Abstract
Cancer-induced bone pain (CIBP) is a common clinical problem in breast cancer patients with bone metastasis. Recent studies shows chemokines are novel targets for treatment of CIBP. In this study, we intra-tibial inoculated with Walker 256 rat mammary gland carcinoma cells into rat bone to established metastatic breast cancer. Then we measured the expression of CXCL10 in the spinal cord of metastatic bone cancer rats, investigated the role of CXCL10 in the development of CIBP, and the underlying mechanism. Results revealed that after intra-tibial inoculation with Walker 256 cells, rats showed up-regulation of CXCL10 and its receptor CXCR3 in the spinal cord. Interestingly, intrathecally injection of recombinant CXCL10 protein induced mechanical allodynia in naïve rats. Blocking the function of CXCL10/CXCR3 pathway via anti-CXCL10 antibody or CXCR3 antagonist prevented the development of CIBP and microglial activation. Moreover, CXCL10-induced mechanical allodynia was rescued by minocycline treatment during the late-stage of CIBP, days 10–14. The regulation of CXCL10 expression involved microglial activation in a manner of autocrine positive feedback. These results suggest that CXCL10 may be a necessary algogenic molecule, especially in the development of CIBP. Its function was partly mediated via spinal microglial activation. This study provides a novel insight into the biological function of chemokine CXCL10 in the molecular mechanism underlying cancer pain. It also provides new target for clinical treatment of metastatic breast cancer-induced bone pain in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is prone to metastasize to bone. About 73 % patients of breast cancer exhibit bone metastases [1]. Cancer-induced bone pain (CIBP) is an intractable problem in breast cancer patients with bone metastasis. Its clinical characteristics are mainly background pain and breakthrough pain [2], which induce a reduced quality of life. The mechanism for generation and maintenance of CIBP involves multiple factors, including immunologic mechanisms and neuron-glial interaction [3]. In the past decade, studies paid much attention to the roles of chemokines in the development of CIBP. Intrathecally administration of neutralizing antibody against monocyte chemotactic protein-1 (MCP-1) attenuated mechanical allodynia in CIBP rats [4]. Furthermore, chemokine CX3CL1/CX3CR1 also participates in pain facilitation in CIBP rats. Blockade of CX3CR1 function in CIBP rats prevented pain facilitation, and also inhibited activation of p38 mitogen-activated protein kinase (MAPK) pathway in microglia [5, 6].
The chemokine (C-X-C motif) receptor CXCR3 has three ligands, including monokine-induced by IFN-γ/CXCL9, IFN-γ-induced protein-10 /CXCL10, and interferon-inducible T cell alpha chemoattractant /CXCL11 [7]. Among these three chemokines, CXCL10 exerts potent biological function, including promoting the chemotactic activity, inducing apoptosis, regulating cell proliferation, angiogenesis, etc. [7–11]. In the tumor microenvironment, CXCL10 is required for migration and invasive motility of cancer cells [12]. CXCL10 also plays roles in regulating the neuro-glial function of the central nervous systems [13, 14]. The previous studies show that CXCL10 was up-regulated in nervous tissues under either inflammatory pain or neuropathic pain conditions [15, 16]. However, to date, little was done to investigate whether CXCL10 participated in the development of cancer pain.
In this present study, we measured the change of CXCL10 and CXCR3 expression in spinal cord of CIBP rats. Next, effect of CXCL10/CXCR3 pathway on mechanical allodynia in CIBP rats was investigated. Furthermore, we detected the underlying mechanism of that how CXCL10 contributed to pain condition in CIBP rats.
Materials and methods
Materials
Walker 256 rat mammary gland carcinoma cells were purchased from the Institute of Cancer Research, Chinese Academy of Medical Science and Peking Union Medical College, China. Recombinant rat CXCL10 protein and rabbit anti-CXCL10 neutralizing antibody were bought from PeproTech (USA). AMG487, a special antagonist of CXCR3, was a kind gift from Amgen (USA). 2-Hydroxypropyl-β-cyclodextrin (HPβCD) and minocycline hydrochloride were bought from Sigma-Aldrich (USA). Goat anti-CXCL10 antibody, goat anti-CXCR3 antibody, and normal rabbit IgG were bought from Santa Cruz (USA). CY3-conjugated goat anti-mouse IgG was bought from ProteinTech Group (USA). NorthernLights donkey anti-goat IgG-NL557 was bought from R&D (USA). Mouse anti-CD11b antibody was bought from Chemicon (USA).
