Abstract
Invasive ductal carcinoma with lobular features (IDC-L) is not recognized as a distinct subtype of breast cancer, and its clinicopathologic features and outcomes are unknown. In this retrospective study, we focused on characterization of clinicopathologic features and outcomes of IDC-L and compared them to invasive ductal carcinoma (IDC) and invasive lobular carcinoma (ILC). 183 cases of IDC-L from 1996 to 2011 were compared with 1,499 cases of IDC and 375 cases of ILC. Available slides of IDC-L (n = 150) were reviewed to quantify the lobular component (≤20, 21–50, 51–80, >80 %), defined as small cells individually dispersed, arranged in linear cords, or in loose aggregates without the formation of tubules or cohesive nests. E-cadherin immunostain was performed to confirm ductal origin. Compared to IDC, IDC-L was more likely to have lower histologic grade (p < 0.001), be positive for estrogen receptor (96 vs. 70 %; p < 0.0001) and progesterone receptor (84 vs. 57 %; p < 0.0001), and less likely to overexpress HER-2/neu (12 vs. 23 %; p = 0.001). Despite these favorable prognostic features, IDC-L had a higher frequency of nodal metastases (51 vs. 34 %; p < 0.0001) and a worse 5-year disease-free survival than IDC (hazard ratio = 0.454; p = 0.0004). ILC and IDC-L had similar clinicopathologic features and outcomes. The proportion of the lobular component in IDC-L had no impact on the size, nodal status, stage, or outcome. Our data suggest that although IDC-L may be a variant of IDC, with >90 % of cases being E-cadherin positive, the clinical and biological characteristics are more similar to that of ILC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is a heterogeneous group of tumors with variable morphology, behavior, response to therapy, and molecular profiles. Invasive ductal carcinoma (IDC) is the most common histologic type comprising 72–80 % of all invasive breast cancers, while invasive lobular carcinoma (ILC) is less common and accounts for 5–15 % of all invasive breast cancers [1–5]. The clinical and biological characteristics of both IDC and ILC have been well described in the literature [1–9]. ILC differs from IDC in risk factors, histologic features, immunophenotype, molecular profiles, and response to systemic therapy [3]. ILC is more frequently hormone receptor positive, demonstrate a higher incidence of synchronous, contralateral primary tumors, present more frequently with multi-centric disease, and metastasize to distinct sites such as meninges, serosa, and retroperitoneum [10–13]. Studies comparing survival of ILC and IDC have provided variable results. Although some studies have shown poorer responses to therapy for ILC and higher tumor recurrence, others have shown improved survival [14, 15].
Invasive ductal carcinomas with lobular features (IDC-L) are commonly observed in daily pathology practice. Despite their frequency, there are no defined criteria or uniform terminology for their diagnosis. It is a category used when there is the presence of a mixture of ductal cytology and growth pattern (pleomorphic cells with cohesive cellular arrangement with or without lumen formation) and lobular pattern (dispersed infiltrating fashion) in the same lesion, or occasional tubules or small nest formation in a lesion that otherwise shows a typical lobular type of infiltration and cytology [16]. In routine practice, many of these tumors are diagnosed as mixed ductal and lobular carcinomas, mammary carcinoma with ductal and lobular features, IDC-L, or simply as ductal or lobular carcinoma. The world health organization (WHO) definition of a mixed ductal and lobular carcinoma is a tumor in which the lobular component comprises at least 50 % of the tumor. E-cadherin expression is typically lost in the lobular component in these cases [16]. In contrast, variable staining for E-cadherin in IDC-L has been reported, but a majority of cases are E-cadherin positive supporting a ductal origin [16]. We will hereinafter refer to mixed ductal and lobular carcinomas, as defined by the WHO, as mixed tumors.
Comprehensive studies of IDC-L are lacking in the literature, and it is unclear whether this subtype is a variant of IDC or a true mixed ductal and lobular carcinoma by WHO criteria. The aim of this current study was to perform a retrospective analysis of a large cohort of patients treated at our institution, focusing on morphology and on characterization of the clinicopathologic features and outcomes of IDC-L to determine if these are true mixed tumors or a variant of ductal carcinoma that may need to be distinguished from invasive ductal and invasive lobular carcinoma.
