Abstract
A native form of mouse monoclonal IgG1 antibody called MAG-1, which recognizes an epitope on provasopressin, has been found to shrink and produce extensive necrosis of human breast tumor xenografts in nu/nu mice. We examined the ability of 90Yttrium-labeled and native MAG-1 to affect the growth in nu/nu mice of cancer xenografts that were estrogen-responsive (from MCF-7 cells) and triple-negative (from MDA-MB231 cells). The growth rates of treated cells were compared to those receiving saline vehicle and those receiving 90Yttrium-labeled and native forms of the ubiquitous antibody, MOPC21. Short-term treatments (4 doses over 6 days) not only with 90Yttrium-MAG-1 but also native MAG-1 produced large reductions in size of rapidly growing tumors of both types, while both 90Yttrium- MOPC21 and native MOPC21 had no effect. Native and 90Yttrium-MAG-1 effects were similar, and arrested tumors recommenced growing soon after treatments stopped. Increasing native MAG-1 treatment to single dosing for 16 consecutive days shrank tumors of both types with no regrowth apparent over a 20-day post-treatment period of observation. Pathological examination of such tumors revealed they had undergone very extensive (>66%) necrosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is one of the leading causes of death among women throughout the world, and each year is responsible for the death of approximately 40,000 women in the United States [1]. Although a number of new treatments for this disease show promise, there is still the need for methods that are more effective. Successful intervention still relies heavily on early detection through mammography and surgical removal with adjuvant therapeutic follow-up largely consisting of chemotherapy and radiation therapy. Approximately two thirds of the cancers are responsive to estrogen and these are typically treated with “anti-estrogenic” compounds like Tamoxafen [2], with aromatase inhibitors such as anastrozole, letrozole, and exemestane, or with combinations of these hormonal therapies [3]. Some cancers display a high expression of Her2/neu (approximately 25%) and patients with these cancers have been shown to respond well to a humanized monoclonal antibody Herceptin, especially when treatment is provided during periods of remission. In addition, a substantial number (approximately 15–20%) of cancers are referred to as “triple-negative tumors,” a name that reflects an absence of estrogen receptors, progesterone receptors, and Her 2/neu. Such tumors tend to be aggressive, and need to be treated with high-dose chemotherapy or a combination of chemotherapies [4, 5].
Vasopressin gene expression has been shown by us to probably represent early oncogenic transformation in breast tissues. This is because no evidence could be found for expression by normal breast tissues, or by various fibrocystic conditions including atypical hyperplasia, while gene-related products were found in all cases examined of pre-invasive breast carcinoma in situ [6]. While vasopressin with vasopressin receptors seems to fulfill a role as part of an autocrine growth loop in these cancers [7, 8], most of the translated precursor, called provasopressin, seems to be incorporated into the plasma membrane as a unique tumor feature [9]. A similar tumor marker on small-cell lung cancer can be targeted in patients with antibodies [10]. A mouse IgG1 monoclonal antibody, MAG-1, directed against a C-terminal segment of provasopressin has been shown by us to give positive immunohistochemical staining with all breast cancer tissues examined, and to highlight the presence of this protein on the surface of breast cancer cells in culture [11, 12]. In this study, we examined the ability of MAG-1 and 90Yttrium-labeled MAG-1 to arrest the growth of breast cancer xenografts in nu/nu mice.
Materials and methods
Tumor cells
The estrogen-responsive human cancer cell line MCF-7 and the triple-negative human cancer cell line MDA-MB231 were obtained from American Type Culture Collection (Rockville, MD) and maintained in DMEM medium (Mediatech, Inc., Herndon, VA), containing 10% fetal bovine serum (Atlanta Biologicals), at 37°C, in an atmosphere of 5% CO2 with medium changes every 3 and 4 days. These cultures were maintained in tissue culture flasks at densities from 1 to 5 × 105 cells/ml.
Antibodies
The mouse monoclonal antibody, MAG-1, is of the IgG1 subclass and has been described previously [13]. It was generated against an 18-mer C-terminal segment of provasopressin and is the subject of a patent application by Woomera Therapeutics Inc. [14]. MOPC21 is also of the IgG1 subclass and is an ubiquitous antibody produced by a mouse myeloma cell line [15]. For this study, both antibodies were purified from culture by protein A affinity chromatography. MAG-1 and MOPC21 were both modified by addition of DTPA, a chelating agent. This was accomplished by reacting each at pH 8.3 overnight with CHXA″-DTPA reagent in tenfold excess [16]. DTPA-modified antibodies were reacted with 90Yttrium chloride, and 90Yttrium-labeled products isolated by Sephadex G25 chromatography.