Animals
Female Sprague-Dawley rats weighing 180–220 g, were supplied by the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology (HUST), Wuhan, China. Rats were kept under controlled conditions (22°C ± 0.5°C, relative humidity 40–60 %, alternate light-dark cycles, food and water ad libitum). All animal procedures were carried out in accordance with the National Institutes of Health guidelines and Ethical Issue of the International Association for the Study of Pain, and were approved by the Experimental Animal Care and Use Committee of Tongji Medical College, HUST (No. 2010-S216).
Surgical procedure of bone cancer models
CIBP modeling was prepared following the previous reports [17, 18]. Briefly, rats were deeply anesthetized with pentobarbital sodium (50 mg/kg, intraperitoneal injection (i.p.)). Then the right tibia was carefully exposed. Walker 256 cells (10 μl, 4 × 106 /ml) or D-Hanks solution (10 μl) were slowly injected into the bone cavity using a 50 μl Hamilton microsyringe. The injection site was closed using bone wax as soon as the syringe was removed. Then the wound was closed after carefully disinfected [17].
Real-time polymerase chain reaction (PCR)
All animals were deeply anesthetized with pentobarbital sodium. Subsequently, each animal’s L3–L5 lumbar region of spinal cord was removed and stored at −80°C for analysis of CXCL10 and CXCR3 mRNA. Primer sequences for the genes of interest were following the previous study [19]. RNA extraction, cDNA synthesis and real-time PCR were performed as reported previously [18]. The expression level of target mRNA was quantified relative to level of the GAPDH (house-keeping gene) using the relative quantification 2−ΔΔCT method.
Immunohistochemical analyses
On day 14 post modeling, rats in each group were deeply anesthetized with pentobarbital sodium and perfused with saline, followed by 4 % paraformaldehyde in 0.1 M phosphate buffer saline (pH 7.4) for immunohistochemistry. L3–L5 spinal cord segments were removed and post-fixed in the same solution for 24 h at 4 °C, then dehydrated in 30 % sucrose solution. After increasing membrane permeability and blocking nonspecific binding, 20 μm-thick sections were incubated overnight at 4 °C with the primary antibodies: mouse anti-CD11b antibody (1:100), goat anti-CXCL10 antibody (1:100), and goat anti-CXCR3 antibody (1:100). Then sections were incubated with donkey anti-goat IgG or CY3-goat anti-mouse IgG for 2.5 h at room temperature. Fluorescent images were captured using confocal microscopy (Olympus, Japan). Optical density mean of CXCL10 and numbers of positive-CXCR3 cells were analyzed by Image Pro Plus. Three slices per sample from four rats of each group were measured.
Intrathecal catheterization and drug delivery
Under the same surgical conditions to CIBP modeling, rats were intrathecally implanted PE-10 catheter to the spinal cord level of the lumbar enlargement, according to the method described previously [20]. Proper intrathecal location was confirmed by a temporary motor block of both hind limbs after the injection of 10 μl 2 % lidocaine. Those rats exhibiting postoperative neurological deficits (e.g., paralysis) or poor grooming were excluded from the experiments. The animals were allowed 7-day recovery period before used in experiments.
For drug dissolution, recombinant rat CXCL10 protein (rrCXCL10) and rabbit anti-CXCL10 neutralizing antibody were dissolved in saline. AMG487 was dissolved in 20 % 2-hydroxypropyl-β-cyclodextrin (HPβCD) as previously described [21], and then diluted into experimental concentration. Minocycline was dissolved in sterile water. All drugs were intrathecally injected (i.t.) in a 10 μl volume followed by a 10 μl saline flush.
