Abstract
Perceptions among women with breast cancer about the relative importance of different potential chemotherapy side effects is not well understood. A survey was performed by women receiving chemotherapy for breast cancer. Grade I/II (mild to moderate) and III/IV (moderate to severe) descriptions of nine common chemotherapy side effects were assigned preference weights using the standard gamble technique. For each hypothetical side effect, patients could choose to stay in the respective side effect state or take a gamble between full health (probability p) or being dead (1 − p). For each side effect, p was varied until the patient was indifferent between these options. The survey also included questions about the importance of survival, slowing cancer growth, and quality of life. This analysis included 69 patients; mean age 54 years (range 35–84), representing all cancer stages. Standard gamble preferences were lowest (i.e., least preferred) for grade III/IV nausea/vomiting (0.621), indicating that patients would, on average, risk a 38 % chance of being dead to avoid having grade III/IV nausea/vomiting for the rest of their lives. The next least preferred side effects were grade III/IV diarrhea (0.677) and grade III/IV sensory neuropathy (0.694). Survival appeared more important than slowing cancer growth and maintaining quality of life across cancer stages. Nevertheless, patients with advanced disease placed less importance on survival (p = 0.09) and higher importance on quality of life (p = 0.05). These standard gamble utilities provide unique insights into chemotherapy toxicities from the patient perspective. Differences in the relative importance of overall survival and quality of life with treatment existed between patients with different stages of disease. These studies should be expanded as the data may also be used to calculate quality-adjusted life expectancy in cost-effectiveness evaluations of breast cancer chemotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women with early-stage and metastatic breast cancer are frequently treated with multiple lines of chemotherapy. Relatively little is known about how patient perceptions of the various possible side effects of chemotherapy affect decisions about ongoing and subsequent treatments [1]. There may be particular side effects that may make a patient decide not to receive a particular agent or to discontinue treatment altogether. Knowing more about patient preferences associated with these side effects could help physicians, patients, and drug funding agencies make appropriate treatment and funding decisions [2].
Patient preferences (or utilities) are a key component in cost-effectiveness analyses that incorporate quality-adjusted life years (QALYs) by combining longevity with quality of life [3]. Specifically, a utility weight is assigned to each health state experienced by a patient on a scale where 0.0 reflects being dead and 1.0 reflects full health [4]. Evaluation of the incremental cost per QALY gained, or cost–utility analyses, is now a standard type of cost-effectiveness analysis [5, 6].
With respect to utility estimates that are relevant to patients with breast cancer, studies that have obtained such estimates have primarily focused on health states occurring in early-stage disease [7, 8], or have obtained such valuations from the general population [9] or nurses [10]. Moreover, we are not aware of any studies that have obtained utilities for individual mild/moderate and severe chemotherapy side effects directly from breast cancer patients receiving chemotherapy. The objective of this study was to obtain utility weights from patients with breast cancer for common side effects associated with adjuvant and as well as palliative chemotherapy.
Methods
This cross-sectional survey was performed at two Canadian Cancer Centres (Ottawa Hospital Cancer Center and Irving Greenberg Family Cancer Centre, Ottawa). Eligible patients were females with breast cancer, of all stage disease, who were currently receiving adjuvant, neoadjuvant, or palliative chemotherapy. Participants had to have adequate written and oral fluency in English and be able to use a computer. After providing written informed consent, participants completed a secure Web survey. Depending upon the patient’s preference, they could complete the survey on a laptop in the chemotherapy unit or at home. The participants completed surveys at their own pace and research team members were available via telephone and/or e-mail if the participant required additional assistance. The study was approved by the Ottawa Hospital Research Ethics Board.
The survey was developed by the study team (NB, MC, KB, and JG) [11] and included questions on demographic/clinical characteristics; agree/disagree questions about the importance of survival, controlling the speed of cancer growth, and quality of life. It also included standard gamble questions, which are designed to obtain preference weights for selected health states. The standard gamble questions in the survey presented different hypothetical health states that each described a side effect of chemotherapy. For each health state, patients were given a choice between two options: the certainty of staying in the hypothetical health state the rest of their life or taking a gamble. The gamble had two possible outcomes: full health (with probability of occurrence p) or immediate death (with probability 1 − p). The probability p was varied in 5 % increments until patients were unable to make a clear choice between staying in the certain state for the rest of their lives and taking the gamble [4]. The objective of the elicitation process is to derive the probability p associated with the indifference point (i.e., utility) between the certain and risky alternative. Figure 1 shows an example of a standard gamble question focusing on the grade I/II motor neuropathy health state.
