Abstract
Although invasive ductal (IDC) and lobular (ILC) breast carcinomas are well characterised in the literature, the biological and clinical significance of mixed tumours with both ductal and lobular components has not been investigated. In the current study, we have examined a well-characterised series of breast carcinoma with a long term follow-up that comprised 140 mixed tumours, 2170 IDC and 380 pure ILC. Results: Mixed tumours constituted 3.6% of all cases. The majority (59%) of the mixed tumours were grade 2 compared to 33% in IDC and 88% in ILC. Positive lymph nodes (LN) were found in 41% and definite vascular invasion (VI) in 26% of the cases. DCIS was detected in 123 (89%) and LCIS in 43 (31%) (both DCIS and LCIS were found in 39 cases). The majority of tumours were predominantly (>50 of tumour area) of ductal type (57%). When compared to pure IDC, mixed tumours showed an association with lower grade, ER positivity and lower frequency of development of distant metastases. When compared to pure ILC, mixed tumours showed an association with higher grade, positive LN metastasis, VI and development of regional metastasis. After adjustment for grade most of these differences were no longer apparent. There was an association between histologic type of carcinoma in LN metastasis and the predominant histologic type of the primary tumour. Mixed tumours showed metastatic patterns similar to that of ILC with frequent metastasis to bone. No clinically meaningful differences in survival were found between these mixed carcinomas and pure IDC or ILC of the breast or between mixed tumours with predominantly ductal or lobular phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer represents a heterogeneous group of tumours with varied morphology, behaviour, and response to therapy. Invasive ductal (IDC) and invasive lobular (ILC) breast carcinomas are the most common types of breast cancer, accounting for 72–80% and 5–15% of invasive breast cancer respectively [1–4], while tumours with mixed histologic types (mixed ductal and lobular) are less common, accounting for 3–5% [5–7]. Several studies have reported that whereas the incidence of IDC has remained stable, the incidence of ILC and mixed ductal and lobular tumours are increasing particularly among postmenopausal women [5, 8]. However, although this increase appears genuinely to be a true rise in the incidence of tumours with lobular morphology, which is probably hormone replacement therapy related [7], it may also be influenced by increased diagnosis of these tumours as a result of either recognition of more non-classical variants of ILC or the adoption of E-cadherin staining in routine practice to distinguish between ILC and IDC.
Although treatment for stage-matched ductal versus lobular carcinomas is similar [9], several studies have shown that ILC is a distinct entity of breast cancer that differs from IDC not only in histological and clinical features [10, 11] but also in the risk factors [12], genomic profiles [13], global transcription programs [14], immunophenotype [15] and response to systemic therapy [11, 16, 17]. Such studies suggest that lobular tumour development and progression may follow a distinct pathway from ductal tumours. ILC is frequently associated with older age, larger tumour size, lower histologic grade, less lymphovascular invasion, absence of E-cadherin expression and positive hormone receptors (HR) [18]. Unlike IDC, ILC more often has ill-defined margins and does not form microcalcifications, making it difficult to detect on screening mammography and ultrasound [19]. ILC has an increased frequency of bilaterality, a higher rate of multiple metastases [1, 2, 4, 20] and unique patterns of metastasis and spread compared with IDC. Some long-term follow-up studies have shown a trend to later locoregional recurrence [11]. In addition, it has been reported that ILC is less responsive to chemotherapy [3, 21], lacks potential benefit of HER2 targeted therapy [16, 17], being typically HER2 negative, but is more often HR positive and responsive to adjuvant hormonal therapy (HT) [11, 22, 23]. Reported prognosis of ILC varies and has been reported to be worse [1, 9, 24–27], no different [17, 28–32], or better [6] than that with IDC.
Although IDC and ILC have been extensively studied and their features are well-characterised in the literature, knowledge about the biological and clinical significance of tumours with mixed ductal and lobular histologic pattern, which are less common, is lacking. Therefore, in this study, we performed a retrospective analysis of a large and well-characterised series of breast cancers with long term follow-up comprising clinicopathologic and outcome information; data on a wide range of proteins of known relevance in breast cancer were also available. In addition, several morphological parameters of tumours with mixed IDC and ILC histologic types were examined. Our aim was to perform a comprehensive characterisation of biological and clinical features of these mixed tumours and to assess differences, if any, between these tumours and pure IDC and ILC tumour types.