Behavioral assessment
Mechanical allodynia was assessed by measuring hind paw withdrawal thresholds (PWTs) to von Frey filament stimulation as previous studies [18, 22]. Briefly, rats were allowed to acclimatize to their surroundings for 30 min before tested. Von Frey filaments, with ascending order of forces (0.4, 0.6, 1, 2, 4, 6, 8, 10, and 15 g), were applied for up to 6 s per filament to the region between the foot pads in the plantar aspect of the right hind paw. Abrupt paw withdrawal, lickings, and shaking were taken to be positive responses. Once a withdrawal response was established, the paw was re-tested after a 5 min-rest, starting with the next descending von Frey hair until no response occurred. The lowest amount of force required to elicit a response was recorded as the PWT (in grams).
Data analysis
Raw data were presented as mean ± SEM. Changes within each group over time were analyzed by using one-way ANOVA, and followed by post hoc comparison (Student-New-man–Keuls test). Significant differences between treatment groups were detected by two-way ANOVA. P < 0.05 was considered to be statistically significant.
Results
CXCL10 and CXCR3 expression were persistently increased in spinal cord of CIBP models
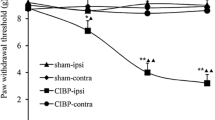
We measured the change of CXCL10 and CXCR3 mRNA expression in the spinal cord of bone cancer models on days 7, 14, and 21 after cancer cells inoculation by real-time PCR, and measured the change of CXCL10 and CXCR3 protein expression in the spinal cord on day 14 by immunohistochemistry. Results revealed that mRNA expressions of CXCL10 and CXCR3 in the spinal cord of CIBP models were increased, as revealed by real-time PCR (Fig. 1a, b). Remarkably, the up-regulation of CXCL10 and CXCR3 was most significant on day 14 (Fig. 1a, b). CXCL10 and CXCR3 protein were also increased in the spinal cord of CIBP rats on day 14, as showed by immunohistochemistry. The optical density mean of CXCL10 was increased in both contralateral and ipsilateral dorsal horn of the spinal cord of CIBP rats (Fig. 2a). CXCR3-positive cells were also increased in both contralateral and ipsilateral dorsal horn in CIBP rats, in which, the up-regulation in the ipsilateral dorsal horn was more remarkable (Fig. 2b).
CXCL10 and CXCR3 expression in the spinal cord were increased in CIBP rats measured by immunofluorescence. CXCL10 (a) and CXCR3 (b) expression were increased in both contralateral (cont) and ipsilateral (ipsi) dorsal horn of the spinal cord of CIBP rats on day 14 post inoculations (c).Optical density mean of CXCL10 and number of CXCR3-positive cells were analyzed by Image Pro Plus. * p < 0.05 versus sham. # p < 0.05 versus CIBP-ipsi. Scale bar 200 μm. n = 3–5 in each group
CXCL10 was required for the development of CIBP
To investigate whether CXCL10 was necessary for inducing pain, naïve rats received rrCXCL10 (10 ng, i.t.) or saline (10 μl, i.t.) treatment (pilot experiment determined dosage). PWTs were measured at 5, 15, 30, 45, 60, 90, and 120 min after injection. Results showed that PWTs decreased immediately after rrCXCL10 injection (2–5 min) from 12.6 g ± 1.0 at 0 min to 2.36 g ± 0.76 at 30 min, an established baseline threshold of drug. The mechanical allodynia induced by rrCXCL10 lasted up to 120 min (Fig. 3a).
CXCL10 directly participated in the development of CIBP (a). The PWTs were decreased immediately after drug injection and lasted for more than 120 min in rrCXCL10-treated rats. * p < 0.05 versus saline-treated rats. # p < 0.05 versus naïve rats (b). Neutralizing anti-CXCL10 antibody (anti-CXCL10) prevented the development of mechanical allodynia in CIBP rats. * p < 0.05 versus CIBP rats. # p < 0.05 versus CIBP-rabbit IgG rats. n = 6–8 in each group
To detect whether CXCL10 was required for the development of CIBP, we inhibited the CXCL10 function via chronic injecting rabbit anti-CXCL10 neutralizing antibody (200 ng, i.t.) or normal rabbit IgG (10 μl, i.t.) once per day from days 1 to 14. PWTs were measured at 30 min after drug injection on days 1, 3, 5, 7, 10, and 14. As shown in Fig. 3b, PWTs failed to decrease until day 14 in anti-CXCL10 neutralizing antibody-treated rats. However, vehicle of normal rabbit IgG treated rats and CIBP rats showed decreased PWTs after day 3 (Fig. 3b).
