Abstract
Cancer-associated fibroblasts (CAFs) are classified into various functional subtypes such as fibroblast activation protein-α (FAP-α), fibroblast specific protein-1 (FSP-1), platelet-derived growth factor receptor-α (PDGFR-α), and PDGFR-β. In this study, we compared the expression of CAF-related proteins in invasive lobular carcinoma (ILC) with those in invasive carcinoma of no special type (NST) and assessed the implications of the differences observed. Using tissue microarrays of 104 ILC and 524 invasive carcinoma (NST) cases, immunohistochemistry for CAF-related proteins [podoplanin, prolyl 4-hydroxylase, FAP-α, FSP-1/S100A4, PDGFR-α, PDGFR-β, and chondroitin sulfate proteoglycan (NG2)] was conducted. In invasive carcinoma (NST), tumor cells expressed a high level of PDGFR-α, whereas ILC tumor cells expressed high levels of podoplanin, prolyl 4-hydroxylase, FAP-α, and FSP-1/S100A4. In stromal cells of invasive carcinoma (NST), high expression levels of prolyl 4-hydroxylase, PDGFR-α, and NG2 were observed, whereas ILC stromal cells expressed high levels of FAP-α, FSP-1/S100A4, and PDGFR-β. In ILC, tumoral FSP-1/S100A4 positivity was associated with higher Ki-67 labeling index (p = 0.010) and non-luminal A type cancer (p = 0.014). Stromal PDGFR-α positivity was associated with lymph node metastasis (p = 0.011). On survival analysis of entire cases, tumoral FSP-1/S100A4 positivity (p = 0.002), stromal podoplanin positivity (p = 0.041), and stromal FSP-1/S100A4 negativity (p = 0.041) were associated with shorter disease-free survival; only tumoral FSP-1/S100A4 positivity (p = 0.044) was associated with shorter overall survival. In ILC, the expression of FAP-α and FSP-1/S100A4 was higher in both tumor and stromal cells than that observed in invasive carcinoma (NST). These results indicate that CAFs are a potential target in ILC treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the most common cancers in females. Among various types of breast cancer, invasive carcinomas can be classified according to histologic subtypes, such as invasive carcinoma of no special type (NST) and invasive lobular carcinoma (ILC) [1]. ILC accounts for approximately 5–15 % of invasive carcinomas [2, 3], and its incidence has increased to a greater extent than that of invasive carcinoma (NST) due to hormone replacement therapy and increased alcohol intake [4, 5]. ILC differs from invasive carcinoma (NST) in several aspects; clinically, ILC shows frequent multiplicity and bilaterality [6, 7], and histologically, non-cohesive cancer cells are observed in ILC due to the loss of E-cadherin [8]. In addition, sites of metastasis with ILC are different from those of invasive carcinoma (NST). For example, bone, gastrointestinal tract, uterus, meninges, ovary, and diffuse serosal involvement are observed frequently with ILC [7, 9, 10].
As research on cancer has progressed, recognition of the importance of the tumor microenvironment has gradually increased. Among several elements comprising the tumor microenvironment, the most important and frequently investigated factor is cancer-associated fibroblasts (CAFs) [11]. CAFs are located adjacent to cancer cells and are associated with tumor initiation, tumor-stimulatory inflammation, metabolism, metastasis, drug responses, and immune surveillance [12]. Despite the important impact of CAFs on cancer, the exact origin of these cells has not been elucidated. In addition, an accurate definition of CAFs remains controversial [11, 12].
Several molecules have been suggested as CAF markers. These include α-smooth muscle actin (αSMA) [13], tenascin-C [14], chondroitin sulfate proteoglycan (NG2) [15], platelet-derived growth factor receptor-α/β (PDGFR-α/β) [16], fibroblast activation protein (FAP) [17], podoplanin [18], prolyl 4-hydroxylase [19], and fibroblast specific protein-1 (FSP-1) [15]. Each CAF marker plays a characteristic role in CAF cross-talk with cancer cells (Table 1). Based on these markers, CAFs can be categorized into various functional subsets. According to one study, CAFs can be divided into 4 types: FAP-α, FSP-1, PDGFR-α, and PDGFR-β. Each type shows different characteristics [20], supporting the hypothesis that CAF phenotypes may be diverse.