Generation of MCF-7 tumor xenografts
Male nu/nu mice 6–7 weeks of age were purchased from Harland and a 17 β-estradiol pellet (0.72 mg/pellet, 60 day release, Innovative Research of America, Sarasota, FL) placed under the skin in the mid-back region with a trachar as recommended by the manufacturer. The trachar pouch was sealed with a single suture to ensure the pellet stayed in place. Three days following this implantation, MCF-7 cells were trypsinized, concentrated into growing medium by centrifugation (4–5 × 107 cells/ml), and injected subcutaneously in the lower right flank quadrant (1–2 × 107 cells per animal) using a 1-ml syringe and 22-gauge needle. Cells were allowed to generate tumor xenografts for 21–24 days before the initiation of the studies. At this time all of the mice receiving cells produced tumors that ranged in length from 0.5 to 0.75 cm. For the following 4 days tumors were evaluated for tumor growth by measuring length, width, and depth with a micrometer and size expressed as the product of all three parameters. Measurements were performed on mice anesthetized with a mixture of isofluorane and oxygen.
Generation of MDA-MB231 tumor xenografts
With no initial priming of male nu/nu animals of age 6–7 weeks, MDA-MB231 cells were prepared in the manner described above for MCF-7 cells and injected subcutaneously in the lower right flank quadrant (2 × 107/cells per animal) using a 1-ml syringe and 22-gauge needle. All animals grew tumors of between 0.5 and 0.75 cm in 25–30 days, which were measured for growth over 4 days prior to treatment.
Treatment with antibodies
Tumor-bearing mice were divided into three groups of eight animals in one short-term treatment study on the effects of 90Yttrium-labeled antibodies, three groups of eight animals in a second short-term treatment study on the effects of naked antibodies, and three groups of four animals in a long-term treatment study on the effects of naked antibodies. Animals were selected to provide a similar range in tumor size for each grouping. For each short-term study, group 1 comprised animals treated with four intra-peritoneal (i.p.) injections of 50 μl of saline vehicle given on days 0, 2, 4, and 6. For one short-term study, animals of group 2 received four i.p. injections of 50 μCi of 90Yttrium-labeled MAG-1 [17] with naked MAG-1 carrier totaling 50 μg antibody/50 μl, and animals of group 3 received four i.p. injections of 50 μCi of 90Yttrium-labeled MOPC21 with naked MOPC21 carrier totaling 50 μg antibody/50 μl on days 0, 2, 4, and 6. The amount of radiolabel employed was based on earlier studies by others [18]. For the second short-term study, groups 2 and 3 comprised treatments on days 0, 2, 4, and 6, with 50 μg/50 μl of naked MAG-1 or naked MOPC21, respectively. Tumor volume was measured daily by micrometry, and the body weight of animals evaluated, for 16 days. For the long-term study, animals were injected i.p. daily for 16 days with, saline vehicle (group 1), naked MAG-1 (group2), or naked MOPC21 (group 3). Tumor size and animal body weight were assessed daily over this period, and daily measurements of tumor volume and body weight were extended for the MAG-1-treated group for an additional 20 days. Possible toxicity of treatment was measured by examining major organs (liver, kidneys) and tumors for necrotic changes at the completion of each study.
Statistical analysis
Longitudinal growth data were evaluated by repeated measures analysis of variance. The independent variables were factors for treatment group and time and for the interaction between treatment and time. In all cases, our analysis focused on comparing MAG1 groups with the control and MOPC21 groups. Separate comparisons were performed between MAG-1 groups, and between saline and MOPC21 groups. A two-sided P-value less than 0.05 was considered statistically significant.
Results
Short-term treatment with naked MAG-1 antibody as well as 90Yttrium-labeled MAG-1 decreases the size of estrogen-responsive tumors
Tumors treated over 6 days with either native MAG-1 or 90Yttrium-labeled MAG-1 not only failed to grow during treatment, but underwent a significant decrease in their original size by up to approximately 40% of original tumor volume during the treatment period. Since results with MAG-1 were similar with each short-treatment method, and since each treatment comprised the same total amount of antibody, we believe that most of the volume effects seen by the end of the treatments are due to naked antibody in both cases. Results are shown in Fig. 1a, b, and are expressed as the percentage change in tumor size with treatment. For the period following treatment, tumors treated with naked antibody began increasing in size following a lapse of 4–5 days, but only approached their original volumes by the end of 9 days of measurement. One difference was that 90Yttrium-labeled MAG-1-treated xenografts showed no sign of regrowth over the same period of observation. Alternatively, the tumors of both saline control groups, and the MOPC21 control group, increased in size by at least three times (>300%), while the 90Yttrium-labeled MOPC21 control group increased by more than 2.5 times, for the period of measurement. In each study, there was no significant difference between saline and MOPC21 antibody treatments.