Materials and methods
Study population
This study was approved by the Institutional Review Board at University of Michigan. Using the keywords “invasive ductal carcinoma with lobular features”, “mammary carcinoma with ductal and lobular features”, and “mixed ductal and lobular carcinoma”, 332 patients with IDC-L were identified from the University of Michigan (UMHS) pathology database from 1996 to 2011. Only patients who received primary surgical or medical treatment at UMHS were included in the study (n = 183). 1,499 patients with IDC, not otherwise specified, and 375 patients with ILC from the UMHS breast cancer tumor registry from 1996 to 2007 were used as the comparison groups. All patients with the diagnosis of ILC from the registry were included during this time period. For patients with IDC, those with special histologic types (tubular, mucinous, and medullary) were excluded and only those with hormone receptor and HER-2/neu status readily obtainable from the database were included. The clinical and biological behavior of this random selection of IDC patients appeared to be representative of this type of breast cancer reported in the literature [3, 17, 18].
Clinical and pathologic data including age, histologic type, tumor size, lymph node status, ER, PR, and HER-2/neu status, vital status, and treatment were obtained from the breast cancer tumor registry database, with search of the medical records to obtain missing information. The lymph node assessment protocol at UMHS is with hematoxylin and eosin sections.
Microscopic review
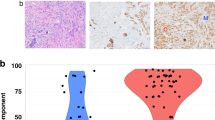
Available slides of IDC-L (150 cases) were reviewed by two pathologists to quantify the lobular component, defined as small cells individually dispersed, arranged in linear cords, or in loose aggregates without the formation of tubules or cohesive nests (see Fig. 1a, b). Semi-quantification of the proportion of the lobular component was grouped as <20, 21–50, 51–80, >80 %. Immunostains for E-cadherin (Clone HECD-1, Zymed) following the manufacturer’s protocol were performed to confirm ductal origin on cases with available tissue blocks (126 cases) (see Fig. 1c).
Statistical methods
Categorical prognostic factors were analyzed using Chi square test or Fisher exact test. ANOVA was used to compare groups with continuous prognostic factors. Prognostic factors included age, size, nodal status, hormone receptor, and HER-2/neu expression, and histologic grade. Disease-specific survival was defined as from date of diagnosis to death with disease. Patients who were alive or died of other causes without disease at the time of death were censored at the time of last follow-up. Disease-free survival was defined from date of diagnosis to disease progression or death. Data were censored at the last follow-up for patients who were disease-free at the time of analysis. Survival functions were estimated using Kaplan–Meier methods. Differences in survival functions were assessed using Cox’s proportional hazards regression. Multivariate Cox regression was also performed to model survival as a function of the histology, after adjusting for size, lymph node status, ER status, and age. Histologic grade was not included in the survival analyses because this information was not available in many of the cases. Survival studies were conducted for a follow-up time of 5 years. All analyses were done using SAS 9.3 software. p < 0.05 was considered as significant.
Results
Patient and tumor characteristics
Table 1 summarizes the clinical and biological tumor characteristics of the three histologic types of breast cancers. Patients with IDC-L presented at a mean age similar to IDC. Compared to IDC, IDC-L were more likely to have lower histologic grade (p < 0.001), be positive for ER (96 vs. 70 %; p < 0.0001) and PR (84 vs. 57 %; p < 0.0001), and less likely to overexpress HER-2/neu (12 vs. 23 %; p = 0.001). Despite having these good prognostic features, there was a higher frequency of nodal metastases (51 vs. 34 %; p < 0.0001). Although IDC-L tumors were larger than IDC tumors, even after correcting for size, the higher frequency of nodal metastases remained significant (p < 0.05). Patients with IDC-L presented at a slightly younger age than ILC (mean 56 vs. 58 years; p = 0.036). Compared to ILC, IDC-L tended to have higher histologic grade (p = 0.003) and was more likely to be PR positive (84 vs. 74 %, p = 0.01). There were no significant differences in ER and HER-2/neu expression, tumor size, or frequency of nodal metastases between IDC-L and ILC. Although not statistically significant, patients with IDC-L and ILC were more likely to present with stage III disease (17 and 22 %) than patients with IDC (13 %).
A comparison between four groups of patients with different proportions of the lobular component (<20, 21–50, 51–80, and >80 %) did not show any statistically significant differences with respect to the mean age, size, lymph node status, stage at presentation, ER status, or outcome (recurrence and survival rates) (Table 2). E-cadherin immunostain demonstrated moderate to strong membrane staining in both the ductal and lobular components in 116 of 126 (92 %) cases confirming ductal origin. The remaining 10 cases showed weak to absent membrane staining in both components.