In breast cancer, several studies have been conducted about the cross-talk between CAFs and cancer cells. CAFs are associated with tumor progression, invasion or metastasis, therapeutic resistance, and prognosis in breast cancer. Due to varying amounts of tumor stroma, it is expected that the impact of CAFs on breast cancer is greater than that on other cancers. CAFs are involved in various clinicopathologic parameters of breast cancer [21]. In a recent study, the tissue microenvironment, such as CAFs and intratumor vessels, differed between invasive carcinoma (NST) and ILC [22]. This finding raises the possibility of differences in CAF phenotypes between invasive carcinoma (NST) and ILC. However, a comparative analysis about CAF-related proteins between invasive carcinoma (NST) and ILC has not been performed. In the current study, we investigated the difference in expression of CAF-related proteins between ILC and invasive carcinoma (NST) and attempted to determine its clinical implications.
Materials and methods
Patient selection and clinicopathologic evaluation
Formalin-fixed paraffin-embedded (FFPE) tissue samples from ILC patients who underwent surgical resection from January 2000 to December 2012 in Severance Hospital, Seoul, South Korea, were used in this study. Cases diagnosed in 2006 as invasive carcinoma (NST) were used as the control group. Patients who underwent neoadjuvant chemotherapy were excluded from this study. All cases were reviewed retrospectively by a breast pathologist (Koo, JS), and histologic evaluations were performed on hematoxylin- and eosin-stained slides. The histological grade was assessed based on the Nottingham grading system [23]. Tumor staging was based on the 7th American Joint Committee on Cancer criteria. Disease-free survival (DFS) time was calculated from the date of the first curative surgery to the date of the first loco-regional or systemic relapse, or death without any type of relapse. Overall survival time was estimated from the date of the first curative operation to the date of the last follow-up, or death from any cause. Clinicopathologic parameters evaluated in each breast cancer patient included age at initial diagnosis, lymph node metastasis, tumor recurrence, distant metastasis, and survival. This study was approved by the Institutional Review Board of the Severance Hospital.
Tissue microarray
After reviewing the hematoxylin- and eosin-stained slides, the most appropriate FFPE tumor tissue samples were collected retrospectively. The most representative tumor area was marked on the FFPE tissue blocks and then extracted using a punch machine. The extracted 3 mm tissue core was transferred to a 6 × 5 recipient block. Two tissue cores were extracted from each case for tissue microarray construction.
Immunohistochemistry
The antibodies used for immunohistochemistry (IHC) on FFPE tissue sections are shown in Table 2. After sectioning the paraffin blocks to 3-μm thickness, the sections were deparaffinized and rehydrated using xylene and ethanol solutions. IHC was conducted with the Ventana Discovery XT automated stainer (Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s protocol. Cell Conditioning 1 buffer (EDTA, pH 8.0, Ventana Medical Systems) was used for antigen retrieval. IHC was performed with inclusion of appropriate positive and negative controls.
Interpretation of IHC results
A cut-off value of 1 % or more positively stained nuclei was used to define estrogen receptor (ER) and progesterone receptor (PR) positivity [24]. Human epidermal growth factor receptor-2 (HER-2) staining was analyzed according to the American Society of Clinical Oncology/College of American Pathologists guidelines, which used the following categories: 0, no immunostaining or incomplete faint/barely perceptible membranous staining in less than 10 % of tumor cells; 1+, incomplete faint/barely perceptible membranous staining in more than 10 % of tumor cells; 2+, incomplete circumferential weak/moderate membranous staining in more than 10 % of tumor cells or complete circumferential intense membranous staining in less than 10 % of tumor cells; and 3+, complete circumferential intense membranous staining in more than 10 % of tumor cells [25]. HER-2 immunostaining was considered positive when strong (3+) membranous staining was observed. Cases scored 0 to 1+ were regarded as negative. Cases showing 2+ HER-2 expression were evaluated for HER-2 amplification by fluorescent in situ hybridization. IHC markers for CAF-related proteins were assessed by light microscopy. The stained slides were evaluated semiquantitatively according to a method reported previously [26]. Tumor and stromal cell staining were assessed as 0, negative or weak immunostaining in <1 % of the tumor/stroma; 1, focal expression in 1–10 % of tumor/stroma; 2, positive in 11–50 % of tumor/stroma; and 3, positive in 51–100 % of tumor/stroma. The evaluation of stained slides was performed on the entire tumor area, and scores of 2 or higher were regarded as positive.
