Abstract
Cyclin D1 (CCND1), a key regulator of cell cycle progression, is overexpressed in many human cancers, including breast cancer. However, the impact of CCND1 overexpression in these cancers remains unclear and controversial. We conducted a systematic literature search in PubMed and EMBASE with the search terms “cyclin D1”, “CCND1”, “breast cancer”, “prognosis”, and potential studies for analysis were selected. Studies with survival data, including progression-free survival (PFS), overall survival (OS) or metastasis-free survival (MFS), were included in this meta-analysis. A total of 33 studies containing 8,537 cases were included. The combined hazard risk (HR) and its 95 % confidence interval (CI) of OS, PFS and MFS were 1.13 (95 % CI 0.87–1.47; P = 0.35), 1.25 (95 % CI 0.95–1.64; P = 0.12), and 1.04 (95 % CI 0.80–1.36; P = 0.76), respectively, for primary breast cancer patients with tumors exhibiting CCND1 overexpression. Interestingly, the impact of CCND1 expression on OS was a 1.67-fold (95 % CI 1.38–2.02; P = 0.00) increased risk for ER-positive breast cancer patients. However, CCND1 overexpression exhibited no association with the PFS or OS of patients who received epirubicin-based neoadjuvant chemotherapy, for which the P values were 0.63 and 0.47, respectively. In summary, CCND1 overexpression impacts the prognosis of ER-positive breast cancer patients, but not patients with unselected primary breast cancer or patients treated with neoadjuvant chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer was the most frequent cancer among women in 2008 (23 % of all cancers), and ranks second overall (10.9 % of all cancers). Despite the development of combined therapeutic modalities and the prolonged overall survival (OS) and progression-free survival (PFS) of breast cancer patients, breast cancer remains the fifth leading cause of overall cancer deaths and the most frequent cause of cancer deaths in women [1]. Thus, identifying specific biomarkers that could serve as prognostic factors for breast cancer patients is crucial for individualized treatments. To date, several biomarkers have been demonstrated to impact the survival of breast cancer patients, including P27 [2], VEGF [3], COX-2 [4], and BCL-2 [5].
Cyclin D1 (CCND1) is located on chromosome 11q13 [6], is a key regulator of cell cycle progression, and functions as an oncogene in many human cancers, including breast cancer. As a G1 cyclin, it is a major positive regulator of the G1 restriction point [7]. Moreover, it contributes to the action of estrogen receptor (ER) in breast cancer patients. As a cellular sensor for the presence of ER, it has been demonstrated to contribute significantly to ER activation in breast cancers [8].
CCND1 overexpression in breast cancer has been reported in various studies [9–30]. However, whether CCND1 represents a prognostic biomarker remains controversial. In this study, we conducted a systematic review and meta-analysis to estimate the effect of CCND1 overexpression on the survival of breast cancer patients.
Materials and methods
Literature research and selection
We identified studies via a literature search using the PubMed and EMBASE databases with “cyclin D1,” “CCND1,” “breast cancer,” and “prognosis” as the search terms for publications published from January 1, 1966, through March 1, 2012. The titles and abstracts of the studies were first scanned to exclude all irrelevant papers. Then, we established the inclusion of the final studies by reading the full text of the remaining articles. Additional articles were identified through the references cited within the first series of selected articles. If more than one study reported the same cases, only the study with the most complete data was included.
The inclusion criteria for the articles examined in this study were as follows: (1) the study should be published in English; (2) total cases should be more than 40; (3) the study should be limited to research on human primary breast cancer; (4) the patients should be female; (5) the study should provide survival information, such as PFS, OS, and metastasis-free survival (MFS); and (6) the minimal follow-up time should be greater than 5 years.
Data extraction and quality assessment
Information was carefully extracted from all of the eligible studies by two independent investigators, according to the inclusion criteria detailed above. OS, MFS, and PFS were selected as the clinical outcomes for prognosis. The following information was collected: the name of the first author, year of publication, source of patients, study design, sample size, histology, stage, CCND1 overexpression (%), the hazard risk (HR) and its 95 % confidence interval (CI) of OS, HR (95 % CI) of PFS, and HR (95 % CI) of MFS.
The studies were assessed for quality using REMARK (Reporting recommendations for tumor MARKer prognostic studies) [31], and the definitions of the 18 items for reporting study quality provided by Chen et. al [32].