Methods
The study population was derived from the Nottingham Tenovus Primary Breast Carcinoma Series of women aged 70 years or less who presented with primary operable invasive breast carcinomas between 1988 and 2004. This is a well-characterised series of patients managed in a single institution with a long term follow-up information available. All patients received standard surgical treatment of either mastectomy or wide local excision with radiotherapy. Adjuvant hormone or chemotherapy treatment was managed on the basis of patients’ tumour prognostic and predictive factor status. Hormone therapy was offered to patients with estrogen receptor (ER) positive tumours and Nottingham Prognostic Index (NPI) scores of 3.4 or greater (moderate and poor prognostic groups).
Patient’s clinical history and tumour characteristics including patients’ age, menopausal status, bilaterality, family history, type and number of primary operation and axillary lymph node surgery, primary tumour size, histologic tumour type [6], histologic grade [33], lymph node (LN) status, vascular invasion (VI), NPI and oestrogen receptor (ER) status were obtained from the database. Survival data including survival time, disease free interval, and development of distant metastasis (DM), local and regional recurrence was maintained on a prospective basis. Patients were followed up at 3-month intervals initially, then 6-monthly and annually for a median period of 68 months (range 1–195). A metastatic search was prompted when the woman presented with symptoms at follow-up, or when metastases were identified during imaging for other clinical conditions. The majority of patients during this time period were imaged with a chest radiograph, liver ultrasound and bone radiography and/or scintigraphy; CT and MRI being used as problem solving tools in selected patients. Breast cancer specific survival (BCSS) was defined as the interval between the operation and death from breast cancer, death being scored as an event, and patients who died from other causes or were still alive were censored at the time of last follow-up. Disease-free interval (DFI) was also calculated from the date of first operation, with first recurrences, local, regional or distant, being scored as an event, and with censoring of other patients at the time of last follow-up or death. Local recurrence was defined as tumour arising in the treated breast or chest wall. Regional recurrence was defined as tumour arising in the axillary or internal mammary lymph nodes (LN).
From the whole series (4412 breast cancers of all types), 140 (3.6%) mixed ductal and lobular carcinomas were identified. These tumours were diagnosed at the time of presentation as mixed tumours and coded as mixed ductal carcinoma of no special type (duct/NST; IDC) and invasive lobular carcinomas (ILC) in the database and therefore, formed the base of this study. These 140 cases were compared to 2048 IDC and 255 ILC of pure histologic type [34–38]. In addition, haematoxylin & eosin stained sections of representative tumour blocks and positive LN from the corresponding cases of these mixed tumours were examined to further assess the morphological features of these tumours and to study the relationship between these features and the histologic tumour type in the metastatic site and tumour behaviour. These tumours were examined for the following morphological parameters; presence and grade of ductal carcinoma in-situ (DCIS), lobular carcinoma in-situ (LCIS) and the predominant histologic invasive tumour type as defined by presence of distinct components of the tumour of either ductal or distinct lobular morphology, irrespective of the proportion or dominance of either component. Data on several other prognostic biomarkers with close relevance to breast cancer were also available. These markers included progesterone (PgR) and androgen (AR) receptors, HER1 (EGFR), HER2, HER4 (cerbB4), p53, P-cadherin, E-cadherin, FHIT protein, neuroendocrine markers (chromogranin-A and synaptophysin), SMA, p63 and basal cytokeratins (CK5/6 and CK14) [34, 36].
Statistical analysis
Statistical analysis was performed using SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). The clinical and biologic characteristics of mixed tumours as compared to both pure ILC and IDC were assessed using contingency tables, χ2 tests, Fisher’s exact tests and student t-tests. BCSS and DFI curves were drawn using Kaplan–Meier estimates, and were compared using log rank tests. Survival rates are presented with their 95% confidence intervals. Multivariate analyses of BCSS and DFI, with stepwise variable selection, were conducted using Cox proportional hazard regression models. A P-value <0.05 was considered significant. Cutoff values for the different IHC biomarkers included in this study were chosen before statistical analysis. Standard cutoffs were used for established prognostic factors and were the same as for previously published data [34, 36].