Tumor phenotype classification
Breast cancer phenotypes were classified based on the IHC results for ER, PR, HER-2, and Ki-67 labeling index (LI), and the fluorescent in situ hybridization results for HER-2. Breast cancer phenotypes were defined as follows [27]: (1) Luminal A type—ER and/or PR positive, HER-2 negative, and Ki-67 LI <14 %; (2) Luminal B type (HER-2 negative)—ER and/or PR positive, HER-2 negative, and Ki-67 LI ≥14 %; and Luminal B type (HER-2 positive)—ER and/or PR positive and HER-2 overexpressed and/or amplified; (3) HER-2 type—ER and PR negative, and HER-2 overexpressed and/or amplified; and (4) Triple negative breast cancer (TNBC) type—ER, PR, and HER-2 negative.
Statistical analysis
Data were analyzed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA). Student’s t test and Fisher’s exact test were used for continuous and categorical variables, respectively. For data analyses involving multiple comparisons, p values were corrected using the Bonferroni multiple comparison procedure. Statistical significance was assumed when p < 0.05. Kaplan–Meier survival curves and log-rank statistics were employed to evaluate time to tumor metastasis and time to survival. Multivariate regression analysis was performed using a Cox proportional hazards model.
Results
Basal characteristics of ILC
The clinicopathologic characteristics of 104 ILC cases are summarized in Table 3. Of these cases, 93 (89.4 %) were the classic type and 11 (10.6 %) were the pleomorphic type. Compared with the classic type, the pleomorphic type was associated with older age (p = 0.010), higher nuclear grade (p < 0.001), higher histologic grade (p < 0.001), higher T stage (p = 0.027), PR negativity (p = 0.016), HER-2 positivity (p = 0.002), higher Ki-67 LI (p < 0.001), and the non-luminal A subtype (p < 0.001).
Expression of CAF-related proteins in ILC according to histologic type
When comparing the expression of CAF-related proteins in ILC according to histologic subtypes, PDGFR-β and NG2 were not expressed in tumor cells. In contrast, PDGFR-α and NG2 were not expressed in stromal cells. There were no significant differences in the expression of CAF-related proteins between the classic and pleomorphic types of ILC (Supplementary Table 1).
Comparison of the expression of CAF-related proteins between invasive carcinoma (NST) and ILC
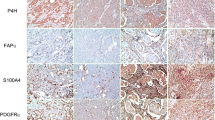
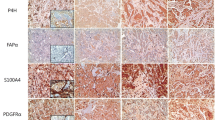
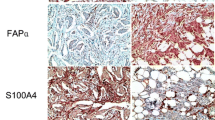
Differences were observed using IHC in the expression of CAF-related proteins between invasive carcinoma (NST) and ILC. In the cancer cell compartment, PDGFR-α was expressed more highly in invasive carcinoma (NST) than that in ILC (p < 0.001), whereas podoplanin, prolyl 4-hydroxylase, FAP-α, and FSP-1/S100A4 were expressed more highly in ILC than those in invasive carcinoma (NST) (p ≤ 0.001). In the stromal cell compartment, the expression of prolyl 4-hydroxylase (p = 0.001) and PDGFR-α (p < 0.001) was higher in invasive carcinoma (NST) than in ILC, while FAP-α, FSP-1/S100A4, and PDGFR-β (p ≤ 0.001) were expressed more highly in ILC than in invasive carcinoma (NST) (Table 4 and Fig. 1). The expression of FSP-1/S100A4 between invasive carcinoma (NST) and ILC differed not only in amount but also in the pattern. In invasive carcinoma (NST), a fascicular pattern was observed, whereas in ILC, a scattered pattern was observed (Fig. 2).
Comparison of the expression of CAF-related proteins between invasive carcinoma (NST) and ILC. In the cancer cell compartment, invasive carcinoma (NST) showed a higher expression of PDGFR-α whereas ILC showed a higher expression of podoplanin, prolyl 4-hydroxylase, FAP-α, and FSP-1/S100A4. In the stromal cell compartment, a higher expression of prolyl 4-hydroxylase, PDGFR-α, and NG2 was observed in invasive carcinoma (NST) compared to ILC. However, ILC showed a higher expression of FAP-α, FSP-1/S100A4, and PDGFR-β. Scale bar represents 300 μm
Comparison of the expression of FSP-1/S100A4 between invasive carcinoma (NST) and ILC. For invasive carcinoma (NST), FSP-1/S100A4-positive cells showed a fascicular pattern with group formation. In contrast, ovoid or round cells were FSP-1/S100A4 positive in ILC. These cells exhibited a scattered but not grouped pattern. Scale bar represents 100 μm
The majority of ILC cases were classified as the luminal type. Thus, we selected luminal type invasive carcinoma (NST) cases and compared the expression of CAF-related proteins with ILC. The results showed that differences in the expression of these proteins between ILC and luminal type invasive carcinoma (NST) were similar to those between ILC and invasive carcinoma (NST) (Table 5).