To identify the health states (side effects) to be included in the standard gamble exercise, the literature and medical labeling information for commonly used chemotherapies in breast cancer were reviewed (NB, MC, KB, and JG). As well, breast cancer web forums also were reviewed to confirm that the selected side effects substantially impacted daily life. A total of 17 health states were selected that included alopecia and both grade I/II and grade III/IV categories of eight side effects. The CTC grading criteria and patients’ own descriptions of the side effects from patient web forums were used to develop the health state descriptions. The survey was pilot tested in five patients before finalization.
To minimize respondent burden due to the large number of side effect health states being assigned standard gamble utilities, two surveys were developed which differed only with respect to the standard gamble section. Specifically, the side effect health states were divided evenly between the two surveys; the two severity level health states, grade I/II and grade III/IV, for each health state, however, always appeared in the same survey. For example, grade I/II diarrhea appeared with grade III/IV diarrhea in the same survey. The target sample size for this study was 70 respondents, of which 35 would be randomized to one of the two surveys (group 1 and group 2). The target sample size was calculated to allow a 95 % confidence interval of ±0.10 around the mean utility estimates [12].
Oversampling was permitted to allow for the target sample size to be reached, after elimination of illogical responses, assigning a more favorable utility to the severe versus the mild level of a side effect. For example, if a patient would take a higher risk of being dead to avoid grade I/II nausea than grade III/IV nausea this would be considered an illogical response. Prior experience with web-based standard gamble surveys has shown that illogical response rates, or assigning the same utility to the vast majority of the health states, may be as high as 20 % [13]. It was determined a priori to exclude such patients because these responses indicate that, for some reason, the standard gamble exercise is not working, either because the patient does not understand it or the exercise is not sensitive enough to detect differences between severity levels. Anticipating that such patients would be excluded from the analysis, the total target sample was 100 patients.
Analysis
The analyses primarily were descriptive analyses, and means, standard deviations, ranges, and percentages were reported, as appropriate. With respect to the standard gamble scoring, for each heath state, the probability p at the indifference point between choosing to stay in the respective health state versus taking a gamble represents the utility score for the health state. Utility scores range from 0.0, reflecting being dead, to 1.0, reflecting full health. For purposes of interpretation, a mean utility of 0.75 indicates that patients would, on average, risk a 25 % chance of being dead to avoid being in the selected health state. Exploratory subgroup analyses were conducted using an analysis of variance test. SAS 9.0 was used for all analyses.
Results
From June to December 2012 a total of 102 patients with breast cancer completed the survey. Of these, 33 (32 %) were excluded from the analysis due to having at least two illogical responses (8 %) or assigning at least 80 % of the health states the same utility (24 %), resulting in an effective sample size of 69 patients. Demographic/clinical characteristics did not differ significantly between the excluded patients versus those included in the analysis (Table 1). The mean age was 54 ± 10.7 (range 35–84 years), and each disease stage was represented. Mean time from diagnosis to participation in the study was 25 + 42 (median-12) months (mean-10 months for adjuvant setting and 40 months for metastatic), and mean number of chemotherapy cycles was 4.30 + 3.05 (4-median) cycles (mean 4 for adjuvant setting and 6 for metastatic). The patients had received an average of 2.3 different chemotherapy combinations and the majority were receiving anthracycline or taxane-based regimens at the time of the survey. As mentioned above two surveys were developed which differed only with respect to the standard gamble section, patient demographic/clinical characteristics were comparable between these two groups.
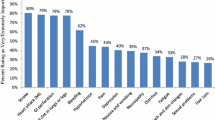
Figure 2 shows the frequencies with which each grade III/IV side effect was rated as the worst or the most acceptable side effect (multiple side effects could be rated as the worst or the most acceptable and thus the percentages add up to more than 100 %). Grade III/IV nausea and/or vomiting requiring a physician or emergency room visit was ranked as the worst side effect (60 %), followed by grade III/IV diarrhea requiring hospitalization (40 %), grade III/IV hand–foot syndrome (37 %), and grade III/IV peripheral neuropathy (34 %). The side effects identified as most acceptable were alopecia (88.4 %) and grade III/IV fatigue (24 %).
Table 2 reports the standard gamble utilities for the side effect health states. The utility for the patients’ current health was 0.769 ± 15.9. The utility was lowest (i.e., least preferred) for grade III/IV nausea/vomiting (0.621), indicating that patients would, on average, risk a 38 % chance of being dead to avoid having grade III/IV diarrhea for the remainder of their lives. The next least preferred states were grade III/IV diarrhea (0.677) and sensory neuropathy (0.694). The most preferred states were grade I/II diarrhea (0.760), hand–foot syndrome (0.754), and mucositis (0.747). No statistically significant differences were observed in the standard gamble utilities by cancer stage, age (under 50, 50–59, and 60 or older), and marital status (data not shown).