a MAG-1 inhibits MCF-7 tumor growth: MAG-1 (filled square) (P < 0.0005, both controls, n = 8 for each group), control (filled circle), and MOPC-21 (triangle). b90Y-MAG-1 inhibits MCF-7 tumor growth: 90Y-MAG-1 (filled square)) (P < 0.0001, both controls), control (filled circle), and 90Y-MOPC-21 (triangle). Arrow denotes last treatment: animals were treated on days 0, 2, 4, and 6. Values, mean ± SEM
Short-term treatment with both forms of MAG-1 antibody also decreases the size of triple-negative tumors
When treated with naked MAG-1 or 90Yttrium-labeled MAG-1, triple-negative tumors, represented by MDA-MB231 cells, behaved like estrogen-responsive tumors. They not only failed to grow during treatment but underwent an even more significant decrease in size by up to 60% of their original volume during this period. The results of both short-term studies with MDA-MB231 tumors are shown in Fig. 2a, b, which represent both changes in tumor volume with treatment but are again represented as percentage change in tumor size. For the period following treatment, tumors treated with naked MAG-1 began increasing in size so they approached the original size by the end of an additional 10 days of measurements. Unlike MCF-7 tumors, tumors of the 90Yttrium-MAG-1-treated group showed a small rise in size toward the end of the observation period that suggested these tumors were commencing regrowth, even though they were still at about 60% of their original size at this point. Alternatively, for the first study, the tumors of both saline and MOPC21 control groups showed no differences and increased in size by at least four times (>400%) over the period of observation. For the second study, likewise, the tumors of the saline group increased by at least four times (>400%), while the 90Yttrium-labeled MOPC21-treated tumors increased to about three times (>300%) their size at the time treatment commenced. There was a small but significant difference between the growth slopes of saline and 90Yttrium-labeled MOPC21-treated tumors (P < 0.05) suggesting that the latter treatment had some minor effects on these tumors, but these are miniscule compared to the effects of 90Yttrium-labeled MAG-1.
a MAG-1 inhibits MDA-MB231 tumor growth: MAG-1 (filled square) (P < 0.0005, both controls, n = 8 for each group), control (filled circle), and MOPC-21 (triangle). b90Y-MAG-1 inhibits MCF-7 tumor growth: 90Y-MAG-1 (filled square), control (filled circle), and 90Y-MOPC-21 (triangle). Arrow denotes last treatment: animals were treated on days 0, 2, 4, and 6. Values, mean ± SEM
Extending and intensifying treatment with naked MAG-1 prevents tumor regrowth
The influence on MCF-7 and MDA-MB231 tumors of extending treatment from 6 to 16 days and treating daily instead of every second day with 50 μg i.p. naked MAG-1 antibody was examined by us. Tumors were again allowed to reach at least 0.5 cm in length before the study started, and controls for this study were tumor-bearing animals treated daily for 16 days with saline vehicle. For MAG-1-treated animals tumor measurement was continued daily for 20 days beyond the final treatment, observations that were precluded for control groups because tumor volumes became too large. Four animals were used in each group of the study. Body weight of each animal was measured daily, and at the end of the study, tumor, liver, and kidneys were examined for possible pathological changes. The results are shown in Fig. 3a, b. MAG-1 treatment of both MCF-7 and MDA-MB231 tumors caused in all cases a large shrinkage and no regrowth for the 20 days of observation following treatment. Saline-treated tumors showed rapid growth so that by the end of 16 days they were about 3.3 and 4.5 times their size at the start of the study (Table 1).
MAG-1 daily treatment (50 μg) for 16 days shrinks and prevents regrowth of a MCF-7 breast tumors (control (filled circle) and MAG-1 (filled square) and b MDA-MB231 breast tumors (control (filled circle) and MAG-1 (filled square): mean ± SEM (P < 0.0005, n = 4 each group). Arrow denotes day of last treatment
MAG-1 antibody is selectively harmful to tumors
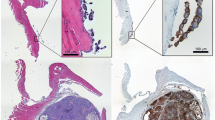
For the short-term studies, fixed tissue examination revealed there were no structural or pathological changes induced in normal tissues by treatments with naked or 90Yttrium-labeled MAG-1, while a substantial amount (30–50%) of necrosis was present in MAG-1-treated tumors of both types compared with untreated tumors where there were little to no (<5%) necrotic changes evident. The MAG-1-treated and untreated MDA-MB231 tumors of the extended study are shown in Fig. 4. Extensive necrosis such that <75% of the treated tumors was observed.