Statistical methods
The methods reported by Parmar et al. [33] for calculating the HR and its 95 % CI for survival data were consistent with those of our previous study [34]. HR describes the relative risk of complications based on a comparison of event rates. Moreover, it allows for including both censoring and time to event to represent the overall reduction in the risk of death compared to the control during the follow-up period. The HR calculations spreadsheet provided by Tierney et al. [35] was used to obtain the HR and its 95 % CI. Some of the following data were collected to summarize the HR from published summary statistics or the data extracted from Kaplan–Meier curves: observed events, expected events, total events, HR rate, variance, the patients in each arm, follow-up details, Kaplan–Meier curves and P value of log rank. Mantel–Haenszel or Cox analyses using Enguage Digitizer 4.1 were used to extract the data from Kaplan–Meier curves. In certain studies, the prognostic value of different variables for clinical outcomes was estimated using both multivariate and univariate analyses. In this case, the results of the multivariate analyses were used to calculate the HR.
Based on Peto’s method [36], χ 2 tests were used to assess heterogeneity. I 2 statistics were performed to assess heterogeneity, where I 2 < 50 % was considered acceptable. If significant heterogeneity was observed, subgroup analysis was conducted to determine the cause of the heterogeneity. If significant heterogeneity persisted, a random-effects model was used for meta-analysis. Otherwise, the fixed-effects model was applied. Sensitivity analysis was performed to confirm the validity of our meta-analysis. Finally, both Begg’s funnel plots and Egger’s tests were performed to assess the publication bias of the literature. All of the tests were two sided, and P values <0.05 were regarded as statistically significant. The results were analyzed and confirmed by two individuals.
Results
Literature search
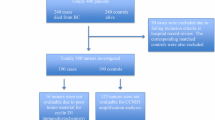
A total of 307 articles were identified. After screening the titles and abstracts, 226 articles were excluded because they were laboratory studies, review articles, case reports, male breast cancer, published in other languages, other tumors, total cases less than 40, or were irrelevant to this study. Eighty articles remained for full text review. Finally, 33 articles were included in this study after the exclusion of studies that lacked prognostic data or that reported repeated cases [37–40] (Fig. 1).
Study characteristics
The characteristics of the 33 selected studies [9–30, 39, 41–50] containing 8,537 cases are shown in Table 1. The studies were published from 1995 to 2013. Among them, 30 studies discussed the impacts of CCND1 overexpression on primary breast cancer patients who did not have any other pre-operative treatment, including radiotherapy, target therapy, endocrine therapy, and chemotherapy. Three studies investigated primary breast cancer patients who received epirubicin-based neoadjuvant chemotherapy. Five studies investigated ER-positive breast cancer patients. In these studies, most patients were hospitalized and diagnosed in the affiliated hospital of a medical college. Choschzick et al. [11] and Rudas et al. [25] only studied ER-positive breast cancer patients, whereas Umekita et al. [28], Elsheikh et al. [42], and Kenny et al. [15] included the survival data of all patients, including ER-positive patients. Several methods were used to assess CCND1 expression, including immunohistochemistry (IHC), polymerase chain reaction (PCR), tissue microarrays (TMAs), and northern blots (NBs). However, IHC was the most commonly used method. CCND1 gene amplification [39, 41–43], CCND1 mRNA overexpression [12, 13, 15, 24, 28, 29], and/or CCND1 protein overexpression were detected in these studies. Most of the studies detected CCND1 overexpression in tissue samples, with the exception of one study, which detected CCND1 in plasma [12]. The IHC evaluation of CCND1 produced variable positivity ranging from 12.9 to 70.1 % for breast cancers, possibly because of the diversity of antibodies and evaluation criteria used. In addition, Rudas et al. [25] reported the results of two randomized controlled trials, which we treated as two studies in the meta-analysis. The median follow-up time ranged from 48 to 132 months, whereas the minimum follow-up time ranged from 2 to 24 months, and the maximum follow-up time ranged from 97 to 176 months.
Main analysis
CCND1 overexpression and prognosis
Twenty-two studies investigated OS in a total of 4,009 unselected cases. Due to significant heterogeneity among the studies (P = 0. 22; I 2− = 63.5 %), a random-effects model was used. However, no statistically significant risk (HR 1.13, 95 % CI 0.87–1.47; P = 0.35) was observed for CCND1 overexpression in breast cancer (Fig 2a). Furthermore, subgroup analysis was performed according to the methods used to detect CCND1 overexpression, analysis methods for survival data, histology, region, and study design. However, none of the results exhibited significant differences.