Responses to the agree/disagree statements regarding the importance of survival, control of tumor growth, and decreasing quality of life showed differences among those in earlier stages of cancer versus those in more advanced stages (Fig. 3). Although not statistically significant, the patients with earlier stage disease appeared to place higher importance on survival than more advanced stage patients. Those with stage 1 disease rated “improving survival is important” as 5.0, and those with stage 4 disease rated this as 4.2 on a 1–5 scale, where 1 reflects “least agree with” and 5 reflects “most agree with” (p = 0.09). In addition, patients with more advanced disease appeared to place higher importance on quality of life than those in earlier stages. For example, patients with stage 4 disease provided a mean rating of 2.9 for “a high quality of life is the most important in my cancer care regardless of my overall survival and the ability of the treatment to slow down my cancer growth” whereas patients with stage 1 disease rated this as 1.8 (p = 0.05). Nevertheless, across the four cancer stages, those statements that referred to the importance of improving survival generally had the highest agreement ratings compared to those referring to the importance of slowing cancer growth or maintaining quality of life.
Discussion
Chemotherapy remains an essential component of breast cancer therapy. However, given the increasing importance placed on patient quality of life it is critical that patients, physicians, and drug funding agencies fully understand the potential impact of treatment on patient quality of life. To this end, this study provides valuable information about commonly occurring side effects associated with chemotherapy from the perspective of patients with different stages of disease. It builds on the current literature by not just focusing on one severity level, but includes mild/moderate as well as severe effects. The utility weights derived from this study could also be used in future cost-effectiveness evaluations to quality-adjusted life expectancy based on the patient perspective.
When utilities are used to quality-adjusted survival in cost-effectiveness analyses, the utility weights for each health state experienced by a patient are multiplied by the duration in the respective health states. Given that the mean utility for the patients’ current health state was 0.769, disutilities in relation to current or full health for each side effect in this sample of patients can be calculated based on the difference between 0.769 and the utility for the health states. These disutilities can be subtracted from the utilities for health states that may be obtained or estimated for current states of target patients in future cost-effectiveness analyses. When more than one side effect occurs at the same time Fu and Kattan [14] recommend using a minimum model, which predicts a joint-state utility as equal to the lower of the two given single state utilities.
In the current study, it is interesting that despite significant progress in development of anti-emetics, nausea and vomiting remain a major concern for breast cancer patients. Grade III/IV nausea/vomiting was rated as the worst side effect by the highest percentage of patients and also had the lowest utility. This finding was consistent with a previous study in ovarian cancer patients [15]. Previous studies have shown alopecia to be rated as a significant side effect by patients [16–18]. This was also observed in this study, in which alopecia was generally less preferred than other grade I/II side effects but more preferred than grade III/IV side effects.
The utility weights obtained through the standard gamble exercise were consistent with the ratings of the least preferred side effects. Grade III/IV nausea, diarrhea, and hand–foot syndrome were rated as the worst side effects, and they also had among the lowest utilities. This finding is consistent with those observed in previous studies evaluating preferences from the perspective of patients with cancer, in which nausea, vomiting, diarrhea, and sensory neuropathy were among the least preferred side effects [11, 16, 17]. It should be noted that grade III/IV nausea and grade III/IV diarrhea were the only two side effects in this study that included a visit to the hospital or emergency room as part of the health state description.
The responses to the agree/disagree items show that maintaining quality of life appears to be increasingly important as disease stage progresses. Nevertheless, the agree/disagree data show that survival is as important for patients on palliative treatment (advanced stages) as for patients receiving adjuvant chemotherapy (earlier stages) regardless of quality of life.
This study has several limitations. Although we used a combination of CTC criteria, information from patient web forums, and patient feedback in the pilot test to informed the health state descriptions, the descriptions still may not represent the average experience for patients experiencing grade I/II or grade III/IV events. In addition, the standard gamble exercise was implemented in a web survey as opposed to face-to-face interviews, and this may have contributed to the high illogical or invariable responses obtained. There is clearly patient selection bias due to this methodology as patients had to be comfortable using a computer. The rate of illogical responses in this study of 32 %, however, largely is in the range of that observed in other internet utility surveys [13, 19]. Moreover, this survey took patients an average of 63 min to complete, which might have resulted in fatigue and thus being less careful about responses. Another potential limitation could be the idea of assigning a particular health state “for the rest of your life” as clearly in practice one could stop chemotherapy with resolution of many side effects. However, in standard gamble, the hypothetical state is assumed to last for the rest of life—this is done to obtain a utility value. However, when applied to clinical trial data, the utility is applied only during the duration of the actual health state. So the fact that these are acute health states are taken into account when the QALY is computed.