Treatment
Of the 183 patients with IDC-L, for the first surgical procedure, 59 patients (32 %) had a mastectomy and 122 (67 %) patients had a lumpectomy. Two patients did not have surgery. Of those that had a lumpectomy as their first surgery, 60 (49 %) required additional surgery to obtain adequate margins.
Of the 183 patients with IDC-L, 110 patients (60 %) received chemotherapy, 147 patients (80 %) received endocrine therapy (tamoxifen and/or aromatase inhibitor), and 14 patients (8 %) received Herceptin. Adjuvant chemotherapy, endocrine therapy, and Herceptin were unknown for 7, 13, and 6 patients, respectively. 128 patients (70 %) received radiation after surgery. Radiation therapy was unknown for ten patients.
Disease-free and disease-specific survival
There were no significant differences in local, regional, and distant recurrence rates between patients with IDC and IDC-L and ILC and IDC-L (see Table 3). Distant metastases, both at diagnosis and/or recurrences, were seen in 16 % (29 of 183) of patients with IDC-L. The most frequent sites were bone (76 %, 22/29) and liver (45 %; 13/29). Other sites of metastases, in order of decreasing frequency, were brain, distant lymph nodes (neck, mediastinal, abdominal), lung, adrenal, skin, renal, peritoneum, and pericardium. 11 of 183 (6 %) patients with IDC-L had contralateral breast cancers—seven with invasive carcinoma and four with ductal carcinoma in situ (DCIS). Contralateral breast cancers are reported to be seen in 4.4–20.9 % of patients with ILC and 3–11.2 % of patients with IDC [3, 18, 19].
Data was available for 2,051 patients (183 IDC-L, 1,494 IDC, and 374 ILC) in the survival analysis. The median follow-up time was 5 years (range 0.024–5 years). Univariate analysis indicated that disease-free survival (DFS) was significantly better for IDC patients when compared to IDC-L, however, no statistical difference was found between ILC and IDC-L. (see Table 4; Fig. 2a). There were no significant differences in disease-specific survival (DSS) between the histologic subtypes (see Table 4; Fig. 2b). In the multivariate analysis, we found that size, lymph node status, and ER status were important prognostic factors associated with DFS and DSS for IDC-L, but age was not. After adjusting for these factors, DFS for the IDC histologic subtype remained significantly better than IDC-L (see Table 4). There were still no differences in DSS between the histologic subtypes.
Disease-free and disease-specific survival plotted from 50 to 100 %. a Disease-free survival. Kaplan–Meier plot of disease-free survival (DFS) of patients with IDC-L and patients with IDC and ILC showing worse DFS for IDC-L compared to IDC, but no difference between IDC-L and ILC. b Disease-specific survival. Kaplan–Meier plot of disease-specific survival (DSS) of patients with IDC-L and patients with IDC and ILC showing no difference in DSS between the groups
Discussion
The management of breast cancer has evolved over the years with multimodality therapy becoming commonplace. These recent advances in breast cancer treatment have required better characterization of the different prognostic and histologic subgroups. Targeted systemic therapy is a goal and better understanding of these differences is essential to direct and individualize treatment decisions. IDC and ILC are the most common histologic types of invasive breast cancers. Several molecular profiling studies, clinical data, and patterns of metastases suggest that these histologic types of breast cancer demonstrate genetic and biological differences. Some breast tumors contain a combination of ductal and lobular morphologies. If such tumors contain 50 % or greater lobular morphology, they may be classified as mixed ductal and lobular carcinoma, or mixed tumors, as defined by the WHO. The clinical and biological significance of mixed tumors is not well characterized [1, 18, 20], however, studies suggest that they may behave different clinically as compared to IDC. The frequency of lymph node metastasis for mixed tumors has been reported to range from 26 [21] to 41 % [22]. In one study, no clinically meaningful difference was seen in survival between IDC and mixed tumors [22]. However, in a different study [23], patients with mixed tumors presented with more advanced disease but had survival superior to patients with IDC, similar to ILC. No published studies, to our knowledge have specifically addressed the behavior of invasive ductal carcinoma with lobular features.