Acute or chronic inhibition of CXCR3 reversed or prevented mechanical allodynia in CIBP models
CXCR3 is the common receptor of CXCL9, CXCL10, and CXCL11. To investigate the role of CXCR3 in the maintenance of CIBP, AMG487-a special antagonist of CXCR3 was employed. On day 14 post cancer cells inoculation, AMG487 (20 μg, i.t.) or vehicle (20 % HPβCD, 10 μl, i.t.) was administrated. In AMG487-rrCXCL10-treated rats, rrCXCL10 (20 ng, i.t.) was injected 30 min after AMG487. PWTs were determined at 0.25, 0.5, 1, 2, 3, and 4 h after last drug injection. Results demonstrated that at 0.5 h time point, AMG487 began to reverse mechanical allodynia in CIBP rats, from 3.00 g ± 1.29 at 0 min to 15.00 g ± 0.00 at 2 h time point, and lasted up to 4 h (Fig. 4a). AMG487 treatment had no effect on the PWTs of sham rats, but prevented rrCXCL10 induced mechanical allodynia (Fig. 4b). These results suggested that CXCL10 induced mechanical allodynia via the chemokine receptor CXCR3. CXCL10/CXCR3 pathway contributed to the maintenance of mechanical allodynia in CIBP rats.
Acute blockade of CXCR3 reversed mechanical allodynia in CIBP rats (a). AMG487 reversed mechanical allodynia in CIBP rats. PWTs of AMG487 treated rats were increased from 30 min and last for at least 4 h after drug injection. * p < 0.05 versus CIBP rats. # p < 0.05 versus vehicle-treated CIBP rats (b). AMG487 did not change the PWTs of sham rats, but prevented rrCXCL10-induced mechanical allodynia in sham rats. n = 6–8 in each group
In order to investigate whether CXCL10 contributes to the development of CIBP via CXCR3, rrCXCL10 (20 ng), AMG487 (20 μg), vehicle (20 % HPβCD, 10 μl) or AMG487/rrCXCL10 were intrathecally injected once per day for 14 days. In AMG487/rrCXCL10 group, rrCXCL10 was injected 30 min after AMG487. PWTs were measured 1 h after last injection at time points 1, 3, 7, 10, and 14 days of post modeling. The PWTs of CIBP rats began diminishing after day 3, suggesting the development of mechanical allodynia. AMG487 rescued the decrease of PWTs in bone cancer rats (Fig. 5a). Interestingly, from day 1, rrCXCL10-treated CIBP rats showed significantly reduced PWTs compared with sham rats. However, rrCXCL10 treatment did not induce mechanical allodynia in AMG487 pre-treated rats (Fig. 5b). These results suggested that chronic blocking CXCR3 function via AMG487 prevented the development of CIBP.
Chronic blockade of CXCR3 prevented the development of CIBP (a). Chronic injection of AMG487 prevented the development of mechanical allodynia of CIBP rats. * p < 0.05 versus CIBP rats. # p < 0.05 versus vehicle-treated CIBP rats (b). AMG487 pre-treatment prevented rrCXCL10-induced mechanical allodynia in CIBP rats. *p < 0.05 versus saline-treated CIBP rats. # p < 0.05 versus rrCXCL10-treated CIBP rats. n=6–8 in each group
Blockade of CXCR3 prevented microglial activation in the spinal cord of CIBP models
The previous studies showed that microglial activation was involved in the development of CIBP. To investigate the change of microglial activation in the AMG487 treated CIBP models, we detected the activation of microglia in the spinal cord by immunohistochemistry. Rats received chronic AMG487 (20 μg, i.t., once daily) or vehicle (20 % HPβCD, 10 μl, i.t.) after bone cancer modeling as above. Rats were sacrificed on day 14, and the spinal cords were removed and sliced. Twenty-μm-thick slices of spinal cord were stained with mouse anti-CD11b antibody. Results showed microglia was significantly activated in the spinal cord of vehicle-treated CIBP rats, displaying a characteristic ramified structure with an increase cytoplasmic and dendrite volume (Fig. 6). However, after chronic AMG487 treatment, the microglial activation was attenuated (Fig. 6).
