Abstract
Various guidelines recommend that women with triple-negative breast cancer should be tested for BRCA1 mutations, but the prevalence of mutations may vary with ethnic group and with geographic region, and the optimal cutoff age for testing has not been established. We estimated the frequencies of BRCA1 and BRCA2 (BRCA) mutations among 190 women with triple-negative breast cancer, unselected for family history, diagnosed at age 50 or less at a single hospital in Mexico City. Patients were screened for 115 recurrent BRCA mutations, which have been reported previously in women of Hispanic origin, including a common large rearrangement Mexican founder mutation (BRCA1 ex9-12del). A BRCA mutation was detected in 44 of 190 patients with triple-negative breast cancer (23 %). Forty-three mutations were found in BRCA1 and one mutation was found in BRCA2. Seven different mutations accounted for 39 patients (89 % of the total mutations). The Mexican founder mutation (BRCA1 ex9-12del) was found 18 times and accounted for 41 % of all mutations detected. There is a high prevalence of BRCA1 mutations among young triple-negative breast cancer patients in Mexico. Women with triple-negative breast cancer in Mexico should be screened for mutations in BRCA1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since 2006, breast cancer has been the leading cause of death from cancer in Mexican women, accounting for 15 % of cancer-related deaths and it is the second cause of death among women aged 30–54 [1, 2]. In Mexico, the ratio of mortality to incidence is twice that of the United States (37 vs. 19 %) [3]. It is predicted that in 2015, there will be 23,764 women diagnosed with breast cancer and 6591 will die from it in Mexico [4]. The mean age at diagnosis of breast cancer in Mexico is 50 years [5–8]. Breast cancers in Mexico are also characterized by a high proportion of the triple-negative subtype, which accounts for 23 % of all breast cancers [7]. This contrasts to the proportion in other countries as Canada, where this subtype comprises about 11 % of unselected breast cancer patients [9].
BRCA testing is not broadly available in Mexico and genetic cancer risk assessment services are not commonly provided. Barriers to implementing genetic counseling and testing for Mexican women include the costs of genetic testing and the lack of public insurance coverage for genetic services. There is limited awareness among providers concerning the benefits of genetic risk assessment and few clinicians have genetic counseling expertise. In Mexico, genetic testing for BRCA mutations is available only in the private sector through laboratories situated outside of the country.
A number of studies have evaluated the prevalence of BRCA mutations in breast cancer patients in several Latin American countries, including Argentina, Brazil, Chile, Colombia, Costa Rica, Mexico, Uruguay, and Venezuela [10–21]. The presence of founder mutations within a defined ethnic group enables rapid and low-cost screening, compared to the cost of the complete sequencing of both BRCA genes [22], but screening only for founder mutations is contingent on a high proportion of all mutations being recurrent. Founder mutations in BRCA1 or BRCA2 have been identified in several Latin American sub-groups, including women from Colombia [13] as well as in Mexican women in the USA [23–25] and in Mexico [18, 19].
The HISPANEL was developed as a genetic screening tool which incorporates 115 recurrent BRCA mutations observed in Hispanic women [18, 23–25]. It is estimated that, among Mexican women with breast or ovarian cancer, the sensitivity of the HISPANEL test is 68 %, compared to full-gene screening [18]. This test can be completed in 72 h at a cost of USD $20 per sample. Recently, we reported a BRCA1 and BRCA2 mutation in 27 % of 33 Mexican women with triple-negative breast cancers [18]. Because of the high prevalence of mutations seen in this small series, we sought to confirm the mutation frequency in a large series of unselected young Mexican women with triple-negative breast cancer.
Materials and methods
Patient population
We conducted a study of young Mexican women with triple-negative breast cancer treated at the National Cancer Institute in Mexico City. Patients diagnosed between January 2006 and January 2012 with triple-negative breast cancer at age 50 years or younger were identified through the hospital database. From a total of 484 patients, 362 were alive, 18 were deceased, and for 104 patients the vital status was unknown. We attempted to contact the living patients. Of these, we were able to contact 204, and the others were not available for follow-up. We excluded nine of them: five were older than 50 years of age at the time of diagnosis and four were not triple-negative breast cancer when the pathology report was re-reviewed. Patients were approached by the research coordinator to participate in the study during an out-patient visit to the hospital or at a scheduled study visit. The research coordinator described the study to the patient and informed her of the implications of genetic testing. After providing written informed consent, the patient was interviewed by the research coordinator for details about her medical and lifestyle history and her family history of cancer. The institutional review boards of the participating centers approved the protocol. A saliva sample was obtained for DNA extraction.
