Abstract
RANK ligand (RANKL) is crucial for the development of mouse mammary glands during pregnancy. RANKL functions as a major paracrine effector of the mitogenic action of progesterone in mammary epithelium via its receptor RANK and has a role in expansion and regenerative potential of mammary stem cells. Pharmacologic inhibition of RANKL attenuates the development of mammary carcinoma and inhibits metastatic progression in multiple mouse models. Primary breast carcinoma samples from the neoadjuvant GeparTrio study were analyzed to correlate the expression of human RANK and RANKL with pathological complete response (pCR), disease-free (DFS), and overall (OS) survival. Pre-treatment FFPE core biopsies (n = 601) were analyzed for percentage and intensity of immunohistochemical RANK and RANKL expression. Antibodies against human RANK (N-1H8; Amgen) and human RANKL (M366; Amgen) were used. RANK protein was expressed in 160 (27 %) patients. Increased RANK expression was observed in 14.5 % of patients and correlated with high tumor grade (p < 0.023) and negative hormone receptor (HR) status (p < 0.001). Patients with high RANK expression showed a higher pCR rate (23.0 % vs. 12.6 %, p = 0.010), shorter DFS (p = 0.038), and OS (p = 0.011). However, prognostic and predictive information was not an independent parameter. Only 6 % of samples expressed RANKL, which was not correlated with any clinical features. Higher RANK expression in the primary tumor is associated with a higher sensitivity to chemotherapy, but also a higher risk of relapse and death. Our study provides a basis for further exploration of the antitumor activity of clinical antibodies against RANKL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2013, breast cancer is the most frequently diagnosed cancer in females with more than 232,000 new cases estimated. In women, the lifetime risk of developing an invasive breast cancer is 12 %. Despite all existing therapies, this disease will cause 14 % of all estimated deaths in the United States and therefore, it is the second most common cause of cancer death among females [1].
Neoadjuvant chemotherapy has become an established treatment option for an increasing number of patients with primary breast cancer [2, 3].
The receptor activator of nuclear factor-κB ligand (RANKL) is a key factor for bone remodeling and metastasis and belongs to the tumor necrosis factor (TNF) superfamily. RANKL is expressed by osteoblasts, but also by extraskeletal tissues, e.g., mammary epithelium [4]. Osteoprotegerin, which is also expressed on osteoblasts, is the decoy receptor of RANKL and the osteoprotegerin:RANKL ratio is important for bone health. RANK, the RANKL receptor, is also found on immature osteoclasts as well as in extraskeletal sites [4]. The relationship between bone destruction and tumor growth in bones has been described as a “vicious cycle,” in which tumor-induced bone destruction by osteoclasts and the consecutive release of growth factors leads to an increased tumor growth [5]. RANKL and its receptor are shown to be essential in the formation of a lactating mammary gland [6]. Moreover, multiple studies have described a role of RANKL during tumorigenesis in breast cancer [7, 8]. Palafox et al. [9] described RANK-dependent changes in tumor stem cells or the induction of epithelial to mesenchymal transition (EMT) that may also contribute to increased metastatic behavior. The role of the RANK–RANKL–osteoprotegerin system was also investigated in other malignancies [10, 11].
Many patients suffering from different malignancies develop bone metastases. The incidence, e.g., in patients with advanced breast or prostate cancer is as high as 70 %. Bone metastases lead to various skeletal-related events (SRE) including bone pain, pathologic fractures, or hypercalcemia of malignancy. Breast cancer patients have the highest incidence of skeletal complications among all cancer patients, leading to the highest rate of irradiation to bone and pathological fractures [12].
Until recently, bisphosphonates (e.g., zoledronic acid, disodium pamidronate) have represented the most relevant pharmacological treatment of bone metastases with hypocalcemia, osteonecrosis of the jaw and impairment of renal function being the clinically most relevant side effect. Denosumab, a fully human monoclonal antibody against RANKL, has been approved for the prevention of SREs. Denosumab prolongs time to first SRE when compared to zoledronic acid as treatment for skeletal complications from bone metastases [13].
Here, we investigated the association of RANK/RANKL expression with the development of metastases in patients with early breast cancer, as well as the predictive value of RANK/RANKL following neoadjuvant chemotherapy.
Therefore, we analyzed expression of RANK and RANKL by immunohistochemistry on pretreatment tumor samples of participants of the neoadjuvant GeparTrio trial.