Results
Of the 140 mixed ductal and lobular carcinoma cases, 82 (59%) were grade 2, 57 (41%) had lymph node positive disease and 57 (40.7%) had lymphovascular invasion (Table 1). Median tumour size was 19 mm (range 6–80 mm). Patients’ age varied from 34 to 70 years (median 57 years). Hormonal therapy was given to 48 patients and chemotherapy to 18 patients. Follow-up data was available for 88 cases of mixed tumours, 1970 IDC and 243 ILC. Of the 88 mixed cases, 20 cases developed locoregional recurrences and 16 cases developed DM (9 cases to the bone, 6 with predominant lobular).
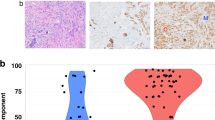
On histologic review, we found that mixed tumours consist of areas with solid/cohesive growth pattern consistent with IDC admixed with areas of discohesive malignant cells with single cell infiltration pattern that are defined as ILC. Importantly, in this study we have only considered tumours with mixed but well defined histologic types and did not include tumours with hybrid morphology that show morphologic features of both lobular and ductal tumours, usually a lobular diffuse infiltrative pattern but with no specific cellular morphology. In our routine practice, such hybrid cases are diagnosed as IDC with lobular features and coded as IDC. According to proportion of each tumour subtype, mixed tumours were classified as predominantly ductal (>50% of the invasive tumour area is IDC; 57%) and predominately lobular (>50% of the invasive tumour area is ILC; 43%) carcinomas. Associated DCIS was found in 123 cases (89%) [13% low grade, 36% intermediate grade and 51% was of high grade]. LCIS was found in 43 cases (31%); 60% of these cases were predominantly lobular. Both DCIS and LCIS were found together in 39 cases; LCIS alone in 4 cases and DCIS alone in 84 cases. Examination of positive LN showed metastasis of ductal morphology in 29 cases (60.5%), lobular in 18 cases (37.5%) and both ductal and lobular in 4 cases (Table 2). In these 4 cases with combined “IDC and ILC” LN metastasis, the primary tumours were predominantly lobular in three and ductal in one. Interestingly, in the 3 cases of predominantly lobular primary tumour and metastatic ductal tumour in the LN, 2 cases were associated with extensive DCIS.
Association between mixed ductal and lobular tumours with clinicopathological variables
Tables 1 and 3 summarize the clinicopathologic and immunophenotypic features of mixed ductal and lobular tumours as compared to ILC and IDC. When compared to ILC, mixed tumours are more frequent in premenopausal women, of higher histologic grade, associated with VI and more likely to receive chemotherapy, and to develop locoregional recurrence and distant metastasis. They are associated with positive expression of E-cadherin, P-cadherin, HER4, neuroendocrine and myoepithelial markers and absence of expression of androgen, BRCA1 and FHIT proteins. Compared to IDC, mixed tumours were more frequently associated with lower histologic grade, positive expression of hormone receptors, and BRCA1, and negative expression of E-cadherin, P-cadherin, p53, HER1 and HER4. Patients with mixed tumours were less likely to receive chemotherapy than those with IDC.
Outcome
Of the 16 patients with mixed tumours who developed DM, 10 cases (63%) metastasized to bone, 4 to the pleura, 2 to the liver and 1 to each of the lung and brain. Three cases showed metastasis to multiple sites including ovaries and peritoneum. Of the 10 cases that metastasized only to bone, the primary tumours of 8 patients showed a predominant lobular histologic type. Of the 21 ILC cases with DM, 11 cases metastasized to bone (52%), 3 cases to the pleura (14%) and only one case to the lung while in IDC, 91 cases (31%) metastasized to bone, 54 (18%) to the liver, 10% to the lung and 6% to the brain. However, no other association between the predominant histologic type of the primary tumours and any other prognostic variable or patients’ outcome was found.
Mixed tumours showed no difference in survival when compared to IDC (Log Rank (LR) = 0.2, P = 0.65, and LR = 0.02, P = 0.88 for BCSS and DFS respectively) but worse survival compared to ILC (LR = 5.16, P = 0.023, LR = 4.5, P = 0.033 for BCSS and DFS respectively) (Figs. 1 and 2). However, these differences were lost in multivariate analysis when tumour grade was included in the model suggesting that the association with outcome is grade dependent.