Laboratory methods
DNA was extracted from saliva samples using Oragene DNA extraction kits in the laboratory of Dr. Narod at Women’s College Hospital in Toronto. An adequate amount of DNA was obtained for 190 of the 195 patients (97 %). Mutation analysis took place in the laboratory of Dr. Weitzel at City of Hope in Duarte, California, and in the laboratory of Dr. Narod at Women’s College Hospital in Toronto. All DNA samples were screened by the HISPANEL assay, a 115 BRCA mutation panel developed from data from U.S. Hispanics [23, 25] other published data on BRCA mutations among Spanish, Hispanic, or South American populations, [26–33] and entries citing Hispanic ancestry in the Breast Cancer Information Core (http://research.nhgri.nih.gov/projects/bic/Member/index.shtml). The HISPANEL uses five multiplex reactions on the Sequenom® (San Diego, CA 92121) MassARRAY platform (MALDI-TOF MS) to detect insertions/deletions and point mutations, and a PCR assay for the BRCA1 ex9-12del mutation. All deleterious mutations were confirmed by Sanger sequencing on an ABI capillary sequencer. Re-sequencing of the original stored sample was subsequently performed before reports were issued to the clinician(s) responsible for a given participant’s care after counseling, as per the IRB-approved protocol.
Results
The median age of the patients at diagnosis was 43 years (range 23–50) and the mean age at interview was 50 years (range 23–65). All patients were diagnosed before the age of 50. Of the 190 patients, five breast cancers were screen-detected by mammography and the others were found by palpation. The median cancer size was 4 cm and 93 % of patients were stage II or above.
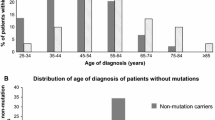
A mutation was found in 44 of 190 patients (23.2 %); 43 had a mutation in BRCA1 and one had a mutation in BRCA2 (Table 1). The prevalence of mutations was 30.3 % among women diagnosed at age 40 and under and was 18.3 % for women diagnosed from age 41 to 50 years. The average age of diagnosis in women with a BRCA1 mutation was 38 years, compared to 41 years for the non-carriers (p = 0.07). Twenty of the 44 women (45 %) with a mutation had a first-or second-degree relative affected with breast cancer and two reported a family history of ovarian cancer (4.5 %). Seven mutations were found in two or more families; together these seven recurrent mutations accounted for 39 of 44 mutations detected (89 %) (Table 1).
Discussion
We estimate the prevalence of BRCA mutations in young triple-negative breast cancer patients from Mexico to be 23 %. The great majority of the detected mutations (97.7 %) were in BRCA1 (n = 43), only a single BRCA2 mutation was found.
We restricted testing to women diagnosed under the age of 50, who comprise approximately 50 % of all breast cancers cases in the region [5–8]. At the time, the study was initiated, NCCN guidelines recommended testing for triple-negative breast cancer, be restricted to patients under the age of 51 years. However, given the high prevalence of mutations that we observed in the age group between 41 and 50 years (18.3 %), it is reasonable to consider testing for BRCA mutations in older women in Mexico. This is in concordance with changes to the NCCN guidelines, which have revised the recommendation for genetic testing for triple-negative breast cancer to patients under the age of 61 [34].
The 44 mutations reported in this study represent 11 different mutations. Of these, seven were seen more than once. The BRCA1 ex9-12del large rearrangement was seen in 18 cases and accounted for 42 % of all mutations in the study. This is the most common reported founder mutation in Mexico [18, 24, 25], but has not been seen in other Latin American countries [11].
The BRCA1 943ins10 mutation was detected in five cases. This is the most commonly reported African founder mutation and is a Bahamian founder mutation [35–37]. It has been reported 34 times in the BIC database in individuals of African or Latin American descent [23, 38]. The BRCA1 R71G mutation was seen in four cases. This is a common founder Spanish mutation [39] and has been recorded 36 times in the BIC database. The BRCA1 2925del4 mutation was also seen four times.
The BRCA1 185delAG mutation was seen in three cases, none of whom identified themselves as being of Jewish ancestry. This mutation is a common founder mutation in the Jewish population and recurrent among non-Jews, including Chilean, Peruvian, and Bahamian women [37, 40, 41]. The BRCA1 185delAG mutation is also common among high-risk breast cancer patients of Mexican descent in the United States (10 % of mutations) [23, 25]. The BRCA1 R1443X mutation, seen four times, has been reported 131 times in the BIC database, mainly in women of Western European origin [38].
The BRCA1 3878delTA mutation, reported twice, has been identified in two Latin American women in the BIC database. The other mutations were seen once each. The Q1200X mutation is very common among Western Europeans. The A1708E mutation is a Spanish and Colombian founder mutation [27, 42].