Materials and methods
Study population
A total of 2,357 women with primary breast cancer (cT2-4, cN0-3, cM0) were enrolled between July 2001 and December 2005 in the neoadjuvant GeparTrio (NCT00544765) [14, 15] and GeparTrio pilot trials [16]. These trials were prospective, multicenter, randomized phase III studies. All participants received initially two cycles of docetaxel, doxorubicin, and cyclophosphamide (TAC), the following treatment was depending on clinical response. Non-responding patients received either four cycles TAC or four cycles vinorelbine and capecitabine. Responders received four additional TAC cycles (pilot study) or were randomized to four or six additional TAC cycles (main study). At that time patients with HER2-positive tumors had no access to trastuzumab. Pathological complete response rate (pCR) was defined as histopathologically complete disappearance of all invasive and non-invasive tumor cells from the breast and axillary tissue removed at surgery (ypT0; ypN0). Overall, the pCR rate was 20.5 % and was four times higher in responding patients than in non-responding patients. The main results of the trial have been published previously [14–17]. This study has been reported according to the REMARK criteria [18].
Immunohistochemical staining
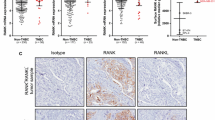
All pretherapeutic core biopsies were formalin-fixed, paraffin-embedded (FFPE) and stored at the German Breast Group tumor bank at the Institute of Pathology, Charité University Hospital, Berlin, Germany. Tumor area was marked on hematoxylin-eosin stained slides for construction of a tissue microarray. Immunhistochemical staining of RANK (N-1H8; Amgen Inc.) and RANKL (M366; Amgen Inc.) was done on the tissue microarrays according to standard operational procedures essentially as described [19]. The specificity of the antibodies was substantiated by concordant signals observed using multiple independent analyses, including IHC, flow cytometry, and Western blots of positive and negative control cells and xenograft samples.
Slides were digitized (Mirax Scan; Zeiss, Jena, Germany) and virtual slides evaluated using the VMscope Slide Explorer (VMscope Berlin, Germany). Additionally, the spots were evaluated conventional using light microscope. The immunohistochemical evaluation was performed independently by two experienced pathologists (DB; BMP). The percentage of positive tumor cells as well as the intensity (negative, weak, moderate, strong) were evaluated and multiplied with one (weak intensity), two (moderate intensity), or three (strong intensity). Finally, the H score as the sum of all products was calculated. In case of a range ≤20, the mean H score was calculated. The cases with a range >20 were discussed and re-evaluated once more. Using the automated cut-off finder (http://molpath.charite.de/cutoff/) [20], an H score of >8.5 was identified as optimal for prediction of pCR, disease-free survival (DFS), and overall survival (OS).
Positive HR status was defined as ≥10 % of tumor cells expressing estrogen receptor and/or progesterone receptor. Positive HER2-status was defined using immunohistochemistry as HER2 3+ (DAKO score) or HER2 2+ with HER2 gene amplification (in situ hybridization).
Statistical evaluation
Statistical evaluation was performed using SPSS Statistic version 20 (IBM Corporation, Armonk, New York, USA). The following clinicopathological parameters were included in the analyses additional to the expression of RANK and RANKL: age, clinical tumor size, histology, grade, clinical nodal status, HR status, HER2 status. These parameters were used as previously published [21]. Correlation between RANK expression and the clinicopathological parameters was assessed using the Pearson’s χ 2 test for trends. The probability of a pCR as a function of RANK expression was determined using univariate and multivariate logistic regression analyses. DFS and OS were analyzed using the Kaplan–Meier-function, cox regression analyses and the log rank test.
Results
Correlations with clinicopathological parameters
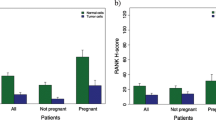
From a total of 2,357 study participants, pretreatment core biopsies from 840 patients were available for TMA construction. For 239 patients, the amount of tumor tissue on the TMA was not sufficient for evaluation; hence FFPE samples from 601 patients were included in the present analyses. Cytoplasmic and/or membranous localized RANK expression was observed in the tumor tissue of about one-third of all included patients (n = 160; 27 %). Figure 1 shows examples for low (≤8.5 H score) and high (>8.5 H score) immunohistochemical RANK expression in invasive breast cancer. Regarding all samples, high RANK expression was observed in 14.5 % patients (n = 87), low rank expression in 85.5 % patients (n = 514).