Inhibiting microglial activation alleviated rrCXCL10-induced mechanical allodynia at later-stage in CIBP models and attenuated CXCL10 up-regulation
To investigate whether microglial activation was involved in rrCXCL10-induced mechanical allodynia, minocycline (50 μg) was intrathecally injected into CIBP rats once per day under stages of development, early- (days 1–7) or later-stage (days 11–14). Similarly, minocycline also was injected to sham rats for 7 days. RrCXCL10 was administrated 1 h after the last minocycline injection. PWTs were measured 30 min after rrCXCL10 injection. Results indicated that minocycline treatment ameliorated pain in both stages but had no effect on sham rats (Fig. 7a, c). Minocycline pre-treatment fully prevented rrCXCL10-induced mechanical allodynia on day 14 (Fig. 7c), but only slightly attenuated rrCXCL10-induced mechanical allodynia on day 7 in CIBP rats and sham rats (Fig. 7a, b). This means rrCXCL10-induced allodynia at the later-stage of CIBP was regulated by microglial activation.
Minocycline prevented rrCXCL10-induced mechanical allodynia in the later-stage of CIBP and inhibited the mRNA expression of CXCL10 in CIBP rats (a). Effect of minocycline treatment on PWTs of the sham rats. # p < 0.05 versus MC-treated rats (b). On day 7 of the early-stage, minocycline had no effect on rrCXCL10-induced mechanical allodynia. * p < 0.05 versus saline-treated rats. # p < 0.05 versus MC-treated rats (c). On day 14 of the later-stage, minocycline treatment prevented mechanical allodynia in CIBP rats, as well as rrCXCL10-treated CIBP rats. * p < 0.05 versus saline-treated rats. # p < 0.05 versus MC-treated rats (d). Expression of CXCL10 mRNA in the spinal cord after minocycline and (or) rrCXCL10 treatment in sham rats. * p < 0.05 versus saline-treated rats. # p < 0.05 versus rrCXCL10-treated rats (e). up-regulation of CXCL10 mRNA in the spinal cord of CIBP rats was partly mediated by minocycline treatment. * p < 0.05 versus saline-treated rats. # p < 0.05 versus MC-saline-treated rats. n=4–6 in each group
To investigate whether inhibiting the function of microglia has influence on the expression of CXCL10 in CIBP models and sham rats, minocycline (50 μg, i.t.) was injected once per day for 7 days. On day 7, rrCXCL10 (20 ng, i.t.) was injected at 1 h after minocycline injection. Two hours after last injection, rats were sacrificed and spinal cords were removed. Then CXCL10 mRNA expression was measured by real-time PCR. The results revealed that rrCXCL10 increased the CXCL10 mRNA expression in both CIBP rats and sham rats. Minocycline pre-treatment partially attenuated both increase of CXCL10 mRNA. However, minocycline had no effect on the CXCL10 expression in sham rats (Fig. 7d, e).
Discussion
This present study demonstrated that (1) the expression of chemokine CXCL10 and its receptor CXCR3 were up-regulated in CIBP rats. (2) CXCL10/CXCR3 contributed to the mechanical allodynia in CIBP rats. CXCL10 directly induced mechanical allodynia in naïve rat. Functionally inhibition of CXCR3 reversed the mechanical allodynia in CIBP rats by single injection of AMG487, and prevented the development of CIBP by chronic AMG487 injection. (3) Meanwhile, activation of microglia in the spinal cord was also reversed by chronic injection of AMG487 in CIBP rats. More importantly, minocycline, inhibitor of microglia, could attenuate rrCXCL10-induced mechanical allodynia at the later-stage of CIBP models. These results showed that CXCL10/CXCR3 mediated the development and maintenance of CIBP partly by microglial activation at least.
Recent studies have shown that chemokines play an important role in CIBP. However, the role of CXCL10 on CIBP was rarely investigated. In this study, our results show that mRNA and protein of CXCL10 and CXCR3 were all up-regulated in CIBP rats. This provides preliminary evidence to the hypotheses that CXCL10 participated in CIBP. Moreover, the expression peaked at day 14 post modeling, remaining high on day 21. According to this finding, we choose day 14 as an optimal time point for behavioral research in subsequent studies.