Our study has several limitations. BRCA1 and BRCA2 mutations were limited to those that have been included in HISPANEL and we did not perform full sequencing. We estimate that HISPANEL will identify at least 68 % of all BRCA1 and BRCA2 mutations in Mexican women with breast cancer [18]. Screening with the HISPANEL for Mexican women is justifiable given the low cost. If full sequencing is completed, the prevalence of BRCA mutations might be even higher than the observed 23 %.
In some cases where there is an exceptional family history and a negative HISPANEL test, full sequencing might be offered. In the present series, 20 women had a family history of breast or ovarian and no BRCA1or BRCA2 mutation; these women might qualify for full sequencing of BRCA1 and BRCA2. In a recent study of Mexican breast cancer patients living in the United States, a strong association was observed between the triple-negative subtype and a family history of breast or ovarian cancer [43]. The genetic basis for this observation is currently under investigation.
Our patients represent those women who were treated at a single hospital in Mexico City and the prevalence and distribution of mutations among cancer patients elsewhere in Mexico might be different. We studied prevalent cases, on average 6 years had passed from diagnosis to genetic testing. Many women in the registry database had died prior to the initiation of the study. Therefore, if the presence of a BRCA1 mutation is associated with a relatively poor survival, our estimate might be subject to survivorship bias and the true prevalence might be even higher.
We identified a BRCA mutation in 23 % of young women with triple-negative breast cancer in Mexico. Previous studies of other populations report prevalences of between 11 and 20 % [44–46]. In the largest study of unselected triple-negative breast cancer patients (n = 1824), 11.2 % had a mutation in BRCA1 or BRCA2 [47]. In patients younger than 50 years of age, the prevalence was 16.6 %. For the group between 50 and 59 years, the prevalence of mutations was 9.6 %.
Few studies had assessed the prevalence of BRCA mutations among Mexican cancer patients, and all included a limited number of patients [15–17]. One recent study of Mexican breast cancer patients unselected for family history or receptor status revealed a prevalence of BRCA mutations that accounted for 4.3 % [19]. In a second Mexican study, BRCA mutations were identified in 15 % (14/96) of breast cancer cases overall and 27 % (9/33) of triple-negative breast cancers [18].
Given the very high prevalence of a small number of founder mutations among unselected Mexican women with triple-negative breast cancers, it is rational to consider testing for BRCA1 mutations to all triple-negative breast cancer patients under age 60. However, to do so, it will require public coverage for preventive services, including genetic testing, as well as for screening for patients and their family members.
The results of this study highlight the potential benefit for BRCA testing of young triple-negative breast cancer patients in Mexico at the time of diagnosis. Ideally, the result of the genetic test will be available to the patient and her doctor prior to the initiation of her treatment. There is increasing evidence that mutation carriers will benefit from a personal approach to treatment which many include tailored chemotherapy, tamoxifen, oophorectomy, and bilateral mastectomy. Byrksi et al. reported that of 107 BRCA1 carriers treated with neoadjuvant cisplatin, 63 % experienced a pathologic complete response (pCR), and all of the women who experienced a pCR remain alive an average of 4 years after the initiation of treatment [48]. Huzarski et al. reported a survival benefit among BRCA1 carriers with breast cancer after oophorectomy [49]. Metcalfe et al. reported a decline in 20-year breast cancer mortality associated with a bilateral mastectomy, compared to unilateral surgery [50]. An additional benefit of testing young women with triple-negative breast cancer is that testing can then be offered to unaffected relatives of mutation carriers.