The MDA-MB231 tumors were harvested 1 day after the end of treatment and analyzed. Histology of MAG-1-treated tumor section (10×) (a) and tumor from control animals (b). Cross-section of saline-treated MDA-MB231 tumor (c), and cross-sections of three tumors from MDA-MB231 cells treated long term with MAG-1 (d, e, and f) and harvested 20 days after treatment
Treatments have no apparent effects on animal well-being
Treatment with either naked MAG-1 or 90Yttrium-labeled MAG-1 had no apparent effect on the well-being of tumor-bearing animals as judged by their behavior and body weights. There were no discernible differences in body weight changes of the period of observation between any of the groups. The daily weights of control and test animals are represented for MDA-MB231 tumor-bearing animals in Fig. 5a, b. There is no observable pathology of the livers and kidney from these MAG-1- and 90Yttrium-MAG-1-treated animals. In addition, histologic evaluation of organ tissues such as the liver and kidneys revealed that there was no damage to these tissues by extensive treatment with MAG-1 (Fig. 6a, b).
Discussion
The dramatic effects on human breast cancer xenografts exhibited by naked MAG-1 antibodies were unexpected by us. The primary reason for studying those effects was to evaluate any contribution they might make to the effects produced by 90Yttrium-labeled MAG-1. However, almost all of the anti-cancer activity of the radioactive antibody preparation seemed to be due to the naked antibody within the preparation. This was further born out by the studies on extended treatments. Tumor xenografts representing both human estrogen-responsive and triple-negative breast cancer underwent large reductions in size and extensive necrosis, with no apparent harmful side-effects. The issue of safety have to be further investigated however, since there is only a 50% homology between human and mouse provasopressins in the C-terminal 18-amino acid region of the molecule used to generate MAG-1 antibody. However, there is good likelihood humanized derivatives of MAG-1 will be safe for use in humans because the mouse antibody did not react with 66 normal human tissues examined by us [20].
The exceptional effects shown by our naked antibody are reminiscent of those demonstrated for mouse 4D5 monoclonal antibody of the IgG2b subclass and its humanized form herceptin. These antibodies are directed against an extracellular portion of the tyrosine kinase receptor Her2 and are reported to cause apoptosis of tumor cells over-expressing this receptor chiefly through compliment-fixation and antibody-directed cell cytotoxicity [17, 19]. However, since MAG-1 is a mouse IgG1 molecule, it is unlikely either of these mechanisms is involved in inducing tumor necrosis with this antibody. Nevertheless, the effects observed are believed to result from a direct action of the antibody on the tumor cells because MAG-1 was found by us to react only with anterior hypothalamus and neurohypophysis, and not with other mouse tissues, nor with 66 normal human tissues [20]. While vasopressin and its receptors play a role in tumor breast cancer growth [7, 8, 12], the exact role of provasopressin as a surface protein remains a mystery and might not be directly connected to the mitogenic action of the hormone. Provasopressin is not a ligand for vasopressin receptors, and could itself, for these tumors, represent a receptor or scaffolding molecule for an as yet unknown ligand. Recently we have demonstrated, using an assay from Promega, that MAG-1 produces dose-dependent cytotoxicity of MCF-7 cells in culture. These preliminary findings, while not erasing the possibility of some antibody-directed cell cytotoxicity being involved, show the in vivo actions of MAG-1 could be direct ones. It is expected that a further understanding of the mechanism of action of the antibody will become clear through intended examination of its effects on specific genes involved with cytotoxicity and apoptosis and through knock-down studies.