In addition, to assess the prognostic value of the different variables in the mixed tumours, univariate and multivariate survival analyses of the mixed tumours as well as of the entire series (3 tumour types) were performed. Univariate analyses show that, in the whole series, grade (1–3), LN stage (1–3), tumour size, VI, and ER status were associated with survival. In the mixed tumours, we found that only grade and ER status were associated with survival. Two additional multivariate analyses were performed, in order to determine the independent prognostic factors in these tumours and to confirm the results of univariate analyses. The first model was used for the entire series and showed that all five parameters were independently associated with survival (histologic grade, LN stage, tumour size, VI and ER status). The second model was used for mixed tumours only. In this model, only ER status and histologic grade were associated with survival (Table 4). When predominantly ductal mixed tumours were compared to predominantly lobular mixed tumours, no difference in BCSS was found (LR = 0.7, P = 0.4). Although predominantly lobular tumours showed a trend towards longer DFI, the difference was not statistically significant (LR = 2.9, P = 0.084).
Discussion
The management of primary breast cancer has changed over the years, and systemic adjuvant therapy is now commonplace. These recent advances in breast cancer treatment have made recognition and characterization of different prognostic groups mandatory. Invasive ductal and lobular (ILC) breast carcinomas are the most common histological types of invasive carcinoma of the breast. Several molecular profiling studies, clinical and follow-up data, and the patterns of metastases suggest that these histological types of breast cancer show genetic and biological differences. However, some tumours show mixed ductal and lobular morphology and the clinical and biological significance of these tumours is currently unrecognised and few studies have addressed these tumours [1, 5, 12].
The incidence of mixed ductal and lobular tumours observed in the present study (3.6%) is in accordance with the range of 2–6% reported in the literature [2–4, 20, 39]. The current study demonstrates that these tumours have clinical and biologic differences compared to the more common and more extensively studied pure IDC and ILC and show features midway between both tumour types. Compared to pure IDC, mixed tumours are associated with markers of good prognosis such as lower histologic grade, positive hormone receptors and negative expression of P-cadherin and p53. Compared to pure ILC, they were more frequent in younger premenopausal patients and were associated with markers of poor prognosis such as higher grade, VI, positive expression of P-cadherin and lack of expression of androgen, BRCA1 and FHIT proteins and they were more likely to develop locoregional recurrence and distant metastasis. Mixed ductal and lobular tumours are more commonly associated with DCIS than LCIS.
One of the major problems of mixed tumours is that the outcome of patients will be determined by the poorer prognostic characteristics of either component type and good prognostic characteristics of one tumour component may have no effect in the presence of the other tumour type. In order to determine the behaviour of these mixed tumours, we assessed several morphologic features of these tumours and compared them with the histologic type and site of metastasis. We found that when these mixed tumours develop metastasis to the LN, the histologic type of the metastatic tumour usually correlates with the predominant histologic type of the primary tumours. When they develop distant metastasis, they frequently metastasize to bone and less often to the liver and lung; a pattern similar to that of ILC, particularly in the tumours with predominant lobular type. Consistent with our results, it has been reported that ILC less often involves the lungs [2, 3, 39] and CNS [39], and liver [19, 20], but is more likely to involve bone [1] than IDC. It has also been documented that metastases to the gastrointestinal system, gynaecologic organs, and peritoneum are more characteristic of lobular carcinoma. In addition, this study confirms and extends the findings of the previous study that has addressed the biologic features of mixed ductal and lobular tumours [1] indicating that these tumours have features between IDC and ILC. They are more likely to be steroid receptor positive and of lower grade than IDC and of younger age and higher grade than ILC patients [13, 40, 41]. The prognosis for mixed tumours compared to IDC or ILC is unclear and no previous studies have addressed this issue. In the present study, the survival of patients with these mixed tumours was found to be similar to that of IDC; and slightly worse than those of ILC; however, this association seemed to be the result of the association of these tumours with a higher histologic grade than ILC.