All ILC cases were classified into two groups according to the number of expressed CAF markers in stromal cells: cases with no more than one expressed CAF marker and those with two or more expressed CAF markers. Comparing the clinicopathologic features between the two groups, significant differences were observed in lymph node metastasis (p = 0.033) and estrogen receptor (ER) status (p = 0.038); in particular, cases expressing no more than one CAF-related protein showed higher proportions of lymph node metastasis and ER negativity (Table 6). In addition, cases expressing two or more CAF-related proteins showed a trend toward younger age (p = 0.066), higher nuclear grade (p = 0.097), HER-2 positivity (p = 0.083), luminal B type (p = 0.079), and pleomorphic type (p = 0.097).
Correlations between CAF-related proteins and clinicopathologic factors in ILC
Our analysis of the relationship between various clinicopathologic factors and the expression of CAF-related proteins in ILC indicated that positivity for tumoral FSP-1/S100A4 was associated with a higher Ki-67 LI (p = 0.010) and the non-luminal A type (p = 0.014). Stromal PDGFR-α positivity was associated with lymph node metastasis (p = 0.011) (Fig. 3).
Impact of the expression status for CAF-related proteins on the prognosis of ILC and invasive breast cancer
To investigate the impact of the expression of CAF-related proteins on the prognosis of ILC, a univariate analysis was performed. The expression of CAF-related proteins was not associated with the DFS or OS times (Supplementary Table 2).
The impact of the expression of CAF-related proteins on the prognosis was analyzed by performing a univariate analysis of all of 628 invasive breast cancer cases. Tumoral FSP-1/S100A4 positivity (p = 0.002), stromal podoplanin positivity (p = 0.041), and stromal FSP-1/S100A4 negativity (p = 0.041) were associated with shorter DFS, and only tumoral FSP-1/S100A4 positivity (p = 0.044) was associated with shorter OS (Table 7; Fig. 4). In addition, another univariate analysis was performed to investigate the impact of the expression of CAF-related proteins on the prognosis based on ER status. In ER-positive breast cancer (n = 441), stromal podoplanin positivity (p = 0.045) was associated with shorter DFS. In ER-negative breast cancer (n = 168), stromal FSP-1/S100A4 negativity (p = 0.023) and tumoral FSP-1/S100A4 positivity (p = 0.023) were associated with shorter DFS (Fig. 4).
Disease-free survival (DFS) and overall survival (OS) according to the expression of CAF-related proteins in invasive breast cancer, ER-positive breast cancer, and ER-negative breast cancer. (a) Stromal podoplanin positivity, (b) tumoral FSP-1/S100A4 positivity, and (c) stromal FSP-1/S100A4 negativity are associated with shorter DFS, and (d) tumoral FSP-1/S100A4 positivity is associated with shorter OS in invasive breast cancer. In ER-positive breast cancer, (e) stromal podoplanin positivity is associated with shorter DFS. In ER-negative breast cancer, (f) stromal FSP-1/S100A4 negativity and (g) tumoral FSP-1/S100A4 positivity are associated with shorter DFS
On multivariate Cox analysis of all of 628 invasive breast cancer cases, higher T stage (hazard ratio 4.057, 95 % CI 1.683–9.779, p = 0.002), tumoral FSP-1/S100A4 positivity (hazard ratio 3.462, 95 % CI 1.414–8.479, p = 0.007), and stromal FSP-1/S100A4 negativity (hazard ratio 2.465, 95 % CI 1.113–5.461, p = 0.026) were independent factors associated with shorter DFS. For OS, higher T stage (hazard ratio 2.239, 95 % CI 1.034–4.849, p = 0.041) and lymph node metastasis (hazard ratio 2.011, 95 % CI 1.014–3.989, p = 0.046) were independent factors associated with shorter OS (Table 8).
Discussion
In this study, we compared the expression of these proteins in ILC and invasive carcinoma (NST). Using IHC, the expression of CAF-related proteins differed between ILC and invasive carcinoma (NST). In the cancer cell compartment, invasive carcinoma (NST) showed higher expression of PDGFR-α whereas a higher expression of podoplanin, prolyl 4-hydroxylase, FAP-α, and FSP-1/S100A4 was observed in ILC. The expression of PDGFR-α was increased in TNBC [28, 29], a subtype of invasive carcinoma (NST). The results of these previous studies accord well with our finding that the expression of PDGFR-α in tumor cells is higher in invasive carcinoma (NST) than that in ILC.