Eleven studies including 3,685 cases were evaluated for the effect of CCND1 overexpression on PFS (Fig 2b). A random-effects model was used to combine HRs because of the heterogeneity observed among the studies (χ 2 = 31.78, P = 0.00, I 2 = 68.5 %). The pooled HR was 1.25 (95 % CI 0.95–1.64; P = 0.12).
The results from four studies (n = 1,941) on the relationship between CCND1 expression and MFS were also negative (Fig 2c). Because low homogeneity (χ 2 = 147.75, P = 0.00, I 2 = 87.1 %) was detected, a random-effects model was used to calculate the HR (1.04, 95 % CI 0.80–1.36; P = 0.76).
CCND1 overexpression and ER-positive patients
A total of five studies including 2,580 cases were evaluated for the impact of CCND1 expression on the OS of ER-positive breast cancer patients. A fixed-effects model was used to combine the HR values. The pooled HR value was 1.67 (95 % CI 1.38–2.02; P = 0.00), with evidence of heterogeneity (χ 2 = 3.73, P = 0.59, I 2 = 0.0 %), suggesting that CCND1 overexpression was associated with increased risk in ER-positive breast cancer patients (Fig 3).
CCND1 overexpression and neoadjuvant chemotherapy
In three studies containing 477 cases, the survival data (PFS and OS) were assessed for the impact of CCND1 overexpression on the prognosis of breast cancer patients who received epirubicin-based neoadjuvant chemotherapy.
A statistically significant risk of CCND1 overexpression in breast cancer was detected with an HR of 1.14 (95 %: 0.68–1.91; 0.63) and significant heterogeneity (χ 2 = 10.79, P = 0.01, I 2 = 81.5 %), indicating that CCND1 overexpression had no impact on the PFS of patients who received epirubicin-based neoadjuvant chemotherapy. Similarly, there was no association between CCND1 overexpression and the OS of patients who received epirubicin-based neoadjuvant chemotherapy (HR 1.15, 95 % CI 0.79–1.66; P = 0.47). All of the results of subgroup survival analysis are shown in Table 2.
Publication bias
No publication bias was detected in our meta-analysis using Begg’s and Egger’s tests. Begg’s funnel plots did not reveal any evidence of obvious asymmetry in this study. Fig 4a shows Begg’s funnel plot of CCND1 overexpression for publication bias in terms of the OS of primary breast cancer patients. Furthermore, the P value of the Egger’s test (P = 0.76) suggested no evidence of publication bias. The Begg’s funnel plot of CCND1 overexpression for the publication bias of ER-positive breast cancer patients is shown in Fig 4b. Egger’s test (P = 0.80) confirmed the results. Moreover, no publication bias was detected in the other sub-group meta-analyses.
Discussion
Cyclin D1, which regulates the G1/S transition, has been shown to accumulate at high levels in late G1 phase of the cell cycle. CCND1 overexpression has previously been reported to be associated with poor prognosis and tumor progression in several different tumor types, including breast cancer [39, 51, 52], because it can promote cell proliferation and differentiation by shortening the G1/S transition. However, no correlation was detected with the prognosis of unselected primary breast cancers in this meta-analysis. Furthermore, eight additional studies [39, 53–60] that were not included in this meta-analysis reported a negative correlation between CCND1 overexpression and the prognosis of breast cancer, although the detailed survival data of these studies are unavailable. Nevertheless, the results of these studies support our finding that CCND1 overexpression has no impact on the prognosis of unselected primary breast cancers.
CCND1 gene amplification and CCND1 protein overexpression frequently occur in breast cancer, although protein overexpression is not always attributed to genetic amplification [39, 42, 43]. Nevertheless, Peurala et al. [24] showed that increased CCND1 protein levels were significantly correlated with increased mRNA expression. Moreover, the protein expression of CCND1 is believed to be more directly affected by CCND1 mRNA overexpression than CCND1 gene amplification [13]. These observations indicate that mechanisms other than genetic amplification are responsible for the altered CCND1 expression, such as ER status.