In this study, there was no difference in survival between mixed tumours with predominantly ductal or predominantly lobular morphology when classified using the arbitrary UK and WHO guidance of these mixed tumours (>50% of the special type component). Moreover, we found it difficult at least in some cases to accurately decide the proportion of each histologic type. This may argue against clinical significance of using this cut-off in routine practice.
Previous studies have demonstrated that E-cadherin expression is lost in the majority of ILC (80–90%) and in the areas of lobular morphology in the mixed tumours, while it is usually positive in IDC (particularly in low grade tumours) and areas of ductal morphology in the mixed tumours [41, 42]. E-cadherin is therefore, one of the major defining features of lobular tumours; rather than a prognostic factor to differentiate between ductal and lobular tumours’ outcome. Although, in the current study, E-cadherin positivity was found in more than a third of mixed tumours, this can be explained by positive staining in the areas with ductal morphology in these tumours and hence the overall score of these cases may reflect staining of ductal component and does not mean absence of lobular component in these tumours.
In conclusion, our results show that mixed ductal and lobular tumours are a distinct entity with features intermediate between ILC and IDC. Assessment of the predominant histologic type of the primary tumour can give an idea about the histologic type of metastatic tumours but does not provide prognostic value in terms of patients’ survival. Although no clinically meaningful differences in survival are observed, mixed tumours showed a worse outcome than pure ILC.
References
Sastre-Garau X, Jouve M, Asselain B et al (1996) Infiltrating lobular carcinoma of the breast. Clinicopathologic analysis of 975 cases with reference to data on conservative therapy and metastatic patterns. Cancer 77(1):113–120
Lamovec J, Bracko M (1991) Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 48(1):28–33
Arpino G, Bardou VJ, Clark GM, Elledge RM (2004) Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 6(3):R149–R156
Borst MJ, Ingold JA (1993) Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery 114(4):637–641. Discussion 41–42
Li CI, Anderson BO, Daling JR, Moe RE (2003) Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289(11):1421–1424
Ellis IO, Galea M, Broughton N et al (1992) Pathological prognostic factors in breast cancer II. Histological type. Relationship with survival in a large study with long-term follow-up. Histopathology 20(6):479–489
Reeves GK, Beral V, Green J, Gathani T, Bull D (2006) Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 7(11):910–918
Li CI, Anderson BO, Porter P et al (2000) Changing incidence rate of invasive lobular breast carcinoma among older women. Cancer 88(11):2561–2569
Molland JG, Donnellan M, Janu NC et al (2004) Infiltrating lobular carcinoma—a comparison of diagnosis, management and outcome with infiltrating duct carcinoma. Breast 13(5):389–396
Yoder BJ, Wilkinson EJ, Massoll NA (2007) Molecular and morphologic distinctions between infiltrating ductal and lobular carcinoma of the breast. Breast J 13(2):172–179
Rakha EA, El-Sayed ME, Powe DG et al (2008) Invasive lobular carcinoma of the breast: Response to hormonal therapy and outcomes. Eur J Cancer 44(1):73–83
Li CI, Daling JR, Malone KE et al (2006) Relationship between established breast cancer risk factors and risk of seven different histologic types of invasive breast cancer. Cancer Epidemiol Biomarkers Prev 15(5):946–954
Cleton-Jansen AM (2002) E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res 4(1):5–8
Korkola JE, DeVries S, Fridlyand J et al (2003) Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res 63(21):7167–7175
Sarrio D, Perez-Mies B, Hardisson D et al (2004) Cytoplasmic localization of p120ctn and E-cadherin loss characterize lobular breast carcinoma from preinvasive to metastatic lesions. Oncogene 23(19):3272–3283
Tubiana-Hulin M, Stevens D, Lasry S et al (2006) Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol 17(8):1228–1233
du Toit RS, Locker AP, Ellis IO et al (1991) An evaluation of differences in prognosis, recurrence patterns and receptor status between invasive lobular and other invasive carcinomas of the breast. Eur J Surg Oncol 17(3):251–257