According to Saika et al, [23] chemokines were inducers of allodynia. Local injection of recombinant MIP-1β produced a long-lasting tactile allodynia via up-regulation of inflammatory molecules. Moreover, inhibiting the function of chemokines and their receptors could attenuate the mechanical allodynia in CIBP or neuropathic pain models [4–6, 24]. In our study, intrathecally injected rrCXCL10 to naïve rat induced tactile allodynia 5 min immediately after drug injection. This suggests CXCL10 may be a rapid-response algogenic molecule. While single injection of AMG487 reversed pain allodynia on day 14 in CIBP rats, repeated injection of AMG487 prevented the development of CIBP. Moreover, rrCXCL10 did not induced allodynia in the AMG487 pre-treated rats. Similarly, repeated injection of anti-CXCL10 neutralizing antibody also prevented allodynia in CIBP rats. These demonstrated that CXCL10 participated in both the development and maintenance of CIBP via receptor CXCR3. Both CXCL10 and CXCR3 may be important targets for analgesic therapy of metastatic breast cancer patients.
Microglial activation is closely implicated in cancer pain and other chronic pain states [18, 25]. Microglia can be activated by phagocytosis or through the activation of constitutively expressed cell surface molecules (IL-1β, IL-6, TNF-α etc.) [26–28]. Studies showed that CXCR3 was distributed in microglia in the central nervous system [29–31]. In this study, microglia was significantly activated in CIBP rats on day 14 post modeling. Blocking CXCR3 prevented both microglia activation and mechanical allodynia. These results demonstrated that the activation of CXCL10/CXCR3 pathway may activate microglia and sequentially induce pain in CIBP rats. Minocycline prevented mechanical allodynia in CIBP rats in the first 7 days after modeling and reversed mechanical allodynia on day 14. This prompts microglia plays roles in both the developing and maintaining of CIBP. On day 14, rrCXCL10-induced mechanical allodynia was inhibited by minocycline pre-treatment, while on day 7, rrCXCL10 still induced pain in minocycline pre-treated CIBP rats. Therefore, the role of CXCL10 in later-stage of CIBP may be mediated via activation of microglia.
In this study, we also found CIBP rats that received minocycline treatment showed partial decrease of CXCL10 mRNA expression compared with CIBP rats. Interestingly, rrCXCL10-induced up-regulation of CXCL10 mRNA was also partially attenuated by minocycline pre-treatment. Therefore, we presume microglia may plays an important role in the regulation of CXCL10 expression. As the receptor of IFN-γ (IFN-γR) was expressed in the membrane in microglia, so when microglia was suppressed, the production of CXCL10 may be decreased. Studies showed that cytokines and chemokines could be regulated by a positive feedback mechanism [32–34]. We presume that the regulation of CXCL10 expression was involved in an autocrine positive feedback loop, which was partly dependent on the function of microglia. However, the details and potential mechanism of this feedback loop were not clear.
Based on all these findings, we speculate that CXCL10 may be an algogenic molecule in the development and maintenance of CIBP, which was mediated by microglial activation. Furthermore, CXCL10 expression may be regulated partly by microglial activation in a manner of autocrine positive feedback loop. CXCL10/CXCR3 pathway may serve as a novel target for clinical treatment of metastatic breast cancer-induced bone pain condition. However, further studies need to be done to investigate the underlying mechanism of this action.