The expression of podoplanin in tumor cells is associated with cancer cell migration and invasion [30, 31], and the expression of prolyl 4-hydroxylase is associated with disease progression and metastasis in breast cancer [32]. The expression of podoplanin and prolyl 4-hydroxylase in tumor cells also correlates with cell motility. Histologically, ILC shows discohesive and infiltrative features [1], and it is expected that ILC will show higher cell motility than invasive carcinoma (NST). ILC does show a higher rate of lymph node metastasis than invasive carcinoma (NST) [33]. In addition, a previous study reported that the expression of a pseudopodial constituent such as α-parvin was only observed in ILC [34]. These previous findings are consistent with the results of the current study.
The current results showed that tumor stroma exhibited different expression levels of CAF-related proteins between invasive carcinoma (NST) and ILC. A higher expression of prolyl 4-hydroxylase, PDGFR-α, and NG2 was observed in invasive carcinoma (NST) stromal cells, while ILC stromal cells showed higher expression of FAP-α, FSP-1/S100A4, and PDGFR-β. These findings suggested the possibility of differences in CAF characteristics of tumor stroma between invasive carcinoma (NST) and ILC. According to a previous study examining differences in the tumor microenvironment between ILC and invasive carcinoma (NST), ILC showed more conspicuous proliferation of CAFs and endothelial cells than invasive carcinoma (NST), whereas invasive carcinoma (NST) showed more prominent maturation of newly formed microvessels than ILC [22, 35]. Alpha-smooth muscle actin was used as a marker of CAF in the previous study [22], while we used seven different CAF markers. Thus, a direct comparison between the two studies is difficult. However, NG2 is a marker for mature pericytes that is only expressed in invasive carcinoma (NST), and PDGFR-β, which is highly expressed in ILC, is a marker for immature pericytes [36]. Thus, our findings correspond well with the results of previous studies. In a study performed using a mouse xenograft model for breast cancer, αSMA-positive CAFs showed 96 % accordance with CAFs expressing PDGFR-β [15]. This finding supports a previous report on the more prominent proliferation of αSMA-positive CAFs in ILC than in invasive carcinoma (NST) [22] and is consistent with our study showing higher PDGFR-β expression in stroma in ILC than in invasive carcinoma (NST). Desmoplastic stroma, which is a frequently observed histologic finding in invasive carcinoma (NST) [37], is caused by PDGFR-α type CAFs [38]. We also found higher expression of PDGFR-α in the stroma of invasive carcinoma (NST), which is consistent with these prior findings.
The expression of FAP-α in breast cancer cells is associated with cell motility and invasion [39, 40]. In addition, FSP-1/S100A4 expression in breast cancer cells correlates with cell motility and invasion [41, 42]. Our results show that the expression of FAP-α and FSP-1/S100A4 in ILC is higher than in invasive carcinoma (NST) in both the cancer cell and stromal compartments. Thus, these findings are consistent with the clinical, histologic, and biologic features of ILC. FAP-α type CAFs are associated with activation of CAFs, modulation of the extracellular matrix, and immunomodulatory functions [20]. In previous studies, increases in the number of αSMA-positive CAFs were more prominent in ILC than those in invasive carcinoma (NST) [22]. This suggests that the number of activated CAFs may increase in ILC. In addition, the immune-related subtype may be one of the two biologically distinct subtypes in ILC [43]. Thus, it is possible that CAFs with an immunomodulatory function may be more prominent in ILC. However, further studies are required to confirm this possibility.
In a previous colocalization study using a mouse xenograft model for breast cancer, αSMA-positive CAFs, and FSP-1/S100A4 CAFs, showed minimal overlap [15]. Although not investigated fully, we expect that the FSP-1/S100A4 type CAFs may have a unique function. For example, the FSP-1/S100A4 type CAF is associated with metastatic colonization [44] and carcinogen protection [45]. Further studies are necessary to elucidate the role of FSP-1/S100A4 in ILC.
In addition to the higher expression of FSP-1/S100A4 in ILC than in invasive carcinoma (NST), we found that the expression patterns of FSP-1/S100A4 differed between both types of breast cancer. In invasive carcinoma (NST), FSP-1/S100A4 was expressed in a fascicular pattern that involved grouped spindle-shaped CAFs. In contrast, FSP-1/S100A4 expression in ILC was scattered in ovoid or round cells. In addition to expression in CAFs and malignant cells in breast cancer tissue, FSP-1/S100A4 is expressed in macrophages [46]. FSP-1/S100A4-type CAFs induce macrophage recruitment in the tumor microenvironment via the secretion of monocyte chemotactic protein-1 [47]. These findings indicate that macrophages in ILC stroma, like CAFs, may be positive for FSP-1/S100A4. Further studies are required to assess this possibility.