Yeatman TJ, Cantor AB, Smith TJ et al (1995) Tumor biology of infiltrating lobular carcinoma. Implications for management. Ann Surg 222(4):549–559. Discussion 59–61
Ferlicot S, Vincent-Salomon A, Medioni J et al (2004) Wide metastatic spreading in infiltrating lobular carcinoma of the breast. Eur J Cancer 40(3):336–341
Winston CB, Hadar O, Teitcher JB et al (2000) Metastatic lobular carcinoma of the breast: patterns of spread in the chest, abdomen, and pelvis on CT. AJR Am J Roentgenol 175(3):795–800
Cocquyt VF, Blondeel PN, Depypere HT et al (2003) Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol 29(4):361–367
Ashikari R, Huvos AG, Urban JA, Robbins GF (1973) Infiltrating lobular carcinoma of the breast. Cancer 31(1):110–116
Mate TP, Carter D, Fischer DB et al (1986) A clinical and histopathologic analysis of the results of conservation surgery and radiation therapy in stage I and II breast carcinoma. Cancer 58(9):1995–2002
Vo TN, Meric-Bernstam F, Yi M et al (2006) Outcomes of breast-conservation therapy for invasive lobular carcinoma are equivalent to those for invasive ductal carcinoma. Am J Surg 192(4):552–555
Korhonen T, Huhtala H, Holli K (2004) A comparison of the biological and clinical features of invasive lobular and ductal carcinomas of the breast. Breast Cancer Res Treat 85(1):23–29
Casolo P, Raspadori A, Drei B et al (1997) Natural history of breast cancer: lobular carcinoma versus ductal carcinoma in our experience. Ann Ital Chir 68(1):43–47 Discussion 48
Peiro G, Bornstein BA, Connolly JL et al (2000) The influence of infiltrating lobular carcinoma on the outcome of patients treated with breast-conserving surgery and radiation therapy. Breast Cancer Res Treat 59(1):49–54
Silverstein MJ, Lewinsky BS, Waisman JR et al (1994) Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer 73(6):1673–1677
Toikkanen S, Pylkkanen L, Joensuu H (1997) Invasive lobular carcinoma of the breast has better short- and long-term survival than invasive ductal carcinoma. Br J Cancer 76(9):1234–1240
Cristofanilli M, Gonzalez-Angulo A, Sneige N et al (2005) Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol 23(1):41–48
Dixon JM, Anderson TJ, Page DL, Lee D, Duffy SW (1982) Infiltrating lobular carcinoma of the breast. Histopathology 6(2):149–161
du Toit RS, Locker AP, Ellis IO et al (1989) Invasive lobular carcinomas of the breast–the prognosis of histopathological subtypes. Br J Cancer 60(4):605–609
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Abd El-Rehim DM, Ball G, Pinder SE et al (2005) High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int J Cancer 116(3):340–350
Rakha EA, El-Sayed ME, Green AR et al (2007) Prognostic markers in triple-negative breast cancer. Cancer 109(1):25–32
Rakha EA, Putti TC, Abd El-Rehim DM et al (2006) Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol 208(4):495–506
Abd El-Rehim DM, Pinder SE, Paish CE et al (2004) Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 203(2):661–671
Abd El-Rehim DM, Pinder SE, Paish CE et al (2004) Expression and co-expression of the members of the epidermal growth factor receptor (EGFR) family in invasive breast carcinoma. Br J Cancer 91(8):1532–1542
Fondrinier E, Guerin O, Lorimier G (1997) A comparative study of metastatic patterns of ductal and lobular carcinoma of the breast from two matched series (376 patients). Bull Cancer 84(12):1101–1107
Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ (2001) Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 115(1):85–98
Berx G, Cleton-Jansen AM, Nollet F et al (1995) E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. Embo J 14(24):6107–6115
Goldstein NS (2002) Does the level of E-cadherin expression correlate with the primary breast carcinoma infiltration pattern and type of systemic metastases? Am J Clin Pathol 118(3):425–434
Author information
Authors and Affiliations
Corresponding authors
Additional information
This study was approved by Nottingham Research Ethics Committee 2 under the title of “Development of a molecular genetic classification of breast cancer”.
Rights and permissions
About this article
Cite this article
Rakha, E.A., Gill, M.S., El-Sayed, M.E. et al. The biological and clinical characteristics of breast carcinoma with mixed ductal and lobular morphology. Breast Cancer Res Treat 114, 243–250 (2009). https://doi.org/10.1007/s10549-008-0007-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-008-0007-4