation to biological profiles in early breast cancer. Breast Cancer Res Treat. 2014; 144(2):307–318. Erratum in: Breast Cancer Res Treat. 2014; 144(2):319
Sundara Rajan S, Hanby AM, Horgan K et al (2014) The potential utility of geminin as a predictive biomarker in breast cancer. Breast Cancer Res Treat 143(1):91–8
Synnestvedt M, Borgen E, Russnes HG et al (2013) Combined analysis of vascular invasion, grade, HER2 and Ki67 expression identifies early breast cancer patients with questionable benefit of systemic adjuvant therapy. Acta Oncol 52(1):91–101
Treré D, Ceccarelli C, Migaldi M et al (2006) Cell proliferation in breast cancer is a major determinant of clinical outcome in node-positive but not in node-negative patients. Appl Immunohistochem Mol Morphol 14(3):314–323
Tutt A, Wang A, Rowland C et al (2008) Risk estimation of distant metastasis in node-negative, estrogen receptor-positive breast cancer patients using an RT-PCR based prognostic expression signature. BMC Cancer 21(8):339
Wiesner FG, Magener A, Fasching PA et al (2009) Ki-67 as a prognostic molecular marker in routine clinical use in breast cancer patients. Breast 18(2):135–141
Xue C, Wang X, Peng R et al (2012) Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci 103(9):1679–1687
Yamamoto S, Ibusuki M, Yamamoto Y et al (2013) Clinical relevance of Ki67 gene expression analysis using formalin-fixed paraffin-embedded breast cancer specimens. Breast Cancer. 20(3):262–270
Zhang X, Li P, Ma W et al (2013) Risk factors of recurrence in small-sized, node negative breast cancer in young women: a retrospective study in Chinese population. Sci China Life Sci 56(4):335–340
Zong Y, Zhu L, Wu J et al (2014) Progesterone receptor status and Ki-67 index may predict early relapse in luminal B/HER2 negative breast cancer patients: a retrospective study. PLoS One 9(8):e95629
Cho U, Kim HE, Oh WJ et al (2015) The long-term prognostic performance of Ki-67 in primary operable breast cancer and evaluation of its optimal cutoff value. Appl Immunohistochem Mol Morphol. doi:10.1097/PAI.0000000000000164
Stuart-Harris R, Caldas C, Pinder SE, Pharoah P (2008) Proliferation markers and survival in early breast cancer: a systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 17(4):323–334
Coates AS, Winer EP, Goldhirsch A et al (2015) Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 26:1533–1546
Cheang MC, Chia SK, Voduc D et al (2009) Ki-67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 101:736–750
Senkus E, Kyriakides S, Penault-Llorca F et al (2013) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi7–23
http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 12 April 2014
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664
Polley MY, Leung SC, McShane LM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906
Polley MY, Leung SC, Gao D et al (2015) An international study to increase concordance in Ki67 scoring. Mod Pathol 28(6):778–786
Penault-Llorca F, André F, Sagan C et al (2009) Ki67 expression and docetaxel efficacy in patients with estrogen receptor-positive breast cancer. J Clin Oncol 27(17):2809–2815
Criscitiello C, Disalvatore D, De Laurentiis M et al (2014) High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast cancer. Breast 23(1):69–75
Sahebjam S, Aloyz R, Pilavdzic D et al (2011) Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br J Cancer 105(9):1342–1345
Acs G, Esposito NN, Kiluk J et al (2012) A mitotically active, cellular tumor stroma and/or inflammatory cells associated with tumor cells may contribute to intermediate or high Oncotype DX Recurrence Scores in low-grade invasive breast carcinomas. Mod Pathol 25(4):556–566
Aktas B, Bankfalvi A, Heubner M et al (2013) Evaluation and correlation of risk recurrence in early breast cancer assessed by Oncotype DX(®), clinicopathological markers and tumor cell dissemination in the blood and bone marrow. Mol Clin Oncol 1(6):1049–1054
Thaker NG, Hoffman KE, Stauder MC et al (2015) The 21-gene recurrence score complements IBTR! Estimates in early-stage, hormone receptor-positive, HER2-normal, lymph node-negative breast cancer. Springerplus 30(4):36
Turner BM, Skinner KA, Tang P, Jackson MC, Soukiazian N, Shayne M, Huston A, Ling M, Hicks DG (2015) Use of modified Magee equations and histologic criteria to predict the Oncotype DX recurrence score. Mod Pathol 28(7):921–931
Cuzick J, Dowsett M, Pineda S et al (2011) Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol 29(32):4273–4278
Sgroi DC, Sestak I, Cuzick J et al (2013) Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 14(11):1067–1076
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have disclosed any potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Petrelli, F., Viale, G., Cabiddu, M. et al. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients. Breast Cancer Res Treat 153, 477–491 (2015). https://doi.org/10.1007/s10549-015-3559-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3559-0