Abbreviations
- CXCL10:
-
C-X-C motif chemokine 10
- CXCR3:
-
C-X-C motif chemokine receptor 3
- rrCXCL10:
-
recombinant rat CXCL10 protein
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- CIBP:
-
cancer-induced bone pain
- PWTs:
-
paw withdrawal thresholds
- HPβCD:
-
2-Hydroxypropyl-β-cyclodextrin
- MC:
-
minocycline
- CD11b:
-
integrin alpha M chain, a special molecule in microglia
References
Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 12(20 Pt 2):6243s–6249s. doi:10.1158/1078-0432.CCR-06-0931
Middlemiss T, Laird BJ, Fallon MT (2011) Mechanisms of cancer-induced bone pain. Clin Oncol 23(6):387–392. doi:10.1016/j.clon.2011.03.003
Vadalouca A, Raptis E, Moka E, Zis P, Sykioti P, Siafaka I (2012) Pharmacological treatment of neuropathic cancer pain: a comprehensive review of the current literature. Pain Pract 12(3):219–251. doi:10.1111/j.1533-2500.2011.00485.x
Hu JH, Zheng XY, Yang JP, Wang LN, Ji FH (2012) Involvement of spinal monocyte chemoattractant protein-1 (MCP-1) in cancer-induced bone pain in rats. Neurosci Lett 517(1):60–63. doi:10.1016/j.neulet.2012.04.026
Hu JH, Yang JP, Liu L, Li CF, Wang LN, Ji FH, Cheng H (2012) Involvement of CX3CR1 in bone cancer pain through the activation of microglia p38 MAPK pathway in the spinal cord. Brain Res 1465:1–9. doi:10.1016/j.brainres.2012.05.020
Yin Q, Cheng W, Cheng MY, Fan SZ, Shen W (2010) Intrathecal injection of anti-CX3CR1 neutralizing antibody delayed and attenuated pain facilitation in rat tibial bone cancer pain model. Behav Pharmacol 21(7):595–601. doi:10.1097/FBP.0b013e32833e7e2a
Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, Stiles JK (2011) CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev 22(3):121–130. doi:10.1016/j.cytogfr.2011.06.001
Giuliani N, Bonomini S, Romagnani P, Lazzaretti M, Morandi F, Colla S, Tagliaferri S, Lasagni L, Annunziato F, Crugnola M, Rizzoli V (2006) CXCR3 and its binding chemokines in myeloma cells: expression of isoforms and potential relationships with myeloma cell proliferation and survival. Haematologica 91(11):1489–1497
Neville LF, Mathiak G, Bagasra O (1997) The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev 8(3):207–219
Sato E, Fujimoto J, Tamaya T (2007) Expression of interferon-gamma-inducible protein 10 related to angiogenesis in uterine endometrial cancers. Oncology 73(3–4):246–251. doi:10.1159/000127422
Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG (2006) CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am J Physiol Lung cell Mol Physiol 290(5):L909–L918. doi:10.1152/ajplung.00430.2005
Shin SY, Nam JS, Lim Y, Lee YH (2010) TNFalpha-exposed bone marrow-derived mesenchymal stem cells promote locomotion of MDA-MB-231 breast cancer cells through transcriptional activation of CXCR3 ligand chemokines. The J Biol Chem 285(40):30731–30740. doi:10.1074/jbc.M110.128124
Muller M, Carter S, Hofer MJ, Campbell IL (2010) Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity—a tale of conflict and conundrum. Neuropathol Appl Neurobiol 36(5):368–387. doi:10.1111/j.1365-2990.2010.01089.x
Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD (2008) Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Nat Acad Sci USA 105(12):4814–4819. doi:10.1073/pnas.0801544105
Strong JA, Xie W, Coyle DE, Zhang JM (2012) Microarray analysis of rat sensory ganglia after local inflammation implicates novel cytokines in pain. PloS one 7(7):e40779. doi:10.1371/journal.pone.0040779
Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R (2010) Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain 148(3):509–518. doi:10.1016/j.pain.2010.01.001
Cao F, Gao F, Xu AJ, Chen ZJ, Chen SS, Yang H, Yu HH, Mei W, Liu XJ, Xiao XP, Yang SB, Tian XB, Wang XR, Tian YK (2010) Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res 1326:162–173. doi:10.1016/j.brainres.2010.02.039
Liu X, Bu H, Liu C, Gao F, Yang H, Tian X, Xu A, Chen Z, Cao F, Tian Y (2012) Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J Huazhong Univ Sci Technolog Med Sci 32(2):291–298. doi:10.1007/s11596-012-0051-5
Steinmetz OM, Sadaghiani S, Panzer U, Krebs C, Meyer-Schwesinger C, Streichert T, Fehr S, Hamming I, van Goor H, Stahl RA, Wenzel U (2007) Antihypertensive therapy induces compartment-specific chemokine expression and a Th1 immune response in the clipped kidney of Goldblatt hypertensive rats. Am J Physiol Renal Physiol 292(2):F876–F887. doi:10.1152/ajprenal.00174.2006