Cytoplasmic RANKL expression was observed in 6 % of tumor samples. High RANK expression was found in less differentiated (G3) (p = 0.023), HR negative (p < 0.001), and highly proliferating tumors (p = 0.001). Regarding the molecular subtypes, high RANK expression was most frequent in triple-negative (HR neg/HER2 neg) breast cancer (p < 0.001). No significant correlations were found between RANK expression and age, tumor size, histological subtype, nodal status, and HER2 status. Table 1 summarizes the correlations with all clinicopathological parameters.
No significant correlations were found between RANKL expression and the clinicopathological parameters mentioned above (data not shown).
Prediction of pCR and survival analyses
Regarding all included patients, patients with high RANK expression had a higher pCR rate compared to patients with low RANK expression (23.0 vs. 12.6 %; p = 0.01). A stratified analysis showed that a significant correlation of pCR rate and RANK expression level was present in younger patients, ductal invasive, smaller, higher grade and HER2 negative tumors, and node-negative disease (Table 1).
In univariate survival analysis 601 patients with a median follow-up time of 5.5 years (0–9.2 years) were included. Patients with high RANK expression had a shorter disease-free (p = 0.038) as well as OS (p = 0.011) compared to patients with low RANK expression (Fig. 2). In the subgroup of patients without a pCR (n = 516), high RANK expression was again associated with a significantly shorter DFS (p = 0.008) and OS (p = 0.003), whereas in pCR patients (n = 85), RANK expression had no prognostic value for either DFS (p = 0.92) or OS (p = 0.777; data not shown). Table 2 gives an overview about relevant clinicopathological parameters and their association with pCR in univariable and multivariable analyses.
The pCR was significantly higher for the subgroups of high RANK expression (p = 0.012), age < 50 years (p = 0.005), less differentiated tumors (G3; p < 0.001), negative HR status (p < 0.001), and positive HER2-status (p = 0.019). Multivariate analysis indicated that a high RANK expression was not independently associated with pCR (p = 0.425). Independent predictors of pCR in multivariable analyses were age <50 years (p = 0.008), less differentiated tumors (G3; p = 0.026), and negative HR status (p < 0.0001; Table 2). In HER-2 negative disease, higher RANK expression predicted a worse outcome (DFS, p = 0.019; OS, p = 0.043; Table 3).
In multivariate Cox regression analysis, nodal status was an independent prognostic factor for DFS (p = 0.03) and OS (p = 0.015), whereas RANK expression was not independently associated with outcome (DFS, p = 0.201; OS, p = 0.258). The only other factor that reached significance for the prediction of OS was the HR status (p = 0.024; Table 4).
Discussion
Our study demonstrates that immunohistochemical expression of RANK is associated with a more aggressive breast cancer type in the neoadjuvant GeparTrio trial. High RANK expression was significantly more frequent in poorly differentiated (G3), HR negative, and highly proliferating tumors. Patients with high RANK expression showed an increased pCR rate and a decreased DFS and OS.
To the best of our knowledge, this is the first analysis of immunohistochemical expression of RANK in a cohort of patients with primary invasive breast cancer who underwent neoadjuvant chemotherapy. Scoring for RANK expression was limited to tumor epithelium in the present study, however, RANK protein is additionally observed in tumor-associated macrophages [9]. In a microarray analysis Santini et al. found significant correlations between higher RANK mRNA levels and basal subtypes (p < 0.0001), larger tumor size (≤2 vs. >2 cm), less differentiated tumors (G1 vs. G3, p < 0.001), and HR negative tumors (p < 0.0001). Furthermore, high RANK expression was significantly associated with the “poor” prognosis group on the basis of the 70-gene signature (p = 0.01) [22]. This transcript analysis would not discriminate between RANK expression within tumor cells versus macrophages but was consistent with our analysis of RANK protein specifically within tumor cells. Complementary to the current study, they ran immunohistochemical analyses and detected significant correlations between positive RANK expression and lymph node metastases (p = 0.05) in a cohort of 92 breast cancer samples. Furthermore they described a significantly higher risk to acquire skeletal metastases (p = 0.023) in RANK positive patients. Additionally, an increased percentage of RANK positive cases developed metastases compared to patients without metastases. Overall, this association between a more aggressive breast cancer subtype and higher RANK expression is similar to our results. Zhang et al. analyzed the immunohistochemical RANK expression of tumor samples from a total of 102 patients with metastatic breast cancer. Except for younger age at diagnosis (≤35 years) no significant correlation between RANK expression and any clinicopathological parameters was found [23]. In both of these studies, the incidence of RANK expression in primary breast cancer was higher (41% [22] and 47 % [23]) than the 27 % incidence reported herein. These discrepancies may be due to the different specificity of IHC reagents or methodologies, independent scoring systems, or differences in tumor histotype distributions analyzed in the different studies. Despite these discrepancies, our present data show that patients with RANK expression showed significantly poor progression-free and disease-specific survival which is consistent with prior analyses.