According to gene expression profiling by DNA microarray, breast cancer is divided into five main molecular classes [61–63]. These classes include basal-like breast cancers (ER-negative, progesterone receptor (PR)-negative, and HER2-negative tumors), HER2-positive cancers, normal breast-like, luminal-A cancers, which are mostly ER-positive, and histologically low-grade luminal-B cancers, which are also mostly ER positive, but might express low levels of hormone receptors, and are often high grade. In retrospective studies, the different genetic subtypes of breast cancer have exhibited different PFS and OS [64, 65]. The strong connection between CCND1 and ER status [14, 42, 44] implies that CCND1 might contribute to the prognosis of ER-positive patients.
In this meta-analysis, CCND1 overexpression was detected to serve as an independent predictor of poor prognosis in ER-positive breast cancer. CCND1 exhibited a strong correlation with ER-positive status in previous studies [14, 42, 44], confirming the important role of CCND1 in ER-positive breast cancer. Furthermore, CCND1 is induced by estrogen and growth factors, and it acts as a cellular sensor for their presence [66]. Thus, it might increase the competitive effect of tamoxifen, which has been proven to be an effective treatment in hormone receptor-positive breast cancer patients and ER-positive breast cancers [67].
Because the neoadjuvant chemotherapy regimens were not entirely consistent, the relationship between increased CCND1 expression and breast cancer patients with neoadjuvant chemotherapy needs to be validated in further studies.
Quality assessment according to REMARK guidelines was conducted for all 33 of the studies included in this meta-analysis. The studies included in this meta-analysis fulfilled, on average, 14 items (range from 10 to 18 items) of the guidelines. Sensitivity and sub-group analyses were performed to ensure that the results were reliable and valid. The rates of breast cancer incidence are reportedly higher in developed regions of the world (except Japan) than in most developing regions, such as eastern Africa [1]. However, there was no association between CCND1 overexpression and the prognosis of breast cancer in different regions. Invasive breast cancer is the most common pathological subtype of breast cancer. However, CCND1 overexpression was not an independent risk factor for invasive breast cancer. Moreover, we performed subgroup analysis according to the univariate or multivariate analyses used to evaluate the prognosis of breast cancer; the results exhibited no significant difference in either case. In summary, the results of the sensitivity and subgroup analysis revealed that no significant changes occurred in the results when poor-quality studies were excluded or in subgroup analyses.
As a meta-analysis, there are some limitations that should be discussed for further consideration. First, a meta-analysis based on individual patient data is the gold standard method. However, to our knowledge, it is rare for a meta-analysis to be based on individual patient data. Obtaining individual patient data for our studies was almost impossible. Therefore, we conducted a meta-analysis of the published literature. Second, a random-effects model was predominantly used for our analyses, except in the cases of ER-positive patients and MFS, due to their significant heterogeneity. Because the random-effects model reduced the effect of large samples with better quality, it was not as stable as the fixed-effects model. Third, certain reports with negative or controversial results might not be reported, and therefore, publication bias is inevitable. Fourth, we conducted a literature search in the PubMed and EMBASE databases, and because we included articles that were published in English only, selection and language bias might exist. Last, the cutoff of methods used to assess ER overexpression was variable between studies, which might contribute in part to the observed heterogeneity.