No significant differences in the expression of CAF-related proteins between classic type and pleomorphic type of ILC in this study. In general, pleomorphic type is more aggressive and shows poor prognosis than classic type ILC [48, 49]. There are several possible causes that can explain the insignificant differences in the expression of CAF-related proteins between the two subtypes. First, such differences could have been caused by limitations in the statistical analysis due to the difference in the number of cases between the two subtypes. Second, there may have been no significant differences in the expression of CAF-related proteins between the two subtypes. One previous study reported no significant differences between the prognosis of the two subtypes after matching patients’ age and the year of diagnosis [50]. In addition, the pleomorphic type is known to show genetic alteration that is similar to that of the classic type [51]. Thus, these findings raise the possibility of insignificant differences in tumor stroma between the two subtypes of ILC, and further studies are required.
Clinically, our study indicates that CAFs are a potential target in cancer therapy. There are several reasons that these cells are a promising drug target [52]. First, compared with cancer cells, CAFs are genetically stable. Second, CAFs show different epigenetic changes from normal stromal cells. Finally, CAFs accompany and support cancer cells through the entire neoplastic spectrum. Thus, we can manage and treat neoplasms in any stage of the disease.
In conclusion, the expression of CAF-related proteins differed between invasive carcinoma (NST) and ILC in both the cancer and stromal cell compartments. ILC showed a particularly elevated expression of FAP-α and FSP-1/S100A4 in both tumor and stromal cells compared to invasive carcinoma (NST). Therapy targeted to the CAF markers used in our study has shown an inhibitory effect on tumor growth [53–56]. This supports our contention that the application of targeted therapy for CAFs can be applied to ILC, which expresses high levels of CAF-related proteins.
References
Tavassoli FA, Devilee P, International Agency for Research on Cancer et al (2003) Pathology and genetics of tumours of the breast and female genital organs. IAPS Press, Lyon
Li CI, Anderson BO, Daling JR et al (2003) Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289(11):1421–1424
Li CI, Uribe DJ, Daling JR (2005) Clinical characteristics of different histologic types of breast cancer. Br J Cancer 93(9):1046–1052. doi:10.1038/sj.bjc.6602787
Tot T (2000) The cytokeratin profile of medullary carcinoma of the breast. Histopathology 37(2):175–181. doi:10.1046/j.1365-2559.2000.00889.x
Reeves GK, Beral V, Green J et al (2006) Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 7(11):910–918. doi:10.1016/s1470-2045(06)70911-1
Lesser ML, Rosen PP, Kinne DW (1982) Multicentricity and bilaterality in invasive breast carcinoma. Surgery 91(2):234–240
Silverstein MJ, Lewinsky BS, Waisman JR et al (1994) Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer 73(6):1673–1677
De Leeuw WJ, Berx G, Vos CB et al (1997) Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol 183(4):404–411. doi:10.1002/(sici)1096-9896(199712)183:4<404:aid-path1148>3.0.co;2-9
Tsutsui S, Ohno S, Murakami S et al (2003) Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery 133(2):219–221. doi:10.1067/msy.2003.32
Uehara H, Takahashi T, Oha M et al (2014) Exogenous fatty acid binding protein 4 promotes human prostate cancer cell progression. Int J Cancer. doi:10.1002/ijc.28903
Franco OE, Shaw AK, Strand DW et al (2010) Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol 21(1):33–39. doi:10.1016/j.semcdb.2009.10.010
Ostman A (2014) Cancer-associated fibroblasts: recent developments and emerging challenges. Semin Cancer Biol 25:1–2. doi:10.1016/j.semcancer.2014.02.004
Desmouliere A, Guyot C, Gabbiani G (2004) The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol 48(5–6):509–517. doi:10.1387/ijdb.041802ad
De Wever O, Nguyen QD, Van Hoorde L et al (2004) Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J 18(9):1016–1018. doi:10.1096/fj.03-1110fje
Sugimoto H, Mundel TM, Kieran MW et al (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5(12):1640–1646
Pietras K, Sjoblom T, Rubin K et al (2003) PDGF receptors as cancer drug targets. Cancer Cell 3(5):439–443