This study is important as it provides a clinical validation of preclinical in vivo and in vitro observations demonstrating the functionality of RANK intrinsic to tumor cells. It has now been demonstrated RANKL will directly stimulate RANK-expressing cancer cells, activating mechanisms relevant to tumorigenesis and metastasis [8]. Several in vivo observations also support the contribution of higher RANK within tumor cells toward increased metastasis to lung and bone [9, 24, 25]. Moreover, blockade of RANKL in mouse models of breast cancer metastasis has been demonstrated to reduce bone and lung metastases [7, 24, 26]. These preclinical studies may provide the molecular mechanisms to explain the observed association of higher RANK expression within primary breast tumor samples and aggressiveness (e.g., higher tumor grade and higher Ki67 index) and/or poorer DFS and OS.
Our study has the limitation that due to the analysis on a TMA only a small area for each tumor could be investigated, however, we were able to evaluate a large cohort from a clinical trial. Previous published analyses also showed the possibility to use these small core biopsies on tissue microarray for immunohistochemical analyses [21, 27, 28].
In summary, we found an association between RANK expression and an aggressive tumor subtype that should be validated in further studies.
References
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63(1):11–30. doi:10.3322/caac.21166
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16(8):2672–2685
Kaufmann M, von Minckwitz G, Rody A (2005) Preoperative (neoadjuvant) systemic treatment of breast cancer. Breast 14(6):576–581
Brown JE, Coleman RE (2012) Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat Rev Clin Oncol 9(2):110–118. doi:10.1038/nrclinonc.2011.197
Roodman GD (2012) Genes associate with abnormal bone cell activity in bone metastasis. Cancer Metastasis Rev 31(3–4):569–578. doi:10.1007/s10555-012-9372-x
Fata JE, Kong YY, Li J, Sasaki T, Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey DL, Boyle WJ, Khokha R, Penninger JM (2000) The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103(1):41–50
Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, Pinkas J, Branstetter D, Dougall WC (2010) RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature 468(7320):103–107. doi:10.1038/nature09495
Dougall WC (2012) Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 18(2):326–335. doi:10.1158/1078-0432.CCR-10-2507
Palafox M, Ferrer I, Pellegrini P, Vila S, Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Munoz P, Vinals F, Tometsko M, Branstetter D, Dougall WC, Gonzalez-Suarez E (2012) RANK induces epithelial-mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis. Cancer Res 72(11):2879–2888. doi:10.1158/0008-5472.CAN-12-0044
Mikami S, Katsube K, Oya M, Ishida M, Kosaka T, Mizuno R, Mochizuki S, Ikeda T, Mukai M, Okada Y (2009) Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J Pathol 218(4):530–539. doi:10.1002/path.2567
Chen G, Sircar K, Aprikian A, Potti A, Goltzman D, Rabbani SA (2006) Expression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulation. Cancer 107(2):289–298. doi:10.1002/cncr.21978
Coleman RE (2004) Bisphosphonates: clinical experience. Oncologist 9(Suppl 4):14–27. doi:10.1634/theoncologist.9-90004-149/suppl_4/14
Ford JA, Jones R, Elders A, Mulatero C, Royle P, Sharma P, Stewart F, Todd R, Mowatt G (2013) Denosumab for treatment of bone metastases secondary to solid tumours: systematic review and network meta-analysis. Eur J Cancer 49(2):416–430. doi:10.1016/j.ejca.2012.07.016
von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Kaufmann M, On behalf of the German Breast G (2008) Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: phase III randomized gepartrio trial. J Natl Cancer Inst 100(8):542–551. doi:10.1093/jnci/djn085
von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Manfred K, For the German Breast G (2008) Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized gepartrio study. J Natl Cancer Inst 100(8):552–562. doi:10.1093/jnci/djn089
von Minckwitz G, Blohmer JU, Raab G, Lohr A, Gerber B, Heinrich G, Eidtmann H, Kaufmann M, Hilfrich J, Jackisch C, Zuna I, Costa SD, German Breast G (2005) In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 16(1):56–63. doi:10.1093/annonc/mdi001