Conclusion
In summary, CCND1 overexpression can serve as an independent prognostic indicator for poor prognosis in ER-positive breast cancer, but cannot distinguish patients with poor OS from groups with favorable prognosis (PFS, OS and MFS) in unselected primary breast cancers, and breast cancer patients with neoadjuvant chemotherapy.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 10 (Internet). Lyon, France: International Agency for Research on Cancer, 2010
Guan X, Wang Y, Xie R, Chen L, Bai J, Lu J, Kuo MT (2010) p27(Kip1) as a prognostic factor in breast cancer: a systematic review and meta-analysis. J Cell Mol Med 14(4):944–953. doi:10.1111/j.1582-4934.2009.00730.x
Wang J, Guo Y, Wang B, Bi J, Li K, Liang X, Chu H, Jiang H (2012) Lymphatic microvessel density and vascular endothelial growth factor-C and -D as prognostic factors in breast cancer: a systematic review and meta-analysis of the literature. Mol Biol Rep 39(12):11153–11165. doi:10.1007/s11033-012-2024-y
Kim HS, Moon HG, Han W, Yom CK, Kim WH, Kim JH, Noh DY (2012) COX2 overexpression is a prognostic marker for Stage III breast cancer. Breast Cancer Res Treat 132(1):51–59. doi:10.1007/s10549-011-1521-3
Callagy GM, Webber MJ, Pharoah PD, Caldas C (2008) Meta-analysis confirms BCL2 is an independent prognostic marker in breast cancer. BMC Cancer 8:153. doi:10.1186/1471-2407-8-153
Motokura T, Arnold A (1993) Cyclin D and oncogenesis. Curr Opin Genet Dev 3(1):5–10
Strauss M, Lukas J, Bartek J (1995) Unrestricted cell cycling and cancer. Nat Med 1(12):1245–1246
Zwijsen RM, Buckle RS, Hijmans EM, Loomans CJ, Bernards R (1998) Ligand-independent recruitment of steroid receptor coactivators to estrogen receptor by cyclin D1. Genes Dev 12(22):3488–3498
Ahnstrom M, Nordenskjold B, Rutqvist LE, Skoog L, Stal O (2005) Role of cyclin D1 in ErbB2-positive breast cancer and tamoxifen resistance. Breast Cancer Res Treat 91(2):145–151. doi:10.1007/s10549-004-6457-4
Bukholm IR, Bukholm G, Nesland JM (2001) Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int J Cancer 93(2):283–287. doi:10.1002/ijc.1311
Choschzick M, Heilenkotter U, Lebeau A, Jaenicke F, Terracciano L, Bokemeyer C, Sauter G, Simon R (2010) MDM2 amplification is an independent prognostic feature of node-negative, estrogen receptor-positive early-stage breast cancer. Cancer Biomark 8(2):53–60. doi:10.3233/DMA-2011-0806
Garcia V, Garcia JM, Pena C, Silva J, Dominguez G, Lorenzo Y, Diaz R, Espinosa P, de Sola JG, Cantos B, Bonilla F (2008) Free circulating mRNA in plasma from breast cancer patients and clinical outcome. Cancer Lett 263(2):312–320. doi:10.1016/j.canlet.2008.01.008
Guo LL, Gao P, Wu YG, Jian WC, Hao CY, Li H, Lin XY (2007) Alteration of cyclin D1 in Chinese patients with breast carcinoma and its correlation with Ki-67, pRb, and p53. Arch Med Res 38(8):846–852. doi:10.1016/j.arcmed.2007.06.004
Hwang TS, Han HS, Hong YC, Lee HJ, Paik NS (2003) Prognostic value of combined analysis of cyclin D1 and estrogen receptor status in breast cancer patients. Pathol Int 53(2):74–80
Kenny FS, Hui R, Musgrove EA, Gee JM, Blamey RW, Nicholson RI, Sutherland RL, Robertson JF (1999) Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res 5(8):2069–2076
Lee A, Park WC, Yim HW, Lee MA, Park G, Lee KY (2007) Expression of c-erbB2, cyclin D1 and estrogen receptor and their clinical implications in the invasive ductal carcinoma of the breast. Jpn J Clin Oncol 37(9):708–714. doi:10.1093/jjco/hym082
Lim SC (2003) Role of COX-2, VEGF and cyclin D1 in mammary infiltrating duct carcinoma. Oncol Rep 10(5):1241–1249
Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC (2000) Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A 97(8):4262–4266. doi:10.1073/pnas.060025397
Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, Howell A, Dowsett M, Landberg G (2012) Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res 14(2):R57. doi:10.1186/bcr3161