References
Data. ISabciM-Ot-N. [cited November 14, 2014]. Available from: http://www.inegi.org.mx/inegi/contenidos/espanol/prensa/Contenidos/estadisticas/2014/mama0.pdf
Knaul FM, Nigenda G, Lozano R, Arreola-Ornelas H, Langer A, Frenk J (2008) Breast cancer in Mexico: a pressing priority. Reprod Health Matters 16:113–123
Chavarri-Guerra Y, Villarreal-Garza C, Liedke PE et al (2012) Breast cancer in Mexico: a growing challenge to health and the health system. Lancet Oncol 13:e335–e343
Weissman BE, Saxon PJ, Pasquale SR, Jones GR, Geiser AG, Stanbridge EJ (1987) Introduction of a normal human chromosome 11 into a Wilms’ tumor cell line controls its tumorigenic expression. Science 236:175–180
Rodriguez-Cuevas S, Guisa-Hohenstein F, Labastida-Almendaro S (2009) First breast cancer mammography screening program in Mexico: initial results 2005-2006. Breast J 15:623–631
Mohar A, Bargallo E, Ramirez MT, Lara F, Beltran-Ortega A (2009) Available resources for the treatment of breast cancer in Mexico. Salud Publica Mex 51(Suppl 2):s263–s269
Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D et al (2011) Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer 117:3658–3669
Villarreal-Garza C, Aguila C, Magallanes-Hoyos MC et al (2013) Breast cancer in young women in Latin America: an unmet, growing burden. Oncologist 18(Suppl):26–34
Trudeau M, Pritchard KI et al (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13:4429–4434
Solano AR, Aceto GM, Delettieres D et al (2012) BRCA1 And BRCA2 analysis of Argentinean breast/ovarian cancer patients selected for age and family history highlights a role for novel mutations of putative south-American origin. Springerplus 1:20
Gomes MC, Costa MM, Borojevic R et al (2007) Prevalence of BRCA1 and BRCA2 mutations in breast cancer patients from Brazil. Breast Cancer Res Treat 103:349–353
Gonzalez-Hormazabal P, Gutierrez-Enriquez S et al (2011) Spectrum of BRCA1/2 point mutations and genomic rearrangements in high-risk breast/ovarian cancer Chilean families. Breast Cancer Res Treat 126:705–716
Hernandez JE, Llacuachaqui M, Palacio GV et al (2014) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from medellin, Colombia. Hered Cancer Clin Pract 12:11
Gutierrez Espeleta GA, Llacuachaqui M, Garcia-Jimenez L et al (2012) BRCA1 and BRCA2 mutations among familial breast cancer patients from Costa Rica. Clin Genet 82(5):484–488
Vidal-Millan S, Taja-Chayeb L, Gutierrez-Hernandez O et al (2009) Mutational analysis of BRCA1 and BRCA2 genes in Mexican breast cancer patients. Eur J Gynaecol Oncol 30:527–530
Calderón-Garcidueñas AL, Ruiz-Flores P, Cerda-Flores RM, Barrera-Saldaña HA (2005) Clinical follow up of Mexican women with early onset of breast cancer and mutations in the BRCA1 and BRCA2 genes. Salud Publica Mex 47(2):110–115
Vaca-Paniagua F, Alvarez-Gomez RM, Fragoso-Ontiveros V et al (2012) Full-Exon pyrosequencing screening of BRCA germline mutations in Mexican women with inherited breast and ovarian cancer. PLoS ONE 7:e37432
Villarreal-Garza C, Alvarez-Gomez RM, Perez-Plasencia C et al (2014) Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico, September 18. Cancer. doi:10.1002/cncr.29058
Torres-Mejia G, Royer R, Llacuachaqui M, et al (2014) Recurrent BRCA1 and BRCA2 mutations in Mexican women with breast cancer. Cancer Epidemiol Biomark Prev
Delgado L, Fernandez G, Grotiuz G et al (2011) BRCA1 and BRCA2 germline mutations in Uruguayan breast and breast-ovarian cancer families. Identification of novel mutations and unclassified variants. Breast Cancer Res Treat 128(1):211–218
Lara K, Consigliere N, Perez J, Porco A (2012) BRCA1 and BRCA2 mutations in breast cancer patients from Venezuela. Biol Res 45:117–130
Narod SA (2009) Screening for BRCA1 and BRCA2 mutations in breast cancer patients from Mexico: the public health perspective. Salud Publica Mex 51(Suppl 2):s191–s196
Weitzel JN, Lagos V, Blazer KR et al (2005) Prevalence of BRCA mutations and founder effect in high-risk Hispanic families. Cancer Epidemiol Biomark Prev 14:1666–1671
Weitzel JN, Lagos VI, Herzog JS et al (2007) Evidence for common ancestral origin of a recurring BRCA1 genomic rearrangement identified in high-risk Hispanic families. Cancer Epidemiol Biomark Prev 16:1615–1620
Weitzel JN, Clague J, Martir-Negron A et al (2013) Prevalence and type of BRCA mutations in Hispanics undergoing genetic cancer risk assessment in the southwestern United States: a report from the Clinical Cancer Genetics Community Research Network. J Clin Oncol 31:210–216
Gallardo M, Silva A, Rubio L et al (2006) Incidence of BRCA1 and BRCA2 mutations in 54 Chilean families with breast/ovarian cancer, genotype-phenotype correlations. Breast Cancer Res Treat 95:81–87