References
American Cancer Society (2008) Cancer facts and figures. American Cancer Society, Atlanta
Jordon VC (2006) Tamoxifen (ICI46, 474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmcol 147:S269–S276
Brueggemeier RW, Hackett JC, Diaz-Cruz ES (2005) Aromatase inhibitors in the treatment of breast cancer. Endocr Rev 26:331–345
De Giorgi U, Rosti G, Frassineti L, Kopf B, Giovanni N, Zumaglini F, Marangolo M (2007) High-dose chemotherapy for triple negative breast cancer. Ann Oncol 18:202–203
Rodenhuis S, Bontenbal M, van Hoesel QGCM et al (2006) Efficacy of high-dose alkylating chemotherapy in HER2/neu-negative breast cancer. Ann Oncol 17:588–596
North WG, Wells W, Fay MJ, Mathew RS, Donnelly EM, Memoli VA (2003) Immunohistochemical evaluation of vasopressin expression in breast fibrocystic disease and ductal carcinoma in situ (DCIS). Endocr Pathol 14:257–262
Taylor AH, Ang VTY, Jenkins JS, Silverlight JJ, Coombes RC, Lauqmani YA (1990) Interaction of vasopressin and oxytocin with human breast cancer cells. Cancer Res 50:7882–7886
North WG, Fay MJ, Du J (1999) MCF-7 breast cancer cells express normal forms of all vasopressin receptors plus an abnormal V2R. Peptides 20:837–842
North WG (2000) Gene regulation of vasopressin and vasopressin receptors in cancer. Exp Physiol 85S:27–40
North WG, Hirsh V, Lisbona R, Schulz J, Cooper B (1989) Imaging of small cell carcinoma using 131I-labelled antibodies to vasopressin associated human neurophysin (VP-HNP). Nucl Med Commun 10:643–651
Keegan BP, Memoli VA, North WG (2002) Targeting the neurophysin-related cell surface antigen on small cell lung cancer using a monoclonal antibody against the glycopeptide region (MAG-1) of provasopressin. Mol Cancer Ther 1:1153–1159
Keegan BP, Akerman BL, Pequeux C, North WG (2006) Provasopressin expression by breast cancer cells: implications for growth and novel treatment strategies. Breast Cancer Res Treat 95:265–277
North WG, Memoli VA, Keegan BP (2005) Immunohistochemical recognition of NRSA on small cell lung cancer with a monoclonal antibody (MAG-1) that recognizes the carboxyl terminus of provasopressin. Appl Immunohistochem Mol Morphol 13:363–366
US Patent Application:# 10/521,091. Inventors: William G. North, Brendan Keegan, Lyn Oligino. Title: Compositions and Uses Thereof For identifying and Targeting Provasopressin-expressing Cancer Cells. Filed: 10/11/2005
Schubert D (1968) Immunoglobulin assembly in a mouse myeloma. Proc Natl Acad Sci USA 60:683–690
Adams GP, Shaller CC, Dadachova E, Simmons HH, Horak EM, Tesfaye A, Klein-Szanto AJ, Marks JD, Brechbiel MW, Weiner LM (2004) A single treatment of yttrium-90-labeled CHX-A″-C6.5 diabody inhibits the growth of established human tumor xenografts in immunodeficient mice. Cancer Res 64:6200–6206
Valabrega G, Montemurro F, Aglietta M (2007) Trastuzumab: mechanism of action, resistance and future perspectives in Her2-overexpressing breast cancer. Ann Oncol 18:977–984
Stein R, Chen S, Goldenberg DM (1997) Advantage of Yttrium-90-labeled over iodine-131-labeled monoclonal antibodies in the treatment of a human lung carcinoma xenograft. Cancer 80:2636–2641
Keegan BP, Memoli VA, Wells WA, North WG (2010) Detection of provasopressin in invasive and non-invasive (DCIS) human breast cancer using a monoclonal antibody directed against the C-terminus (MAG-1). Breast Cancer: Basic and Clin Res 4:15–22
Hudis CA (2007) Trastuzumab-mechanism of action and use. N Engl J Med 357:1664–1666
Acknowledgment
This study was supported in part by USPHS SBIR Phase I Grant: 1R43CA119483-01 to Woomera Therapeutic Inc.
Conflict of interest statement
Concerning the signed COI, all authors with the exception of William G. North and Roy H.L. Pang have no commercial interest relating to the material in the manuscript. However, as explained in the referenced patent applicant and by institute designation, Drs. North and Pang do have a commercial interest in Woomera Therapeutics Inc., the patent applicant. Dr. North is a full-time faculty member of Dartmouth Medical School, but is also the President of Woomera Therapeutics Inc. and holds >40% interest in that company. Dr. Pang is the CEO and CSO of the company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
North, W.G., Pang, R.H.L., Gao, G. et al. Native MAG-1 antibody almost destroys human breast cancer xenografts. Breast Cancer Res Treat 127, 631–637 (2011). https://doi.org/10.1007/s10549-010-1009-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1009-6