McIntosh GG, Anderson JJ, Milton I, Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW, Horne CH (1995) Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene 11(5):885–891
Michalides R, Hageman P, van Tinteren H, Houben L, Wientjens E, Klompmaker R, Peterse J (1996) A clinicopathological study on overexpression of cyclin D1 and of p53 in a series of 248 patients with operable breast cancer. Br J Cancer 73(6):728–734
Millar EK, Dean JL, McNeil CM, O’Toole SA, Henshall SM, Tran T, Lin J, Quong A, Comstock CE, Witkiewicz A, Musgrove EA, Rui H, Lemarchand L, Setiawan VW, Haiman CA, Knudsen KE, Sutherland RL, Knudsen ES (2009) Cyclin D1b protein expression in breast cancer is independent of cyclin D1a and associated with poor disease outcome. Oncogene 28(15):1812–1820. doi:10.1038/onc.2009.13
Pelosio P, Barbareschi M, Bonoldi E, Marchetti A, Verderio P, Caffo O, Bevilacqua P, Boracchi P, Buttitta F, Barbazza R, Dalla Palma P, Gasparini G (1996) Clinical significance of cyclin D1 expression in patients with node-positive breast carcinoma treated with adjuvant therapy. Ann Oncol 7(7):695–703
Peurala E, Koivunen P, Haapasaari KM, Bloigu R, Jukkola-Vuorinen A (2013) The prognostic significance and value of cyclin D1, CDK4 and p16 in human breast cancer. Breast Cancer Res 15(1):R5. doi:10.1186/bcr3376
Rudas M, Lehnert M, Huynh A, Jakesz R, Singer C, Lax S, Schippinger W, Dietze O, Greil R, Stiglbauer W, Kwasny W, Grill R, Stierer M, Gnant MF, Filipits M (2008) Cyclin D1 expression in breast cancer patients receiving adjuvant tamoxifen-based therapy. Clin Cancer Res 14(6):1767–1774. doi:10.1158/1078-0432.CCR-07-4122
Takano Y, Takenaka H, Kato Y, Masuda M, Mikami T, Saegusa M, Okayasu I (1999) Cyclin D1 overexpression in invasive breast cancers: correlation with cyclin-dependent kinase 4 and oestrogen receptor overexpression, and lack of correlation with mitotic activity. J Cancer Res Clin Oncol 125(8–9):505–512
Tobin NP, Lundgren KL, Conway C, Anagnostaki L, Costello S, Landberg G (2012) Automated image analysis of cyclin D1 protein expression in invasive lobular breast carcinoma provides independent prognostic information. Hum Pathol 43(11):2053–2061. doi:10.1016/j.humpath.2012.02.015
Umekita Y, Ohi Y, Sagara Y, Yoshida H (2002) Overexpression of cyclinD1 predicts for poor prognosis in estrogen receptor-negative breast cancer patients. Int J Cancer 98(3):415–418. doi:10.1002/ijc.10151
Utsumi T, Yoshimura N, Maruta M, Takeuchi S, Ando J, Mizoguchi Y, Harada N (2000) Correlation of cyclin D1 MRNA levels with clinico-pathological parameters and clinical outcome in human breast carcinomas. Int J Cancer 89(1):39–43. doi:10.1002/(SICI)1097-0215(20000120)89:1<39:AID-IJC7>3.0.CO2-T
van Diest PJ, Michalides RJ, Jannink L, van der Valk P, Peterse HL, de Jong JS, Meijer CJ, Baak JP (1997) Cyclin D1 expression in invasive breast cancer. Correlations and prognostic value. Am J Pathol 150(2):705–711
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 93(4):387–391. doi:10.1038/sj.bjc.6602678
Chen M, Cai E, Huang J, Yu P, Li K (2012) Prognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 21(7):1126–1134. doi:10.1158/1055-9965.EPI-12-0020
Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17(24):2815–2834. doi:10.1002/(SICI)1097-0258(19981230)17:24<2815:AID-SIM110>3.0.CO2-8
Xu XL, Ling ZQ, Chen SZ, Li B, Ji WH, Mao WM (2013) The impact of E-cadherin expression on the prognosis of esophageal cancer: a meta-analysis. Dis Esophagus. doi:10.1111/dote.12024
Tierney JFSL, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8:16
Yusuf S, Peto R, Lewis J, Collins R, Sleight P (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27(5):335–371
Dublin EA, Patel NK, Gillett CE, Smith P, Peters G, Barnes DM (1998) Retinoblastoma and p16 proteins in mammary carcinoma: their relationship to cyclin D1 and histopathological parameters. Int J Cancer 79(1):71–75. doi:10.1002/(SICI)1097-0215(19980220)79:1<71:AID-IJC14>3.0.CO2-K