Yaksh TL, Rudy TA (1976) Chronic catheterization of the spinal subarachnoid space. Physiol Behav 17(6):1031–1036
Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM (2006) Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res 66(15):7701–7707. doi:10.1158/0008-5472.CAN-06-0709
Fox A, Medhurst S, Courade JP, Glatt M, Dawson J, Urban L, Bevan S, Gonzalez I (2004) Anti-hyperalgesic activity of the cox-2 inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain 107(1–2):33–40
Saika F, Kiguchi N, Kobayashi Y, Fukazawa Y, Kishioka S (2012) CC-chemokine ligand 4/macrophage inflammatory protein-1beta participates in the induction of neuropathic pain after peripheral nerve injury. Eur J Pain 16(9):1271–1280. doi:10.1002/j.1532-2149.2012.00146.x
Liou JT, Yuan HB, Mao CC, Lai YS, Day YJ (2012) Absence of C-C motif chemokine ligand 5 in mice leads to decreased local macrophage recruitment and behavioral hypersensitivity in a murine neuropathic pain model. Pain 153(6):1283–1291. doi:10.1016/j.pain.2012.03.008
Watkins LR, Maier SF (2002) Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 82(4):981–1011. doi:10.1152/physrev.0 0011.2002
Guillot X, Semerano L, Decker P, Falgarone G, Boissier MC (2012) Pain and immunity. Joint, bone, spine : revue du rhumatisme 79(3):228–236. doi:10.1016/j.jbspin.2011.10.008
Schomberg D, Olson JK (2012) Immune responses of microglia in the spinal cord: contribution to pain states. Exp Neurol 234(2):262–270. doi:10.1016/j.expneurol.2011.12.021
Yao M, Chang XY, Chu YX, Yang JP, Wang LN, Cao HQ, Liu MJ, Xu QN (2011) Antiallodynic effects of propentofylline Elicited by interrupting spinal glial function in a rat model of bone cancer pain. J Neurosci Res 89(11):1877–1886. doi:10.1002/jnr.22711
Van Der Meer P, Goldberg SH, Fung KM, Sharer LR, Gonzalez-Scarano F, Lavi E (2001) Expression pattern of CXCR3, CXCR4, and CCR3 chemokine receptors in the developing human brain. J Neuropathol Exp Neurol 60(1):25–32
Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H (2004) CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci 24(39):8500–8509. doi:10.1523/JNEUROSCI.2451-04.2004
Vinet J, de Jong EK, Boddeke HW, Stanulovic V, Brouwer N, Granic I, Eisel UL, Liem RS, Biber K (2010) Expression of CXCL10 in cultured cortical neurons. J Neurochem 112(3):703–714. doi:10.1111/j.1471-4159.2009.06495.x
Kroeze KL, Boink MA, Sampat-Sardjoepersad SC, Waaijman T, Scheper RJ, Gibbs S (2012) Autocrine regulation of re-epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. J Invest Dermatol 132(1):216–225. doi:10.1038/jid.2011.245
Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, Mehrad B (2010) Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol 185(10):6190–6197. doi:10.4049/jimmunol.1002064
Korkaya H, Liu S, Wicha MS (2011) Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots. Clinical Cancer Res 17(19):6125–6129. doi:10.1158/1078-0432.CCR-10-2743
Acknowledgments
We are grateful to Amgen for kindly providing AMG487 to us. We also gratefully acknowledge the expertise of Ms. Amber Chen (Project Intern, Department of Neuroscience, Baylor College of Medicine, USA) in the proof-reading of the manuscript. This work was supported by grants from the Natural Science Foundation of China (Nos. 81070890, 30872441, 81371250, 81171259, 81100832 and 30901395), and also supported by 2010 Clinical Key Disciplines Construction Grant from the Ministry of Health of China.
Conflict of interest
The authors declare that they do not have financial relationships with any of the organizations that sponsored the research, and they do not have any other real or apparent conflict(s) of interest that may have a direct bearing on the subject matter of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Huilian Bu and Bin Shu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Bu, H., Shu, B., Gao, F. et al. Spinal IFN-γ-induced protein-10 (CXCL10) mediates metastatic breast cancer-induced bone pain by activation of microglia in rat models. Breast Cancer Res Treat 143, 255–263 (2014). https://doi.org/10.1007/s10549-013-2807-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2807-4