In conclusion, this study provides insight on chemotherapy side effects from the perspective of patients receiving chemotherapy for breast cancer. The findings help in better understanding patient preferences in oncology and may be used to enhance the medical decision-making process. Given the widespread use of chemotherapy in breast cancer patients, it is essential that we are fully aware of what the most significant side effects of treatment are from the patient’s perspective so that we can better set patient expectations with respect to health-related quality of life.
References
Jansen SJ, Stiggelbout AM, Wakker PP, Vliet Vlieland TP, Leer JW, Nooy MA, Kievit J (1998) Patients’ utilities for cancer treatments: a study of the chained procedure for the standard gamble and time tradeoff. Med Decis Mak 18(4):391–399
Chao C, Studts JL, Abell T, Hadley T, Roetzer L, Dineen S, Lorenz D, YoussefAgha A, McMasters KM (2003) Adjuvant chemotherapy for breast cancer: how presentation of recurrence risk influences decision-making. J Clin Oncol 21(23):4299–4305. doi:10.1200/jco.2003.06.025
Brazier J, Deverill M (1998) The use of health-related quality of life instruments in economic evaluation in health services research methods. A guide to best practice. BMJ Books, London
Torrance GW (1986) Measurement of health state utilities for economic appraisal. J Health Econ 5(1):1–30
Drummond MF, Iglesias CP, Cooper NJ (2008) Systematic reviews and economic evaluations conducted for the National Institute for Health and Clinical Excellence in the United Kingdom: a game of two halves? Int J Technol Assess Health Care 24(2):146–150. doi:10.1017/s0266462308080203
Cheng TF, Wang JD, Uen WC (2012) Cost-utility analysis of adjuvant goserelin (Zoladex) and adjuvant chemotherapy in premenopausal women with breast cancer. BMC Cancer 12:33. doi:10.1186/1471-2407-12-33
Melnikow J, Birch S, Slee C, McCarthy TJ, Helms LJ, Kuppermann M (2008) Tamoxifen for breast cancer risk reduction: impact of alternative approaches to quality-of-life adjustment on cost-effectiveness analysis. Med Care 46(9):946–953. doi:10.1097/MLR.0b013e318179250f
Mansel R, Locker G, Fallowfield L, Benedict A, Jones D (2007) Cost-effectiveness analysis of anastrozole vs tamoxifen in adjuvant therapy for early stage breast cancer in the United Kingdom: the 5-year completed treatment analysis of the ATAC (‘arimidex’, tamoxifen alone or in combination) trial. Br J Cancer 97(2):152–161. doi:10.1038/sj.bjc.6603804
Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J (2006) Health state utilities for metastatic breast cancer. Br J Cancer 95(6):683–690. doi:10.1038/sj.bjc.6603326
Brown RE, Hutton J, Burrell A (2001) Cost effectiveness of treatment options in advanced breast cancer in the UK. Pharmacoeconomics 19(11):1091–1102
Beusterien K, Grinspan J, Tencer T, Brufsky A, Visovsky C (2012) Patient preferences for chemotherapies used in breast cancer. Int J Women’s Health 4:279–287. doi:10.2147/ijwh.s31331
Furlong W, Feeney D, Torrance G (1990) Guide to design and development of health state utility instrumentation. Center for Health Economics Policy Analysis Working Paper Series. Hamilton (Ontario): McMaster University Paper No. 90-9
Beusterien K, Leigh N, Jackson C, Miller R, Mayo K, Revicki D (2005) Integrating preferences into health status assessment for amyotrophic lateral sclerosis: the ALS Utility Index. Amyotroph Lateral Scler Other Motor Neuron disord 6(3):169–176. doi:10.1080/14660820410021339
Fu AZ, Kattan MW (2008) Utilities should not be multiplied: evidence from the preference-based scores in the United States. Med Care 46(9):984–990. doi:10.1097/MLR.0b013e3181791a9c
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13(4):219–227. doi:10.1007/s00520-004-0710-6
Griffin AM, Butow PN, Coates AS, Childs AM, Ellis PM, Dunn SM, Tattersall MH (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7(2):189–195
Coates A, Abraham S, Kaye SB, Sowerbutts T, Frewin C, Fox RM, Tattersall MH (1983) On the receiving end–patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 19(2):203–208
Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, Sawyer WT (1999) Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 7(2):59–65
Chang WT, Collins ED, Kerrigan CL (2001) An internet-based utility assessment of breast hypertrophy. Plast Reconstr Surg 108(2):370–377
Acknowledgments
We would like to thank all the patients and their families for their help with this project. Funding for this study was received in the form of an unrestricted educational Grant from Eisai Pharmaceuticals.
Conflict of interest
Susan Dent—speaking honoraria (Eisai). There is no conflict of interest for the other authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuchuk, I., Bouganim, N., Beusterien, K. et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Res Treat 142, 101–107 (2013). https://doi.org/10.1007/s10549-013-2727-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2727-3