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Kummel S, Paepke S, Schneeweiss A, Untch M, Zahm DM, Mehta K, Loibl S (2013) Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol 31(29):3623–3630. doi:10.1200/JCO.2012.45.0940
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of NCIEWGoCD (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. doi:10.1007/s10549-006-9242-8
Wood CE, Branstetter D, Jacob AP, Cline JM, Register TC, Rohrbach K, Huang LY, Borgerink H, Dougall WC (2013) Progestin effects on cell proliferation pathways in the postmenopausal mammary gland. Breast Cancer Res 15(4):R62. doi:10.1186/bcr3456
Budczies J, Klauschen F, Sinn BV, Gyorffy B, Schmitt WD, Darb-Esfahani S, Denkert C (2012) Cutoff Finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 7(12):e51862. doi:10.1371/journal.pone.0051862
von Minckwitz G, Müller BM, Loibl S, Budczies J, Hanusch C, Darb-Esfahani S, Hilfrich J, Weiss E, Huober J, Blohmer JU, du Bois A, Zahm DM, Khandan F, Hoffmann G, Gerber B, Eidtmann H, Fend F, Dietel M, Mehta K, Denkert C (2011) Cytoplasmic poly(adenosine diphosphate-ribose) polymerase expression is predictive and prognostic in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol 29(16):2150–2157. doi:10.1200/JCO.2010.31.9079
Santini D, Schiavon G, Vincenzi B, Gaeta L, Pantano F, Russo A, Ortega C, Porta C, Galluzzo S, Armento G, La Verde N, Caroti C, Treilleux I, Ruggiero A, Perrone G, Addeo R, Clezardin P, Muda AO, Tonini G (2011) Receptor activator of NF-kB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One 6(4):e19234. doi:10.1371/journal.pone.0019234
Zhang L, Teng Y, Zhang Y, Liu J, Xu L, Qu J, Hou K, Yang X, Liu Y, Qu X (2012) Receptor activator for nuclear factor κ B expression predicts poor prognosis in breast cancer patients with bone metastasis but not in patients with visceral metastasis. J Clin Pathol 65(1):36–40. doi:10.1136/jclinpath-2011-200312
Blake ML, Tometsko M, Miller R, Jones JC, Dougall WC (2014) RANK expression on breast cancer cells promotes skeletal metastasis. Clin Exp Metastasis 31(2):233–245. doi:10.1007/s10585-013-9624-3
Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell SK, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F (2012) Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol 10(7):e1001363. doi:10.1371/journal.pbio.1001363
Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470(7335):548–553. doi:10.1038/nature09707
Sinn BV, von Minckwitz G, Denkert C, Eidtmann H, Darb-Esfahani S, Tesch H, Kronenwett R, Hoffmann G, Belau A, Thommsen C, Holzhausen HJ, Grasshoff ST, Baumann K, Mehta K, Dietel M, Loibl S (2013) Evaluation of mucin-1 protein and mRNA expression as prognostic and predictive markers after neoadjuvant chemotherapy for breast cancer. Ann Oncol 24(9):2316–2324. doi:10.1093/annonc/mdt162
Loibl S, Muller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, Ataseven B, du Bois A, Fissler-Eckhoff A, Gerber B, Kulmer U, Alles JU, Mehta K, Denkert C (2011) Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 130(2):477–487. doi:10.1007/s10549-011-1715-8
Acknowledgments
We would like to thank Ms. Ines Koch and Ms. Britta Beyer for their excellent technical assistance as well as Ms. Britta Dahl for proofreading.
Conflict of interest
D.B. declares that he receives remuneration as an employee of Amgen, Inc. and got stock ownership in Amgen, Inc.. S.L. declares to be a consultant/advisory role of Amgen. M.W. declares to be a consultant/advisory role of DAKO and he receives funding from DAKO and Biotest. W.C.D. declares that he receives remuneration as an employee of Amgen, Inc., got stock ownership in Amgen, Inc. and receives funding from Amgen, Inc.. G.V.M. declares that he receives remuneration of Amgen, Inc. and receives funding from Amgen, Inc.. The other author’s declare that they have no conflict of interest.
Ethical Standard
The experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pfitzner, B.M., Branstetter, D., Loibl, S. et al. RANK expression as a prognostic and predictive marker in breast cancer. Breast Cancer Res Treat 145, 307–315 (2014). https://doi.org/10.1007/s10549-014-2955-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-014-2955-1