Gillett CE, Smith P, Peters G, Lu X, Barnes DM (1999) Cyclin-dependent kinase inhibitor p27Kip1 expression and interaction with other cell cycle-associated proteins in mammary carcinoma. J Pathol 187(2):200–206. doi:10.1002/(SICI)1096-9896(199901)187:2<200:AID-PATH228>3.0.CO;2-M
Husdal A, Bukholm G, Bukholm IR (2006) The prognostic value and overexpression of cyclin A is correlated with gene amplification of both cyclin A and cyclin E in breast cancer patient. Cell Oncol 28(3):107–116
Lim SC, Lee MS (2002) Significance of E-cadherin/beta-catenin complex and cyclin D1 in breast cancer. Oncol Rep 9(5):915–928
Bostner J, Ahnstrom Waltersson M, Fornander T, Skoog L, Nordenskjold B, Stal O (2007) Amplification of CCND1 and PAK1 as predictors of recurrence and tamoxifen resistance in postmenopausal breast cancer. Oncogene 26(49):6997–7005. doi:10.1038/sj.onc.1210506
Elsheikh S, Green AR, Aleskandarany MA, Grainge M, Paish CE, Lambros MB, Reis-Filho JS, Ellis IO (2008) CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res Treat 109(2):325–335. doi:10.1007/s10549-007-9659-8
Rodriguez C, Hughes-Davies L, Valles H, Orsetti B, Cuny M, Ursule L, Kouzarides T, Theillet C (2004) Amplification of the BRCA2 pathway gene EMSY in sporadic breast cancer is related to negative outcome. Clin Cancer Res 10(17):5785–5791. doi:10.1158/1078-0432.CCR-03-0410
Aaltonen K, Amini RM, Landberg G, Eerola H, Aittomaki K, Heikkila P, Nevanlinna H, Blomqvist C (2009) Cyclin D1 expression is associated with poor prognostic features in estrogen receptor positive breast cancer. Breast Cancer Res Treat 113(1):75–82. doi:10.1007/s10549-008-9908-5
Jacquemier J, Charafe-Jauffret E, Monville F, Esterni B, Extra JM, Houvenaeghel G, Xerri L, Bertucci F, Birnbaum D (2009) Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res 11(2):R23. doi:10.1186/bcr2249
Kreike B, Hart G, Bartelink H, van de Vijver MJ (2010) Analysis of breast cancer related gene expression using natural splines and the Cox proportional hazard model to identify prognostic associations. Breast Cancer Res Treat 122(3):711–720. doi:10.1007/s10549-009-0588-6
Perez-Tenorio G, Karlsson E, Waltersson MA, Olsson B, Holmlund B, Nordenskjold B, Fornander T, Skoog L, Stal O (2011) Clinical potential of the mTOR targets S6K1 and S6K2 in breast cancer. Breast Cancer Res Treat 128(3):713–723. doi:10.1007/s10549-010-1058-x
Bonnefoi H, Diebold-Berger S, Therasse P, Hamilton A, van de Vijver M, MacGrogan G, Shepherd L, Amaral N, Duval C, Drijkoningen R, Larsimont D, Piccart M (2003) Locally advanced/inflammatory breast cancers treated with intensive epirubicin-based neoadjuvant chemotherapy: are there molecular markers in the primary tumour that predict for 5-year clinical outcome? Ann Oncol 14(3):406–413
Chen S, Chen CM, Yu KD, Yang WT, Shao ZM (2012) A prognostic model to predict outcome of patients failing to achieve pathological complete response after anthracycline-containing neoadjuvant chemotherapy for breast cancer. J Surg Oncol 105(6):577–585. doi:10.1002/jso.22140
Wachter DL, Fasching PA, Haeberle L, Schulz-Wendtland R, Dimmler A, Koscheck T, Renner SP, Lux MP, Beckmann MW, Hartmann A, Rauh C, Schrauder MG (2013) Prognostic molecular markers and neoadjuvant therapy response in anthracycline-treated breast cancer patients. Arch Gynecol Obstet 287(2):337–344. doi:10.1007/s00404-012-2534-9
Mahdey HM, Ramanathan A, Ismail SM, Abraham MT, Jamaluddin M, Zain RB (2011) Cyclin D1 amplification in tongue and cheek squamous cell carcinoma. Asian Pac J Cancer Prev 12(9):2199–2204
Wang MT, Chen G, An SJ, Chen ZH, Huang ZM, Xiao P, Ben XS, Xie Z, Chen SL, Luo DL, Tang JM, Lin JY, Zhang XC, Wu YL (2012) Prognostic significance of cyclinD1 amplification and the co-alteration of cyclinD1/pRb/ppRb in patients with esophageal squamous cell carcinoma. Dis Esophagus 25(7):664–670. doi:10.1111/j.1442-2050.2011.01291.x
Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, Ryden L, Gallagher WM, O’Brien SL (2006) CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res 12(21):6421–6431. doi:10.1158/1078-0432.CCR-06-0480
Muss HB, Bunn JY, Crocker A, Plaut K, Koh J, Heintz N, Rincon M, Weaver DL, Tam D, Beatty B, Kaufman P, Donovan M, Verbel D, Weiss L (2007) Cyclin D-1, interleukin-6, HER-2/neu, transforming growth factor receptor-II and prediction of relapse in women with early stage, hormone receptor-positive breast cancer treated with tamoxifen. Breast J 13(4):337–345. doi:10.1111/j.1524-4741.2007.00440.x
Nielsen NH, Emdin SO, Cajander J, Landberg G (1997) Deregulation of cyclin E and D1 in breast cancer is associated with inactivation of the retinoblastoma protein. Oncogene 14(3):295–304. doi:10.1038/sj.onc.1200833
Reed W, Florems VA, Holm R, Hannisdal E, Nesland JM (1999) Elevated levels of p27, p21 and cyclin D1 correlate with positive oestrogen and progesterone receptor status in node-negative breast carcinoma patients. Virchows Arch 435(2):116–124
Reed W, Sandstad B, Holm R, Nesland JM (2003) The prognostic impact of hormone receptors and c-erbB-2 in pregnancy-associated breast cancer and their correlation with BRCA1 and cell cycle modulators. Int J Surg Pathol 11(2):65–74
Peters MG, Vidal Mdel C, Gimenez L, Mauro L, Armanasco E, Cresta C, Bal de Kier Joffe E, Puricelli L (2004) Prognostic value of cell cycle regulator molecules in surgically resected stage I and II breast cancer. Oncol Rep 12(5):1143–1150
Olsson A, Borgquist S, Butt S, Zackrisson S, Landberg G, Manjer J (2012) Tumour-related factors and prognosis in breast cancer detected by screening. Br J Surg 99(1):78–87. doi:10.1002/bjs.7757
Nicolini A, Campani D, Miccoli P, Spinelli C, Carpi A, Menicagli M, Ferrari P, Gadducci G, Rossi G, Fini M, Giavaresi G, Bonazzi V, Giardino R (2004) Vascular endothelial growth factor (VEGF) and other common tissue prognostic indicators in breast cancer: a case-control study. Int J Biol Markers 19(4):275–281
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lonning PE, Borresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98(19):10869–10874. doi:10.1073/pnas.191367098
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100(14):8418–8423. doi:10.1073/pnas.0932692100
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. doi:10.1038/35021093
Bennis S, Abbass F, Akasbi Y, Znati K, Joutei KA, El Mesbahi O, Amarti A (2012) Prevalence of molecular subtypes and prognosis of invasive breast cancer in north-east of Morocco: retrospective study. BMC Res Notes 5:436. doi:10.1186/1756-0500-5-436
Su Y, Zheng Y, Zheng W, Gu K, Chen Z, Li G, Cai Q, Lu W, Shu XO (2011) Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study. BMC Cancer 11:292. doi:10.1186/1471-2407-11-292
Gao CY, Zelenka PS (1997) Cyclins, cyclin-dependent kinases and differentiation. BioEssays 19(4):307–315. doi:10.1002/bies.950190408
Stendahl M, Kronblad A, Ryden L, Emdin S, Bengtsson NO, Landberg G (2004) Cyclin D1 overexpression is a negative predictive factor for tamoxifen response in postmenopausal breast cancer patients. Br J Cancer 90(10):1942–1948. doi:10.1038/sj.bjc.6601831
Acknowledgments
The authors thank Jian-Guo Feng at Zhejiang Cancer Hospital (Zhejiang Cancer Research Institute) for data statistics assistance. This study was supported by Province important technology and science (Special feature of major province scientific and technological 2011), No. 2011C13039-1, 2011–2014, and the establishment and NSFC general program, No. 81172081, 2012.01-2015-12.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, XL., Chen, SZ., Chen, W. et al. The impact of cyclin D1 overexpression on the prognosis of ER-positive breast cancers: a meta-analysis. Breast Cancer Res Treat 139, 329–339 (2013). https://doi.org/10.1007/s10549-013-2563-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2563-5