We observed that IDC-L was associated with patient and tumor characteristics that were more similar to ILC than with IDC. IDC-L had lower histologic grade, higher rates of hormone receptor positivity and lower rates of HER-2/neu overexpression than IDC. Despite these features that are typically considered to be prognostically favorable, IDC-L had a higher rate of nodal metastasis than IDC. The presence of lobular morphology in any proportion, similar to micropapillary morphology, may be an unfavorable histologic feature [24]. 16 % (29/183) of patients with IDC-L had distant metastases at diagnosis and/or recurrences. Similar to the data on mixed tumors reported by Rakha et al. [22], our data showed frequent metastases to bone (76 %, 22/29). This propensity for metastasis to the bone is more characteristic of ILC. Lee et al. [19] reported a bony metastasis rate of 72.7 % in ILC and 36.5 % in IDC in patients with systemic recurrence of disease. In our study, a majority of patients with IDC-L (67 %) had a lumpectomy as their initial surgical procedure. Interestingly, re-excision was required in 49 % of these patients to achieve adequate margins. The re-excision rate for positive/close margins on all breast cancers at our institution is 25 % as reported by Sabel et al. [25]. In the study by Moore et al. [26] comparing the re-excision rates of ILC and IDC, the re-excision rates were 51 and 15 %, respectively. In our experience, as well as in the literature [26, 27], ILCs are typically less well defined, both grossly and radiographically, tend to have more infiltrative borders, and are more often multifocal than IDCs, making margin control more difficult. IDC-L appears to be similar to ILC in this respect and likely accounts for the need for re-excision. Finally, although only DFS was statistically significantly worse for IDC-L than IDC, our survival analysis suggests that IDC-L and ILC have a trend for a worse outcome than IDC.
Diagnosing IDC-L is often subjective and the criteria for diagnosis are not well defined. Review of the literature and experience at our institution have demonstrated that mixed ductal and lobular carcinoma and IDC-L are not clearly defined [21–23]. At our institution, IDC-L is used inconsistently and is often used interchangeably with mixed ductal and lobular carcinoma and mammary carcinoma with ductal and lobular features. In the study of mixed tumors by Rakha et al. [22], only tumors with “mixed but well defined histologic types” were included and those with hybrid morphology that showed morphologic features of both lobular and ductal tumors, which was described as a “lobular diffuse infiltrative pattern but with no specific cellular morphology”, were excluded as these cases were diagnosed in their practice as IDC-L. In our study, the lobular component of the IDC-L was defined as small cells individually dispersed, arranged in linear cords, or in loose aggregates without the formation of tubules or cohesive nests.
The absence of E-cadherin staining is one of the major defining features of lobular tumors and previous studies have demonstrated loss of expression in the majority of ILC (80–90 %) and in the areas of lobular morphology in the mixed tumors, while positive in IDC and in the ductal morphology of mixed tumors [16]. In the study by Rakha et al. [22], 70 % of the mixed tumors had absent or reduced E-cadherin immunostaining. In a separate study by Suryadevara et al. [21] on mixed ductal and lobular carcinoma, the histologic features were not defined, but E-cadherin was positive in 90 % of cases. Acs et al. [16] described three patterns of E-cadherin expression in 41 cases of IDC-L: “lobular-like” which was characterized by a complete or almost complete loss of E-cadherin staining (10 cases, 24 %); “ductal-like” demonstrating uniform membrane expression of E-cadherin throughout the tumor (24 cases, 58 %); and “intermediate” where there was focally complete loss of E-cadherin staining (7 cases, 17 %). These “intermediate” cases were subsequently re-classified as mixed ductal and lobular carcinoma. In our current study, E-cadherin was moderately to strongly positive in 92 % of cases (116 of 126), with immunoreactivity appreciated in both the ductal and lobular components. 10 cases demonstrated weak to absent E-cadherin immunostaining in both components.
To our knowledge, this is the first comprehensive study of IDC-L. Although IDC-L does not fulfill the WHO criteria for a true mixed ductal and lobular carcinoma, it may be considered as within the spectrum of mixed tumors as there is overlap in clinicopathologic features reported in the literature. Limitations of this study include its retrospective nature and potential for selection bias, as well as the short median follow-up time of 5 years. However, important conclusions are borne from our study: (1) It may be important to distinguish invasive ductal carcinoma with lobular features from invasive ductal carcinoma as our data suggests that the presence of lobular morphology may be a poor prognostic feature. (2) E-cadherin immunostain may not be necessary to determine whether the cells with lobular morphology are lobular or ductal in origin as the clinicopathologic features and outcomes of invasive ductal carcinoma with lobular features and invasive lobular carcinoma are similar.