Kraman M, Bambrough PJ, Arnold JN et al (2010) Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330(6005):827–830. doi:10.1126/science.1195300
Kawase A, Ishii G, Nagai K et al (2008) Podoplanin expression by cancer associated fibroblasts predicts poor prognosis of lung adenocarcinoma. Int J Cancer 123(5):1053–1059. doi:10.1002/ijc.23611
Kojima Y, Acar A, Eaton EN et al (2010) Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 107(46):20009–20014. doi:10.1073/pnas.1013805107
Cortez E, Roswall P, Pietras K (2014) Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol 25:3–9. doi:10.1016/j.semcancer.2013.12.010
Mao Y, Keller ET, Garfield DH et al (2013) Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev 32(1–2):303–315. doi:10.1007/s10555-012-9415-3
Nakagawa S, Miki Y, Miyashita M et al (2016) Tumor microenvironment in invasive lobular carcinoma: possible therapeutic targets. Breast Cancer Res Treat 155(1):65–75. doi:10.1007/s10549-015-3668-9
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Hammond ME, Hayes DF, Dowsett M et al (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28(16):2784–2795. doi:10.1200/jco.2009.25.6529
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. doi:10.1200/jco.2013.50.9984
Henry LR, Lee HO, Lee JS et al (2007) Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res 13(6):1736–1741. doi:10.1158/1078-0432.ccr-06-1746
Goldhirsch A, Wood WC, Coates AS et al (2011) Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 22(8):1736–1747. doi:10.1093/annonc/mdr304
Jansson S, Bendahl PO, Grabau DA et al (2014) The three receptor tyrosine kinases c-KIT, VEGFR2 and PDGFRalpha, closely spaced at 4q12, show increased protein expression in triple-negative breast cancer. Plos One 9(7):e102176. doi:10.1371/journal.pone.0102176
Zhu Y, Wang Y, Guan B et al (2014) C-kit and PDGFRA gene mutations in triple negative breast cancer. Int J Clin Exp Pathol 7(7):4280–4285
Martin-Villar E, Megias D, Castel S et al (2006) Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J Cell Sci 119(Pt 21):4541–4553. doi:10.1242/jcs.03218
Grau SJ, Trillsch F, Tonn JC et al (2015) Podoplanin increases migration and angiogenesis in malignant glioma. Int J Clin Exp Pathol 8(7):8663–8670
Xiong G, Deng L, Zhu J et al (2014) Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer 14:1. doi:10.1186/1471-2407-14-1
Wasif N, Maggard MA, Ko CY et al (2010) Invasive lobular vs. ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol 17(7):1862–1869. doi:10.1245/s10434-010-0953-z
Ito M, Hagiyama M, Mimae T et al (2014) alpha-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res Treat 144(1):59–69. doi:10.1007/s10549-014-2859-0
Catteau X, Simon P, Noel JC (2014) Myofibroblastic stromal reaction and lymph node status in invasive breast carcinoma: possible role of the TGF-beta1/TGF-betaR1 pathway. BMC Cancer 14:499. doi:10.1186/1471-2407-14-499
Song S, Ewald AJ, Stallcup W et al (2005) PDGFRbeta + perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol 7(9):870–879. doi:10.1038/ncb1288
Iacobuzio-Donahue CA, Argani P, Hempen PM et al (2002) The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res 62(18):5351–5357
Shao ZM, Nguyen M, Barsky SH (2000) Human breast carcinoma desmoplasia is PDGF initiated. Oncogene 19(38):4337–4345. doi:10.1038/sj.onc.1203785
Huang Y, Simms AE, Mazur A et al (2011) Fibroblast activation protein-alpha promotes tumor growth and invasion of breast cancer cells through non-enzymatic functions. Clin Exp Metastasis 28(6):567–579. doi:10.1007/s10585-011-9392-x
Jia J, Martin TA, Ye L et al (2014) FAP-alpha (Fibroblast activation protein-alpha) is involved in the control of human breast cancer cell line growth and motility via the FAK pathway. BMC Cell Biol 15:16. doi:10.1186/1471-2121-15-16
Jenkinson SR, Barraclough R, West CR et al (2004) S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br J Cancer 90(1):253–262. doi:10.1038/sj.bjc.6601483
Wang L, Wang X, Liang Y et al (2012) S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim Pol 59(4):593–598
Michaut M, Chin SF, Majewski I et al (2016) Integration of genomic, transcriptomic and proteomic data identifies two biologically distinct subtypes of invasive lobular breast cancer. Sci Rep 6:18517. doi:10.1038/srep18517
O’Connell JT, Sugimoto H, Cooke VG et al (2011) VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108(38):16002–16007. doi:10.1073/pnas.1109493108