Torres D, Rashid MU, Gil F et al (2007) High proportion of BRCA1/2 founder mutations in Hispanic breast/ovarian cancer families from Colombia. Breast Cancer Res Treat 103:225–232
Vogel KJ, Atchley DP, Erlichman J et al (2007) BRCA1 and BRCA2 genetic testing in Hispanic patients: mutation prevalence and evaluation of the BRCAPRO risk assessment model. J Clin Oncol 25:4635–4641
Jara L, Ampuero S, Santibanez E et al (2004) Molecular analysis of the eighteen most frequent mutations in the BRCA1 gene in 63 Chilean breast cancer families. Biol Res 37:469–481
Diez O, Osorio A, Duran M et al (2003) Analysis of BRCA1 and BRCA2 genes in Spanish breast/ovarian cancer patients: a high proportion of mutations unique to Spain and evidence of founder effects. Hum Mutat 22:301–312
de la Hoya M, Gutierrez-Enriquez S, Velasco E et al (2006) Genomic rearrangements at the BRCA1 locus in Spanish families with breast/ovarian cancer. Clin Chem 52:1480–1485
Blesa JR, Garcia JA, Ochoa E (2000) Frequency of germ-line BRCA1 mutations among Spanish families from a Mediterranean area. Hum Mutat 15:381–382
Campos B, Diez O, Domenech M et al (2001) BRCA2 mutation analysis of 87 Spanish breast/ovarian cancer families. Ann Oncol 12:1699–1703
Green ED, Olson MV (1990) Chromosomal region of the cystic fibrosis gene in yeast artificial chromosomes: a model for human genome mapping. Science 250:94–98
Hall MJ, Reid JE, Burbridge LA, Pruss D, Deffenbaugh AM et al (2009) BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast -ovarian cancer. Cancer 115(10):2222–2233
Olopade OI, Fackenthal JD, Dunston G, Tainsky MA, Collins F, Whitfield-Broome C (2003) Breast cancer genetics in African Americans. Cancer 97(1 Suppl):236–245
Donenberg T, Lunn J, Curling D et al (2011) A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat 125:591–596
Breast Cancer Information Core (BIC). An open access online breast cancer mutation data base. 2010
Vega A, Campos B, Bressac-De-Paillerets B et al (2001) The R71G BRCA1 is a founder Spanish mutation and leads to aberrant splicing of the transcript. Hum Mutat 17:520–521
Jara L, Ampuero S, Santibanez E et al (2006) BRCA1 and BRCA2 mutations in a South American population. Cancer Genet Cytogenet 166:36–45
Abugattas J, Llacuachaqui M, Allende YS et al (2014) Prevalence of BRCA1 and BRCA2 mutations in unselected breast cancer patients from Peru. Clin Genet. doi:10.1111/cge.12505
Torres D, Umaña A, Robledo JF et al (2009) Estudio de factores genéticos para cáncer de mama en Colombia, Spanish. Univ Med Bogotá (Colombia) 50:297–301
Martinez ME ea. Family history of breast and ovarian cancer prevalence and its association with triple negative subtype in hispanic women. American Association for Cancer Research Science of Cancer Health Disparities Meeting; San Antonio, TX November 9–12, 2014
Young SR, Pilarski RT, Donenberg T et al (2009) The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer 9:86
Fostira F, Tsitlaidou M, Gogas H, et al (2010) Prevalence of BRCA1 mutations among 284 women with triple-negative breast cancer. ASCO Meeting Abstracts 28(15_suppl %U http://meeting.ascopubs.org/cgi/content/abstract/28/15_suppl/1511):1511
Gonzalez-Angulo AM, Timms KM et al (2011) Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer, February 22. Clin Cancer Res 17(5):1082–1089
Couch FJ, Hart SN, Sharma P, et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol In press
Byrski T, Huzarski T, Dent R et al (2014) Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat 147:401–405
Huzarski T, Byrski T, Gronwald J et al (2013) Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol 31:3191–3196
Metcalfe K, Gershman S, Ghadirian P et al (2014) Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ 348:g226
Acknowledgments
The project was supported in part by the Breast Cancer Research Foundation, Avon Foundation grant #02-2013-044, American Cancer Society grant #RSGT-00-263-01, and Award Number RC4A153828 from the National Cancer Institute (J. Weitzel).
Author information
Authors and Affiliations
Corresponding author
Additional information
Villarreal-Garza C and Weitzel JN have authors contributed equally to this study.
Rights and permissions
About this article
Cite this article
Villarreal-Garza, C., Weitzel, J.N., Llacuachaqui, M. et al. The prevalence of BRCA1 and BRCA2 mutations among young Mexican women with triple-negative breast cancer. Breast Cancer Res Treat 150, 389–394 (2015). https://doi.org/10.1007/s10549-015-3312-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3312-8