References
Sastre-Garau X, Jouve M, Asselain B et al (1996) Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 75(1):113–120
Lamovec J, Bracko M (1991) Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 48(1):28–33
Arpino G, Bardou VJ, Clark GM, Elledge RM (2004) Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 6(3):R149–R156
Borst MJ, Ingold JA (1993) Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 114(4):637–642
Li CI, Uribe DJ, Daling JR (2005) Clinical characteristics of different histologic types of breast cancer. Br J Cancer 93(9):1046–1052
Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW (1982) Infiltrating lobular carcinoma of the breast. Histopathology 6(2):149–161
Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW (1992) Pathological prognostic factors in breast cancer. II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 20(6):479–489
Li CI, Anderson BO, Daling JR, Moe RE (2003) Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289(11):1421–1424
Reeves GK, Beral V, Green J, Gathani T, Bull D (2006) Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 7(11):910–918
Lesser ML, Rosen PP, Kinne DW (1982) Multicentricity and bilaterality in invasive breast carcinoma. Surgery 91(2):234–240
Nesland JM, Holm R, Johannessen JV (1985) Ultrastructural and immunohistochemical features of lobular carcinoma of the breast. J Pathol 145(1):39–52
Mendelson EB, Harris KM, Doshi N, Tobon H (1989) Infiltrating lobular carcinoma: mammographic patterns with pathologic correlation. AJR Am J Roentgenol 153(2):265–271
Kumar ND, Hart WR (1982) Metastases to the uterine corpus from extragenital cancers. A clinicopathologic study of 63 cases. Cancer 50(10):2163–2169
Silverstein MJ, Lewinsky BS, Waisman JR et al (1994) Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer 73(6):1673–1677
Li CI, Moe RE, Daling JR (2003) Risk of mortality by histologic type of breast cancer among women aged 50–79 years. Arch Intern Med 163(18):2149–2153
Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ (2001) Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 115(1):85–98
Wasif N, Maggard MA, Ko CY, Giuliano AE (2010) Invasive lobular versus ductal breast cancer: a stage-matched comparison of outcomes. Ann Surg Oncol 17(7):1862–1869
Korhonen T, Huhtala H, Holli K (2004) A comparison of the biological and clinical features of invasive lobular and ductal carcinomas of the breast. Breast Cancer Res Treat 85(1):23–29
Lee JH, Park S, Park HS, Park BW (2010) Clinicopathological features of infiltrating lobular carcinomas comparing with infiltrating ductal carcinomas: a case control study. World J Surg Oncol 8:34
Li CI, Daling JR, Malone KE et al (2006) Relationship between established breast cancer risk factors and risk of seven different histologic types of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 15(5):946–954
Suryadevara A, Paruchuri LP, Banisaeed N, Dunington G, Rao KA (2010) The clinical behavior of mixed ductal/lobular carcinoma of the breast: a clinicopathologic analysis. World J Surg Oncol 8:51
Rakha EA, Gill MS, El-Sayed ME et al (2009) The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res Treat 114(2):43–50
Af Bharat, Gao F, Margenthaler JA (2009) Tumor characteristics and patient outcomes are similar between invasive lobular and mixed invasive ductal/lobular breast cancers but differ from pure invasive ductal breast cancers. Am J Surg 198(4):516–519
Nassar H, Wallis T, Andea A, Dey J, Adsay V, Vischer D (2001) Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol 14(9):836–841
Sabel MS, Jorns JM, Wu A, Myers J, Newman LA, Breslin TM (2012) Development of an intraoperative pathology consultation service at a free-standing ambulatory surgical center: clinical and economic impact for patients undergoing breast cancer surgery. Am J Surg 204(1):66–77
Moore MM, Borossa G, Imbrie JZ et al (2000) Association of infiltrating lobular carcinoma with positive surgical margins after breast-conservation therapy. Ann Surg 231(6):877–882
Pestalozzi BC, Zahrieh D, Mallon E et al (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol 26(18):3006–3014
Acknowledgments
The authors gratefully acknowledge the UMHS Cancer Center Research Histology & Immunoperoxidase Lab for performing the E-cadherin immunostains and Steven C. Smith, M.D., Ph.D. for his assistance in the preparation of Fig. 2.
Conflict of interest
The authors have no disclosures or conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arps, D.P., Healy, P., Zhao, L. et al. Invasive ductal carcinoma with lobular features: a comparison study to invasive ductal and invasive lobular carcinomas of the breast. Breast Cancer Res Treat 138, 719–726 (2013). https://doi.org/10.1007/s10549-013-2493-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2493-2