Zhang J, Chen L, Liu X et al (2013) Fibroblast-specific protein 1/S100A4-positive cells prevent carcinoma through collagen production and encapsulation of carcinogens. Cancer Res 73(9):2770–2781. doi:10.1158/0008-5472.can-12-3022
Cabezon T, Celis JE, Skibshoj I et al (2007) Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int J Cancer 121(7):1433–1444. doi:10.1002/ijc.22850
Zhang J, Chen L, Xiao M et al (2011) FSP1 + fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178(1):382–390. doi:10.1016/j.ajpath.2010.11.017
Eusebi V, Magalhaes F, Azzopardi JG (1992) Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol 23(6):655–662
Bentz JS, Yassa N, Clayton F (1998) Pleomorphic lobular carcinoma of the breast: clinicopathologic features of 12 cases. Mod Pathol 11(9):814–822
Narendra S, Jenkins SM, Khoor A et al (2015) Clinical outcome in pleomorphic lobular carcinoma: a case-control study with comparison to classic invasive lobular carcinoma. Ann Diagn Pathol 19(2):64–69. doi:10.1016/j.anndiagpath.2015.01.005
Simpson PT, Reis-Filho JS, Lambros MB et al (2008) Molecular profiling pleomorphic lobular carcinomas of the breast: evidence for a common molecular genetic pathway with classic lobular carcinomas. J Pathol 215(3):231–244. doi:10.1002/path.2358
Ohlund D, Elyada E, Tuveson D (2014) Fibroblast heterogeneity in the cancer wound. J Exp Med 211(8):1503–1523. doi:10.1084/jem.20140692
Haubeiss S, Schmid JO, Murdter TE et al (2010) Dasatinib reverses cancer-associated fibroblasts (CAFs) from primary lung carcinomas to a phenotype comparable to that of normal fibroblasts. Mol Cancer 9:168. doi:10.1186/1476-4598-9-168
Brennen WN, Isaacs JT, Denmeade SR (2012) Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 11(2):257–266. doi:10.1158/1535-7163.mct-11-0340
Santos AM, Jung J, Aziz N et al (2009) Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest 119(12):3613–3625. doi:10.1172/jci38988
Scott AM, Wiseman G, Welt S et al (2003) A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res 9(5):1639–1647
Neri S, Ishii G, Hashimoto H et al (2015) Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer 137(4):784–796. doi:10.1002/ijc.29464
Slany A, Haudek-Prinz V, Meshcheryakova A et al (2014) Extracellular matrix remodeling by bone marrow fibroblast-like cells correlates with disease progression in multiple myeloma. J Proteome Res 13(2):844–854. doi:10.1021/pr400881p
Garin-Chesa P, Old LJ, Rettig WJ (1990) Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA 87(18):7235–7239
Lee HO, Mullins SR, Franco-Barraza J et al (2011) FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11:245. doi:10.1186/1471-2407-11-245
Liao D, Luo Y, Markowitz D et al (2009) Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. Plos One 4(11):e7965. doi:10.1371/journal.pone.0007965
Erez N, Truitt M, Olson P et al (2010) Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 17(2):135–147. doi:10.1016/j.ccr.2009.12.041
Pietras K, Pahler J, Bergers G et al (2008) Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. Plos Med 5(1):e19. doi:10.1371/journal.pmed.0050019
Crawford Y, Kasman I, Yu L et al (2009) PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell 15(1):21–34. doi:10.1016/j.ccr.2008.12.004
Ehnman M, Missiaglia E, Folestad E et al (2013) Distinct effects of ligand-induced PDGFRalpha and PDGFRbeta signaling in the human rhabdomyosarcoma tumor cell and stroma cell compartments. Cancer Res 73(7):2139–2149. doi:10.1158/0008-5472.can-12-1646
Pietras K, Ostman A, Sjoquist M et al (2001) Inhibition of platelet-derived growth factor receptors reduces interstitial hypertension and increases transcapillary transport in tumors. Cancer Res 61(7):2929–2934
Pietras K, Rubin K, Sjoblom T et al (2002) Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res 62(19):5476–5484
Cooke VG, LeBleu VS, Keskin D et al (2012) Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell 21(1):66–81. doi:10.1016/j.ccr.2011.11.024
Funding
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1420080). This research was also supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT, and Future Planning (2015R1A1A1A05001209).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, C.K., Jung, W.H. & Koo, J.S. Expression of cancer-associated fibroblast-related proteins differs between invasive lobular carcinoma and invasive ductal carcinoma. Breast Cancer Res Treat 159, 55–69 (2016). https://doi.org/10.1007/s10549-016-3929-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3929-2